The Water or Hydrological Cycle Water Cycle Water













- Slides: 18

The Water or Hydrological Cycle

Water Cycle �Water cycle path of water through the environment �From the earth to the atmosphere and back to the earth �Importance of water: �Absorbs and releases water = stable global weather patterns �Medium for reactions �Solvent � 60% of cell’s mass

Properties of Water � All living things need water � Water can be found in the biosphere in three states” 1. 2. 3. Solid (snow or ice) Liquid Gas ( vapour) � Water is continuously entering and leaving living systems

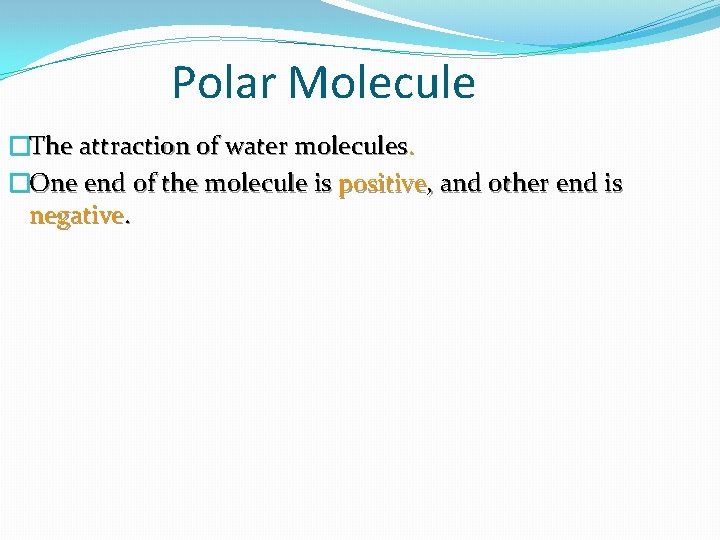
Polar Molecule �The attraction of water molecules. �One end of the molecule is positive, and other end is negative.

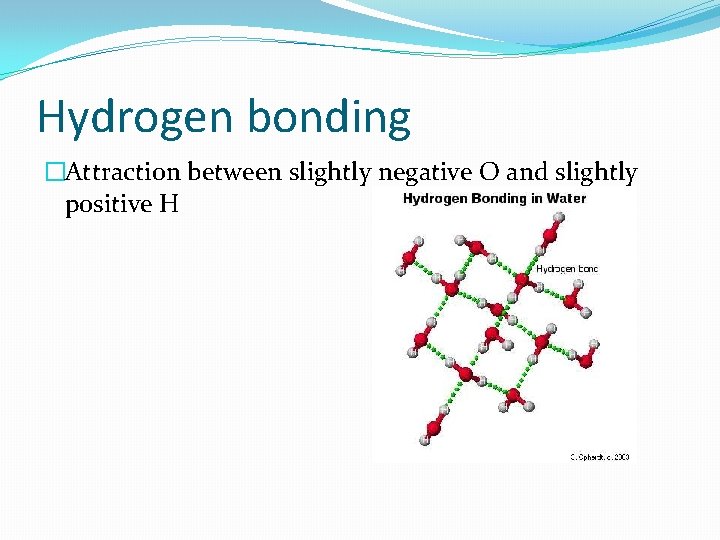
Hydrogen bonding �Attraction between slightly negative O and slightly positive H

Higher Boiling and Freezing �Takes more energy to make molecules let go of each other and change state �Liquid to gas �Solid to liquid



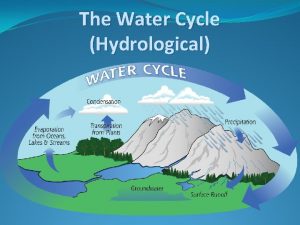
Hydrological Cycle Terms – Abiotic Components �Precipitation �atmospheric water falling in the form of �rain, snow, sleet, hail, dew. . . �Groundwater �water on the surface or just below the surface �lakes, rivers, oceans, aquifers, artesian wells… �Evaporation �water that has been heated by the sun rises as water vapor. �Condensation �vapor into droplets when cooled

More Hydrological Cycle Terms – Biotic Components �Cellular Respiration �the process in which living organisms convert the energy of sugars into energy used by body processes �Transpiration �the loss of water through the leaves of plants

Water’s Role �Moves from atmosphere to earth through precipitation �Surface runoff and groundwater flows to other bodies of water �Water enters the atmosphere through transpiration and evaporation

Role of living things �Plants: �Return water to the atmosphere through transpiration and evaporation �Animals: �Return water to the atmosphere through gas exchange, perspiration, evaporation, excretion, cellular respiration

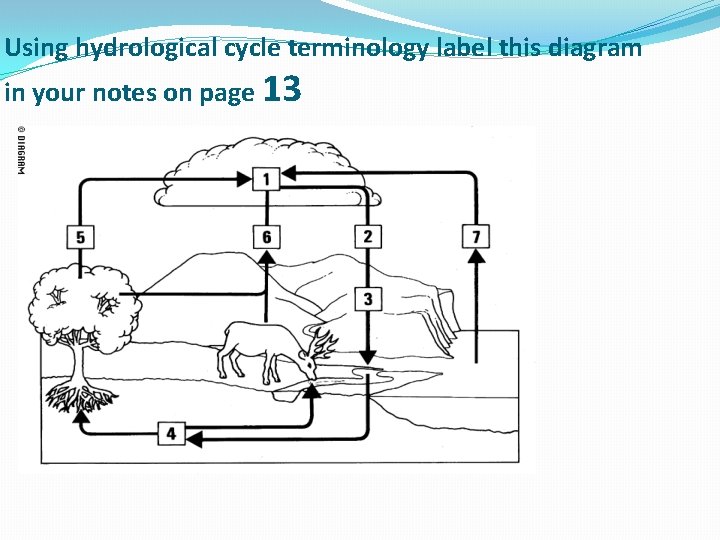
Using hydrological cycle terminology label this diagram in your notes on page 13

Water Cycle 1. Water in clouds (condensation) 2. Rain and snow (precipitation) 3. Water drains into river and soil (run off) 4. Water taken up by plants and animals (through groundwater) 5. Water loss by transpiration 6. Water loss by respiration 7. Evaporation

Water Beneath the Soil �Freshwater comes from two sources �Ground water: rain fall seeps into the soil and filters downwards due to gravity �Surface water: precipitation that collects above ground in lakes and rivers

Ground water is created by, �Percolation: water filters downward between the particles in the soil �Eventually the water fills the lower level of the soil and a water table forms above impenetrable bedrock or clay �As water seeps downwards it dissolves organic matter and minerals called leaching

Acid Deposition �Industrial plants burn metals & fossil fuels. �When they undergo combustion, sulfur is released into the air �This is usually in the form of SO 2 �Some industrial plants as well as automobiles also release nitrogen wastes �usually nitrous oxides (NOx)

So. �Sulfur dioxide and nitrous oxides can condense with water vapor in the air to form … �tiny droplets of acid. �This is called acid rain!! �Not all the SO 2 or NOx combine with H 2 O(g). �Some will circulate in the air and fall to the ground in the form of dry acid deposition.
 Condensation process example
Condensation process example Hydrological cycle diagram
Hydrological cycle diagram National meteorological and hydrological services
National meteorological and hydrological services Vietnam meteorological and hydrological administration
Vietnam meteorological and hydrological administration Croatian meteorological and hydrological service
Croatian meteorological and hydrological service Hydrological
Hydrological Hydrological prediction center
Hydrological prediction center Water and water and water water
Water and water and water water Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Glasgow thang điểm
Glasgow thang điểm Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng