Nuclear Reactions Cf E Higher Physics Nuclear Reactions



- Slides: 16

Nuclear Reactions Cf. E Higher Physics

Nuclear Reactions �Nuclear reactions are the processes that occur in the large nucleii of unstable elements. �The main reactions we will look at are: �Alpha, Beta & Gamma radiation �Induced nuclear fission �Nuclear fusion But first lets look at the Periodic Table and element symbols………….

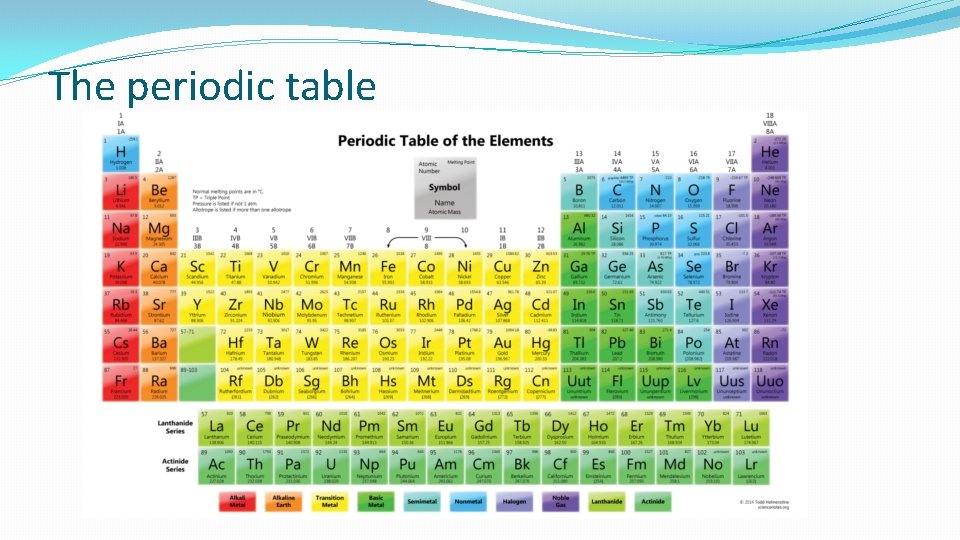
The periodic table

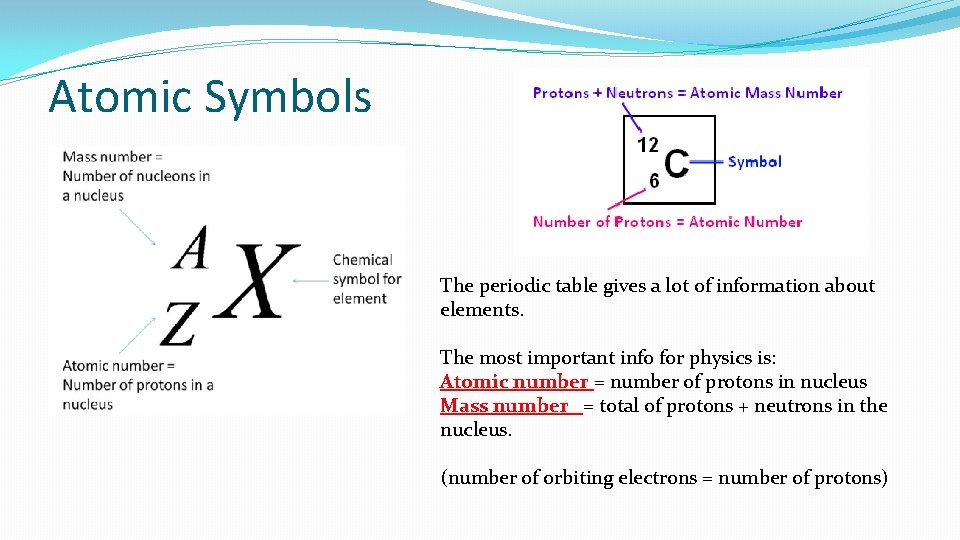
Atomic Symbols The periodic table gives a lot of information about elements. The most important info for physics is: Atomic number = number of protons in nucleus Mass number = total of protons + neutrons in the nucleus. (number of orbiting electrons = number of protons)

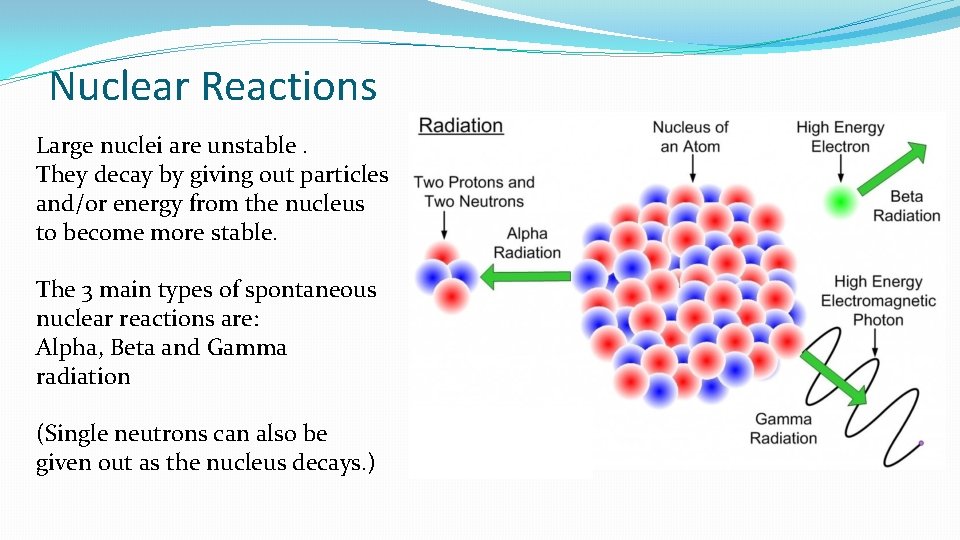
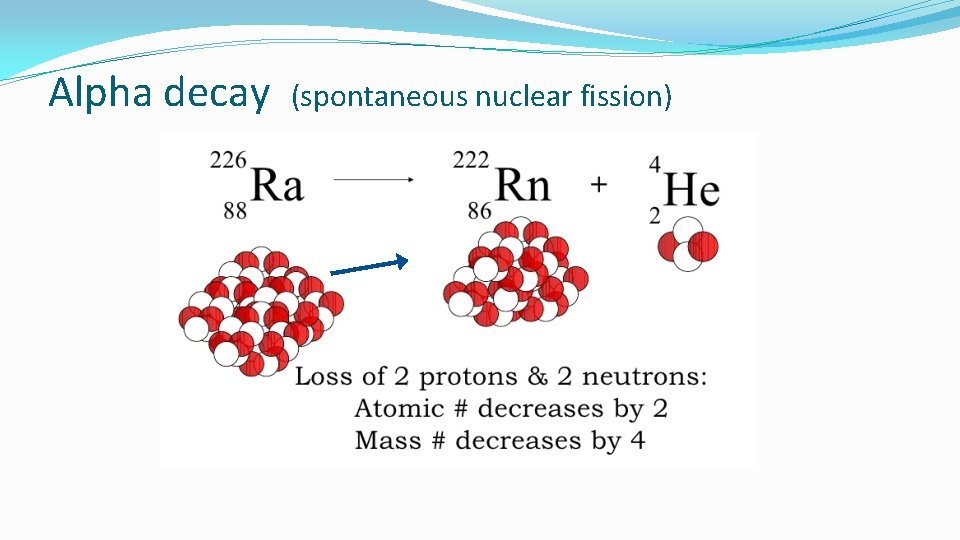
Nuclear Reactions Large nuclei are unstable. They decay by giving out particles and/or energy from the nucleus to become more stable. The 3 main types of spontaneous nuclear reactions are: Alpha, Beta and Gamma radiation (Single neutrons can also be given out as the nucleus decays. )

Alpha decay (spontaneous nuclear fission)

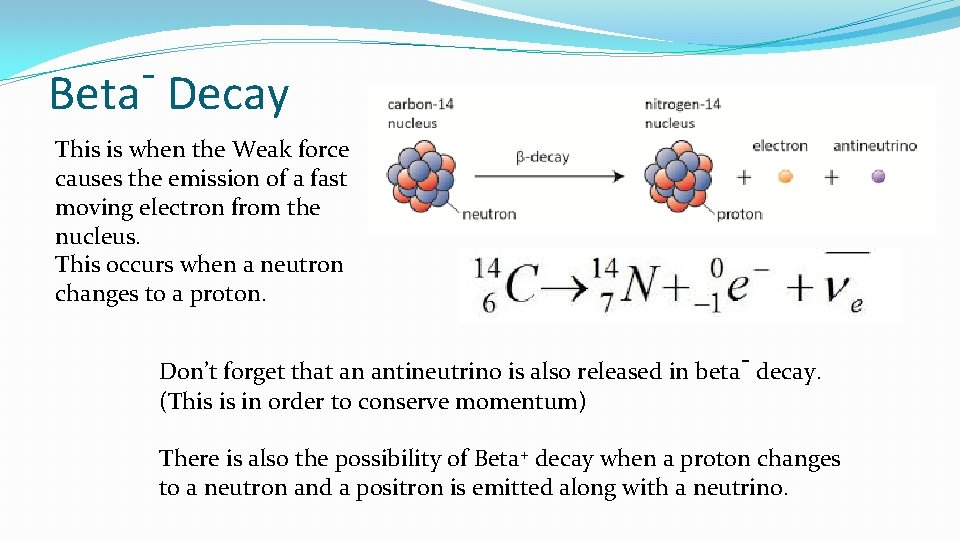
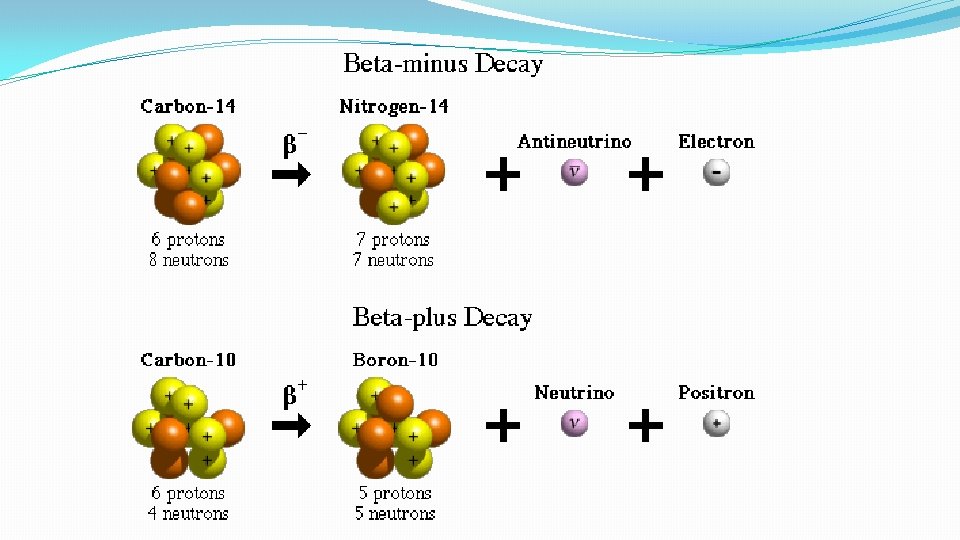
Beta Decay This is when the Weak force causes the emission of a fast moving electron from the nucleus. This occurs when a neutron changes to a proton. Don’t forget that an antineutrino is also released in beta- decay. (This is in order to conserve momentum) There is also the possibility of Beta+ decay when a proton changes to a neutron and a positron is emitted along with a neutrino.


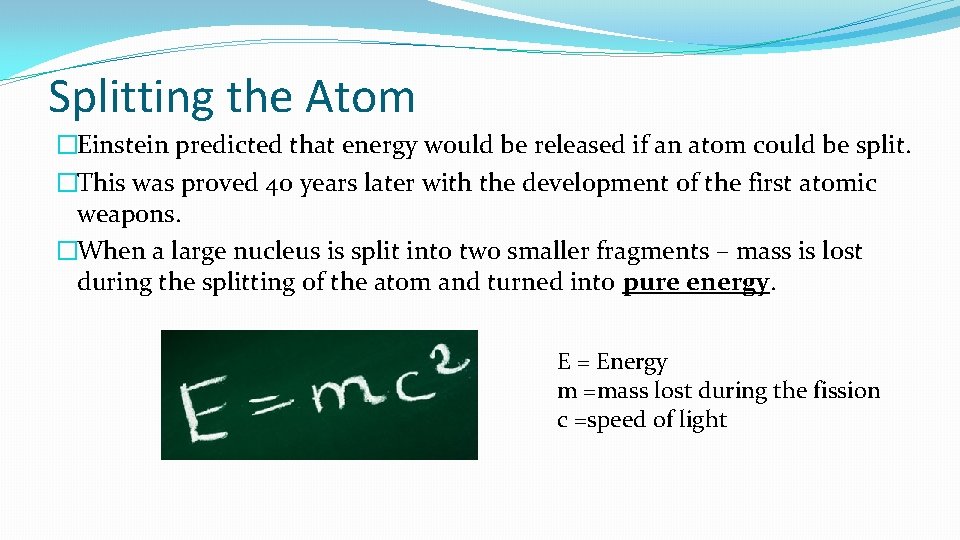
Splitting the Atom �Einstein predicted that energy would be released if an atom could be split. �This was proved 40 years later with the development of the first atomic weapons. �When a large nucleus is split into two smaller fragments – mass is lost during the splitting of the atom and turned into pure energy. E = Energy m =mass lost during the fission c =speed of light

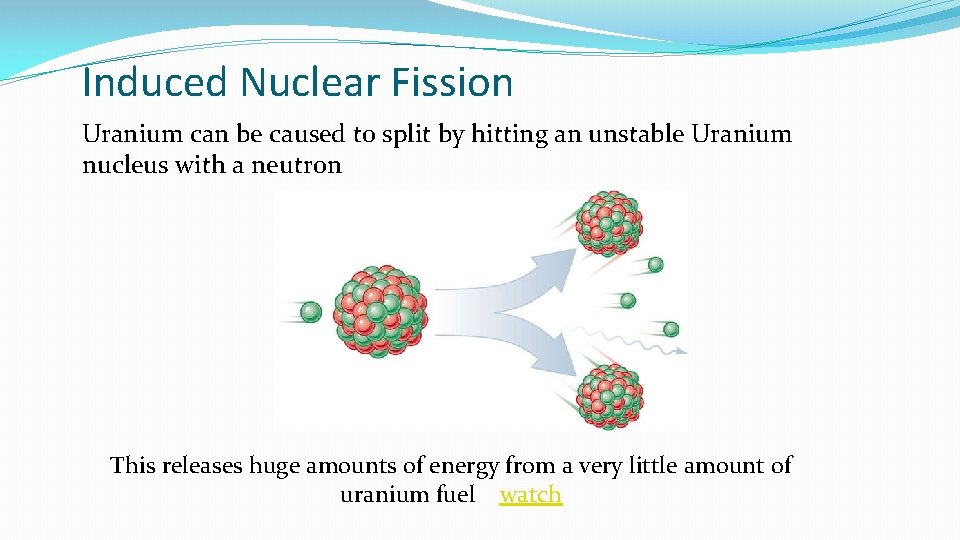
Induced Nuclear Fission Uranium can be caused to split by hitting an unstable Uranium nucleus with a neutron This releases huge amounts of energy from a very little amount of uranium fuel watch

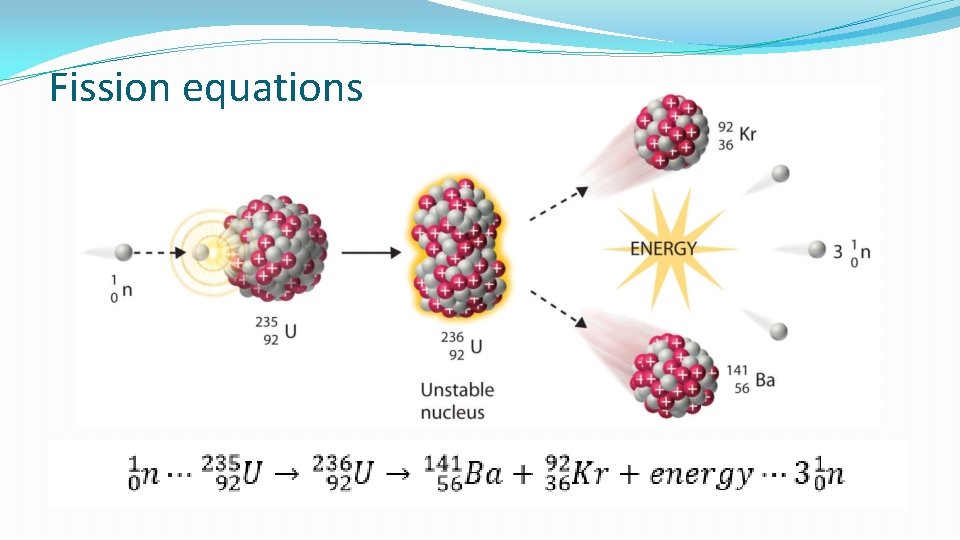
Fission equations

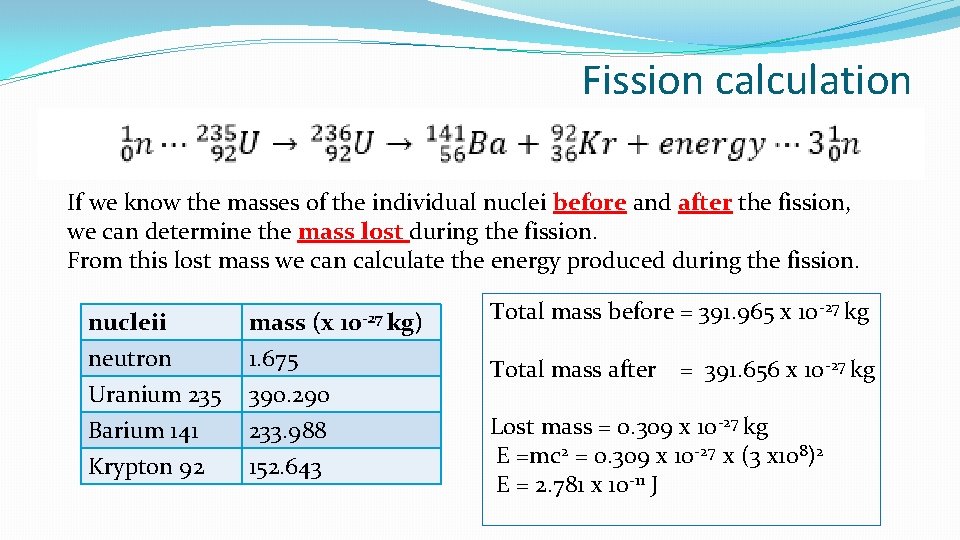
Fission calculation If we know the masses of the individual nuclei before and after the fission, we can determine the mass lost during the fission. From this lost mass we can calculate the energy produced during the fission. nucleii neutron Uranium 235 Barium 141 mass (x 1. 675 390. 290 233. 988 Krypton 92 152. 643 10 -27 kg) Total mass before = 391. 965 x 10 -27 kg Total mass after = 391. 656 x 10 -27 kg Lost mass = 0. 309 x 10 -27 kg E =mc 2 = 0. 309 x 10 -27 x (3 x 108)2 E = 2. 781 x 10 -11 J

Uses of fission �Nuclear power stations �Nuclear submarines �Nuclear weapons Watch watch

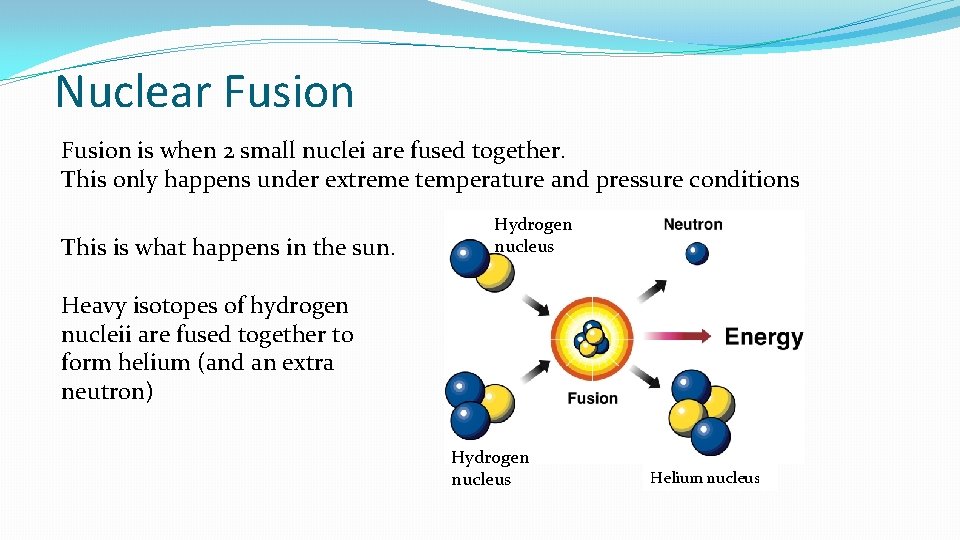
Nuclear Fusion is when 2 small nuclei are fused together. This only happens under extreme temperature and pressure conditions This is what happens in the sun. Hydrogen nucleus Heavy isotopes of hydrogen nucleii are fused together to form helium (and an extra neutron) Hydrogen nucleus Helium nucleus

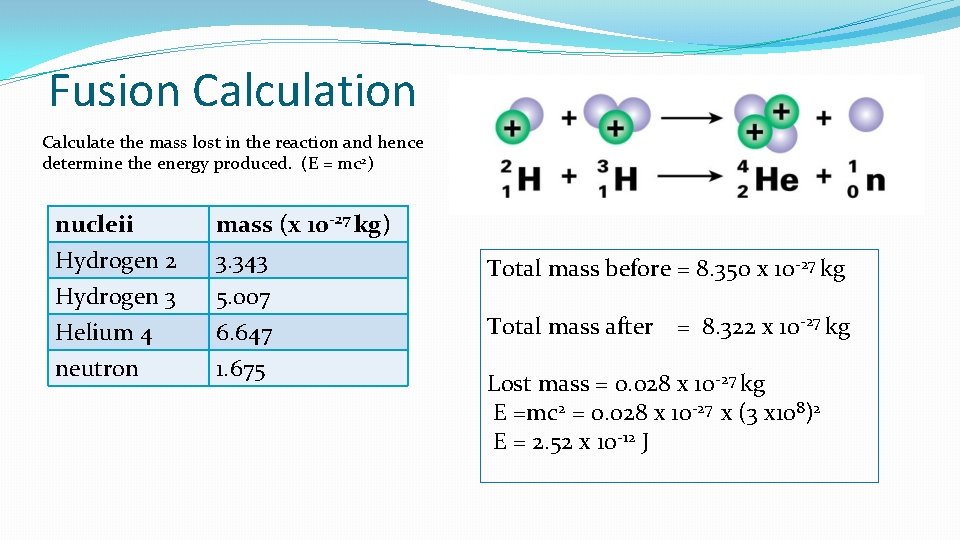
Fusion Calculate the mass lost in the reaction and hence determine the energy produced. (E = mc 2) nucleii Hydrogen 2 Hydrogen 3 Helium 4 mass (x 10 -27 kg) 3. 343 5. 007 6. 647 neutron 1. 675 Total mass before = 8. 350 x 10 -27 kg Total mass after = 8. 322 x 10 -27 kg Lost mass = 0. 028 x 10 -27 kg E =mc 2 = 0. 028 x 10 -27 x (3 x 108)2 E = 2. 52 x 10 -12 J

Atomic data �All of the data you require will be on the data sheet in the exam, but here’s a quick list � Charge on the electron = 1. 6 x 10 -19 C (negatively charged) (Charge on a proton is the same but it is positively charged) �Mass of the electron = 9. 11 x 10 -31 kg �Mass of a proton = 1. 673 x 10 -27 kg �Mass of a neutron= 1. 675 x 10 -27 kg Any other data you require will be given in the question………………. .