National Viral Hepatitis Control Program Elimination of Hepatitis











- Slides: 22

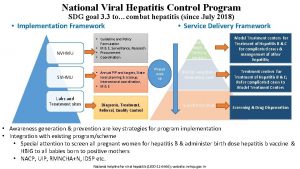
National Viral Hepatitis Control Program

• Elimination of Hepatitis C by 2030 • Reduction in the infected population, morbidity and mortality associated with Hepatitis B and C • Reduce the risk, morbidity and mortality due to Hepatitis A and E.

• Enhance community awareness on hepatitis and lay stress on preventive measures • Provide early diagnosis and management of viral hepatitis at all levels of healthcare • Develop capacities for implementation of standard diagnostic and treatment protocols

• Strengthen the existing infrastructure facilities – build capacities of existing human resource – raise additional human resources, only where required • Develop linkages with the existing National programs • Develop a web-based “Viral Hepatitis Information and Management System” to maintain a registry of persons affected with viral hepatitis and its sequelae.

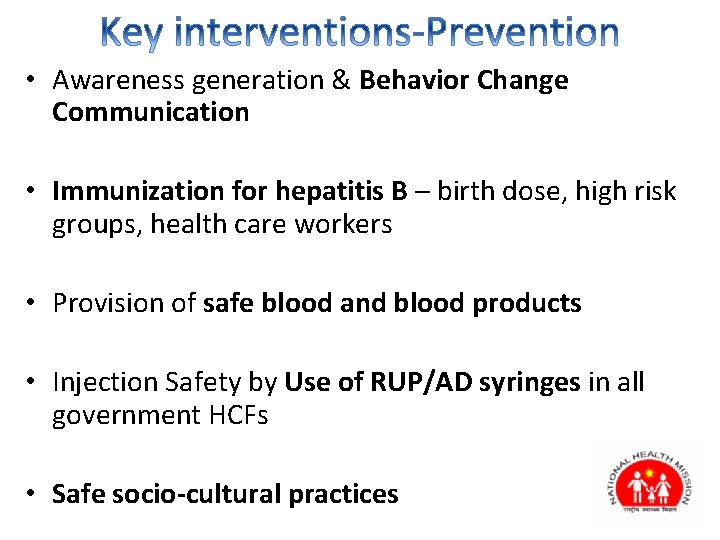
• Awareness generation & Behavior Change Communication • Immunization for hepatitis B – birth dose, high risk groups, health care workers • Provision of safe blood and blood products • Injection Safety by Use of RUP/AD syringes in all government HCFs • Safe socio-cultural practices


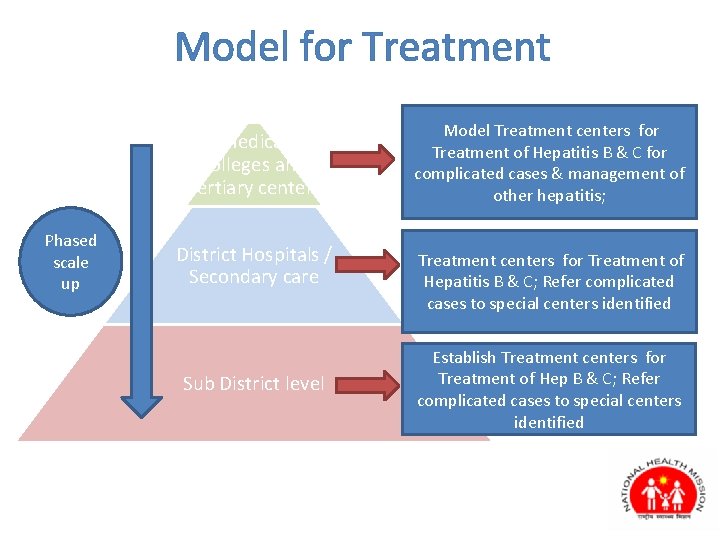
Model for Treatment Medical colleges and Tertiary centers Phased scale up District Hospitals / Secondary care Sub District level Model Treatment centers for Treatment of Hepatitis B & C for complicated cases & management of other hepatitis; Treatment centers for Treatment of Hepatitis B & C; Refer complicated cases to special centers identified Establish Treatment centers for Treatment of Hep B & C; Refer complicated cases to special centers identified

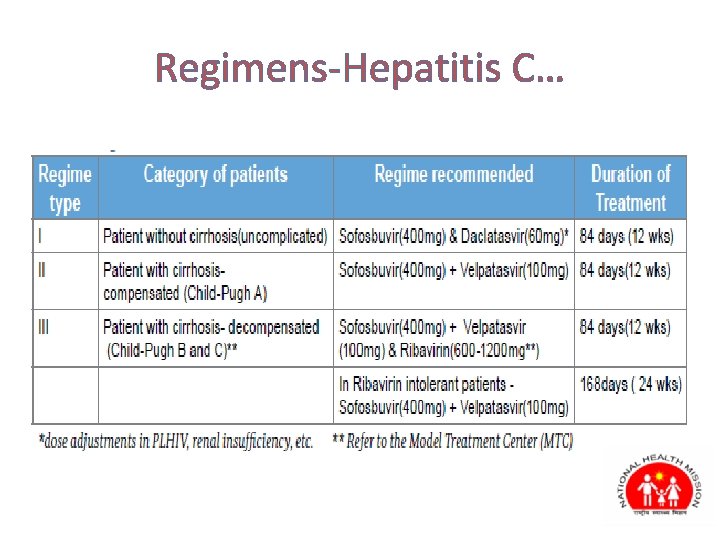
Regimens-Hepatitis C…

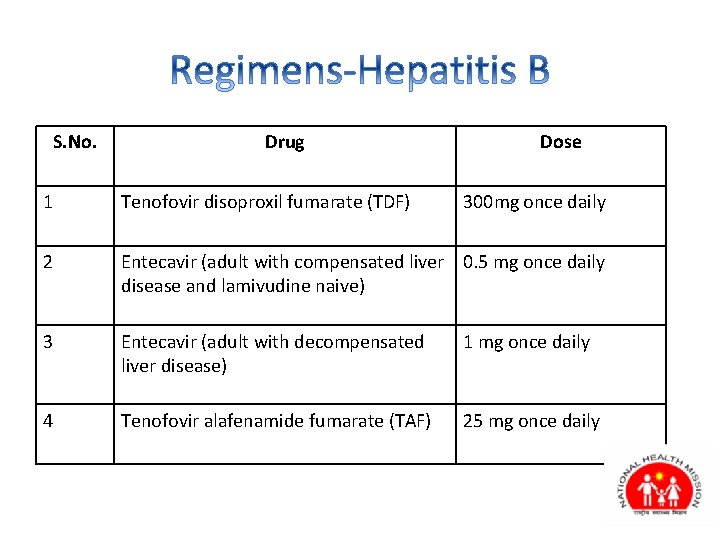
S. No. Drug Dose 1 Tenofovir disoproxil fumarate (TDF) 300 mg once daily 2 Entecavir (adult with compensated liver 0. 5 mg once daily disease and lamivudine naive) 3 Entecavir (adult with decompensated liver disease) 1 mg once daily 4 Tenofovir alafenamide fumarate (TAF) 25 mg once daily

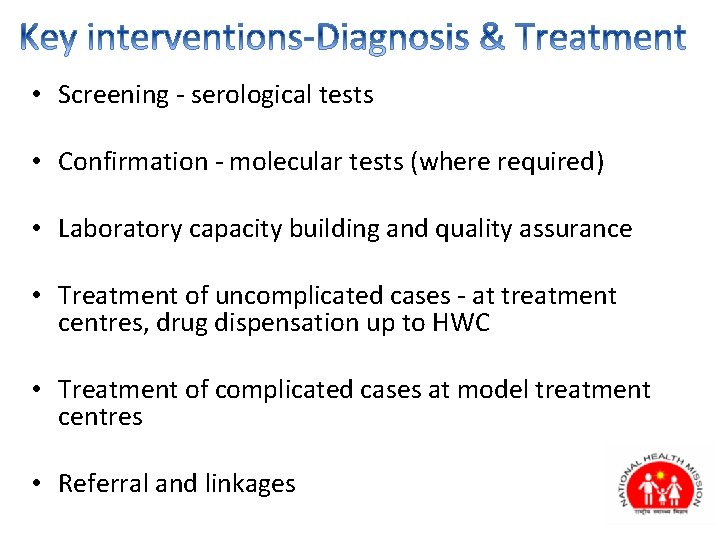
• Screening - serological tests • Confirmation - molecular tests (where required) • Laboratory capacity building and quality assurance • Treatment of uncomplicated cases - at treatment centres, drug dispensation up to HWC • Treatment of complicated cases at model treatment centres • Referral and linkages

• National Action Plan for combating viral hepatitis conceptualized and released • Program launched by the Hon’ble HFM in July, 2018 on the occasion of the World Hepatitis Day. Guidelines to standardize the operations of the program and laboratory and treatment protocols were released 1. National Guidelines for Diagnosis and Management of viral hepatitis 2. National Laboratory Guidelines for testing of viral hepatitis 3. National Viral Hepatitis Control Program-Operational guidelines • Advocacy event ‘India’s Response to Viral Hepatitis’ was held on 24 th Feb. , 2019 wherein goodwill ambassador of WHO SEARO was invited as guest on honour. The following were released during that event in Mumbai a. Technical Guidelines for Diagnosis and Management of Hepatitis B b. National Action Plan-Combating Viral Hepatitis in India c. National Viral Hepatitis Control Program website

• Two workshops have been held at the national level for the nodal officers to sensitize them about the services provided under the program. • Video-conferences held twice for the state principal secretaries, mission directors and state nodal officers for monitoring the program • Around 250 experts in the field of microbiology, gastroenterology, hepatology and medicine have been trained as master trainers from all the states/UTs at the national level to cascade down the training till lowest level of service delivery.

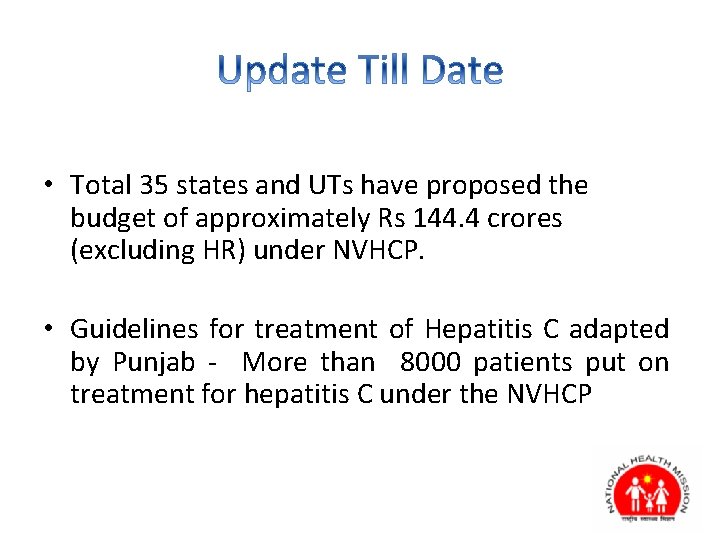
• Total 35 states and UTs have proposed the budget of approximately Rs 144. 4 crores (excluding HR) under NVHCP. • Guidelines for treatment of Hepatitis C adapted by Punjab - More than 8000 patients put on treatment for hepatitis C under the NVHCP

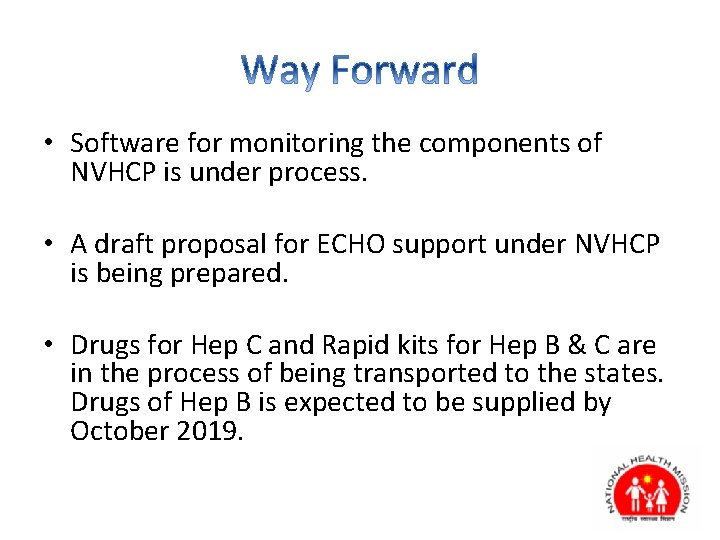
• Software for monitoring the components of NVHCP is under process. • A draft proposal for ECHO support under NVHCP is being prepared. • Drugs for Hep C and Rapid kits for Hep B & C are in the process of being transported to the states. Drugs of Hep B is expected to be supplied by October 2019.


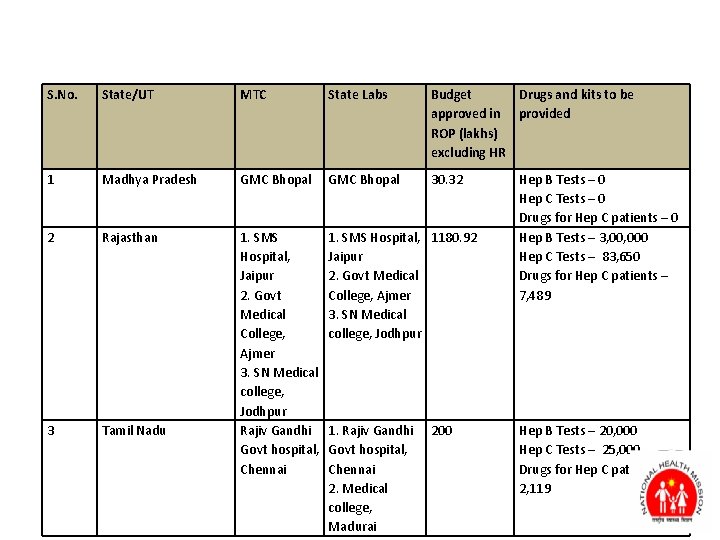
S. No. State/UT MTC State Labs Budget Drugs and kits to be approved in provided ROP (lakhs) excluding HR 1 Madhya Pradesh GMC Bhopal 30. 32 2 Rajasthan 1. SMS Hospital, 1180. 92 Jaipur 2. Govt Medical College, Ajmer 3. SN Medical college, Jodhpur 3 Tamil Nadu 1. SMS Hospital, Jaipur 2. Govt Medical College, Ajmer 3. SN Medical college, Jodhpur Rajiv Gandhi Govt hospital, Chennai 1. Rajiv Gandhi Govt hospital, Chennai 2. Medical college, Madurai 200 Hep B Tests – 0 Hep C Tests – 0 Drugs for Hep C patients – 0 Hep B Tests – 3, 000 Hep C Tests – 83, 650 Drugs for Hep C patients – 7, 489 Hep B Tests – 20, 000 Hep C Tests – 25, 000 Drugs for Hep C patients – 2, 119

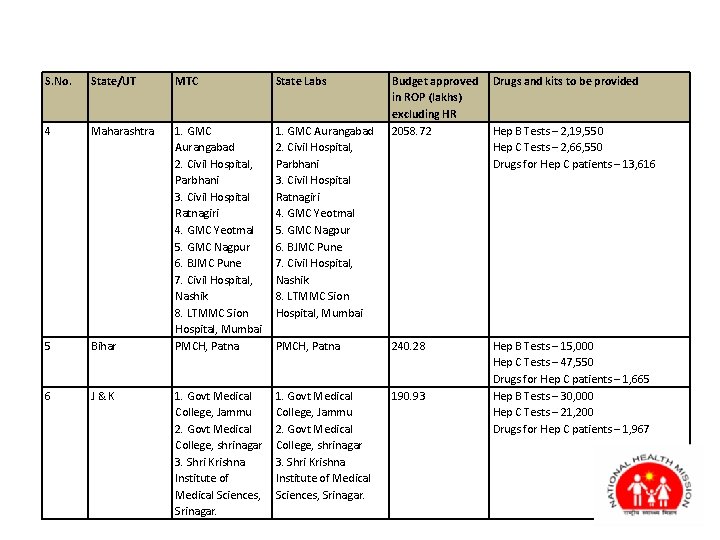
S. No. State/UT MTC State Labs Budget approved in ROP (lakhs) excluding HR 2058. 72 Drugs and kits to be provided 4 Maharashtra 1. GMC Aurangabad 2. Civil Hospital, Parbhani 3. Civil Hospital Ratnagiri 4. GMC Yeotmal 5. GMC Nagpur 6. BJMC Pune 7. Civil Hospital, Nashik 8. LTMMC Sion Hospital, Mumbai 5 Bihar 1. GMC Aurangabad 2. Civil Hospital, Parbhani 3. Civil Hospital Ratnagiri 4. GMC Yeotmal 5. GMC Nagpur 6. BJMC Pune 7. Civil Hospital, Nashik 8. LTMMC Sion Hospital, Mumbai PMCH, Patna 240. 28 1. Govt Medical College, Jammu 2. Govt Medical College, shrinagar 3. Shri Krishna Institute of Medical Sciences, Srinagar. 190. 93 Hep B Tests – 15, 000 Hep C Tests – 47, 550 Drugs for Hep C patients – 1, 665 Hep B Tests – 30, 000 Hep C Tests – 21, 200 Drugs for Hep C patients – 1, 967 6 J & K 1. Govt Medical College, Jammu 2. Govt Medical College, shrinagar 3. Shri Krishna Institute of Medical Sciences, Srinagar. Hep B Tests – 2, 19, 550 Hep C Tests – 2, 66, 550 Drugs for Hep C patients – 13, 616

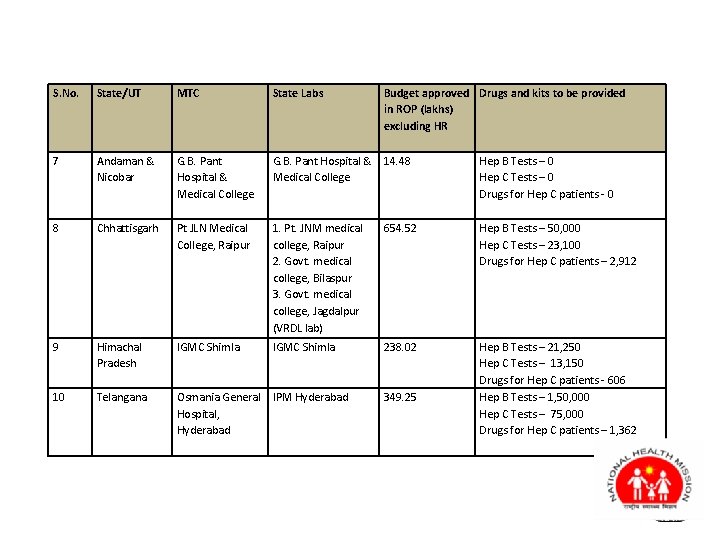
S. No. State/UT MTC State Labs Budget approved Drugs and kits to be provided in ROP (lakhs) excluding HR 7 Andaman & Nicobar G. B. Pant Hospital & Medical College G. B. Pant Hospital & 14. 48 Medical College Hep B Tests – 0 Hep C Tests – 0 Drugs for Hep C patients - 0 8 Chhattisgarh Pt JLN Medical College, Raipur 1. Pt. JNM medical college, Raipur 2. Govt. medical college, Bilaspur 3. Govt. medical college, Jagdalpur (VRDL lab) 654. 52 Hep B Tests – 50, 000 Hep C Tests – 23, 100 Drugs for Hep C patients – 2, 912 9 Himachal Pradesh IGMC Shimla 238. 02 10 Telangana Osmania General IPM Hyderabad Hospital, Hyderabad Hep B Tests – 21, 250 Hep C Tests – 13, 150 Drugs for Hep C patients - 606 Hep B Tests – 1, 50, 000 Hep C Tests – 75, 000 Drugs for Hep C patients – 1, 362 349. 25

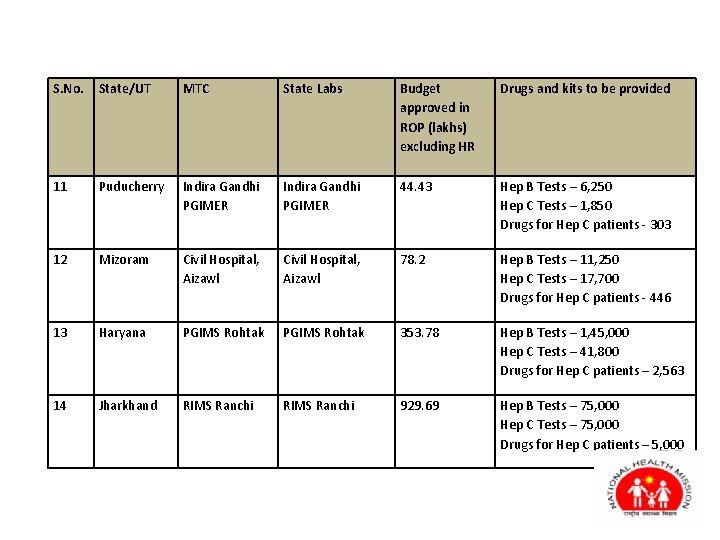
S. No. State/UT MTC State Labs Budget approved in ROP (lakhs) excluding HR Drugs and kits to be provided 11 Puducherry Indira Gandhi PGIMER 44. 43 Hep B Tests – 6, 250 Hep C Tests – 1, 850 Drugs for Hep C patients - 303 12 Mizoram Civil Hospital, Aizawl 78. 2 Hep B Tests – 11, 250 Hep C Tests – 17, 700 Drugs for Hep C patients - 446 13 Haryana PGIMS Rohtak 353. 78 Hep B Tests – 1, 45, 000 Hep C Tests – 41, 800 Drugs for Hep C patients – 2, 563 14 Jharkhand RIMS Ranchi 929. 69 Hep B Tests – 75, 000 Hep C Tests – 75, 000 Drugs for Hep C patients – 5, 000

S. No. State/UT MTC State Labs Budget approved Drugs and kits to be provided in ROP (lakhs) excluding HR 15 Delhi Lok Nayak Hospital, Delhi (MAMC) Lok Nayak Hospital, Delhi (MAMC) 195. 57 16 Karnataka Bangalore Medical College & Research Institute, Bangalore 1) Bangalore Medical 62. 17 College, Bangalore, 2)Mysore Medical College, Mysore, 3) Belgaum Institute of Medical Sciences, Belgaum 4) Gulbarga Institute of Medical Sciences, Gulbarga. Hep B Tests – 1, 11, 750 Hep C Tests – 64, 400 Drugs for Hep C patients – 1, 665 Hep B Tests – 0 Hep C Tests – 0 Drugs for Hep C patients - 0

• State Steering committee under Principal Secy Health for guiding the program • State Viral Hepatitis Management Unit – Nodal officer – Key officers from Blood, SACS, Immunisation, Maternal Health, infection control in the unit – Hiring of contractual staff if not available • Supplementary proposal for Hepatitis B whole blood test kits and drugs for Hepatitis B

Thank You
 Hepatitis viral
Hepatitis viral Overview of the national tuberculosis elimination program
Overview of the national tuberculosis elimination program Gaussjordan
Gaussjordan Dbcs in ophthalmology
Dbcs in ophthalmology Citizens energy group
Citizens energy group Aerochamber definition
Aerochamber definition Replicação viral ciclo lítico e lisogênico
Replicação viral ciclo lítico e lisogênico Section 24-1 viral structure and replication
Section 24-1 viral structure and replication Inklüzyon cisimcikleri
Inklüzyon cisimcikleri Egg inoculation diagram
Egg inoculation diagram Viral inoculation in embryonated egg
Viral inoculation in embryonated egg Egg inoculation diagram
Egg inoculation diagram Spasmodic croup
Spasmodic croup Variola varicela
Variola varicela Decapsidação
Decapsidação Viral load sample collection
Viral load sample collection Que causa la meningitis
Que causa la meningitis Causes of viral hemorrhagic fever
Causes of viral hemorrhagic fever Inmunidad
Inmunidad Viral infection
Viral infection Viral
Viral Viral recombination
Viral recombination Vaccins à vecteur viral
Vaccins à vecteur viral