National Cervical Screening Program Update Tuesday 15 May













- Slides: 18

National Cervical Screening Program Update Tuesday, 15 May 2018

Contents 1. National Cervical Screening Program (NCSP): Update • • • Cervical screening for women under 25 years Cervical screening for women aged 70 years and older Clarification of cervical cancer symptoms NCSP promotion in General Practice Self-collection Toolkit for under-screened and never-screened women 2. National Cancer Screening Register (NCSR): Update • Release 2 update • Accessing test results, screening histories and participant follow-up • Update on screening correspondence 3. Further information and contact 2

National Cervical Screening Program: Update Cervical screening for women under 25 years Background • Some women under the age of 25 will present expecting a routine Cervical Screening Test, having previously had Pap tests. It is important that these women are aware that cervical screening is no longer recommended in asymptomatic women under 25 and that Medicare does not fund routine cervical screening in this group. • Cervical cancer in women under 25 years of age is rare, and screening has not changed the incidence of cervical cancer or mortality from this disease in this age group in Australia since 1991. It usually takes 10 -15 years for a persistent HPV infection to develop into cervical cancer. • Commencing screening at age 25 will reduce the investigation and treatment of common cervical abnormalities that would usually resolve by themselves. 3

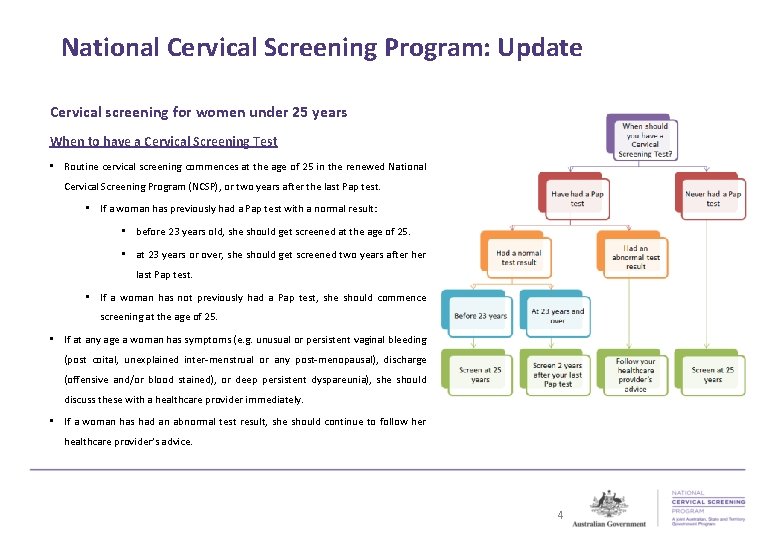
National Cervical Screening Program: Update Cervical screening for women under 25 years When to have a Cervical Screening Test • Routine cervical screening commences at the age of 25 in the renewed National Cervical Screening Program (NCSP), or two years after the last Pap test. • If a woman has previously had a Pap test with a normal result: • before 23 years old, she should get screened at the age of 25. • at 23 years or over, she should get screened two years after her last Pap test. • If a woman has not previously had a Pap test, she should commence screening at the age of 25. • If at any age a woman has symptoms (e. g. unusual or persistent vaginal bleeding (post coital, unexplained inter-menstrual or any post-menopausal), discharge (offensive and/or blood stained), or deep persistent dyspareunia), she should discuss these with a healthcare provider immediately. • If a woman has had an abnormal test result, she should continue to follow her healthcare provider’s advice. 4

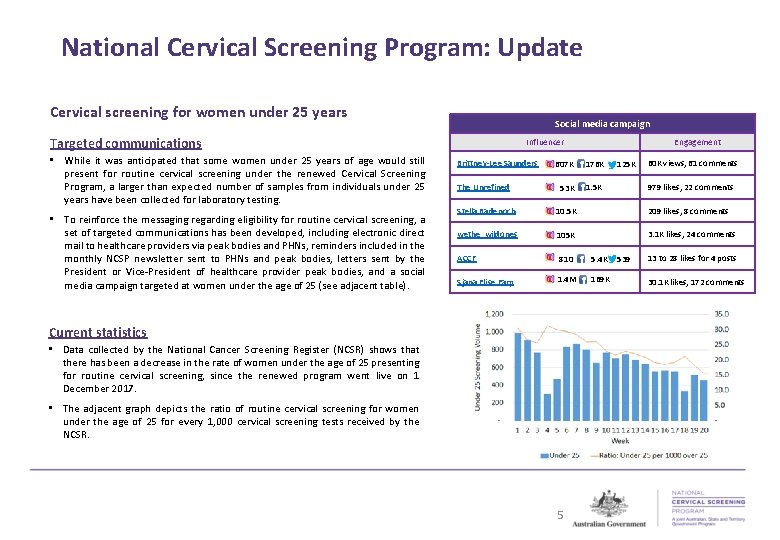
National Cervical Screening Program: Update Cervical screening for women under 25 years Social media campaign Targeted communications • While it was anticipated that some women under 25 years of age would still present for routine cervical screening under the renewed Cervical Screening Program, a larger than expected number of samples from individuals under 25 years have been collected for laboratory testing. • To reinforce the messaging regarding eligibility for routine cervical screening, a set of targeted communications has been developed, including electronic direct mail to healthcare providers via peak bodies and PHNs, reminders included in the monthly NCSP newsletter sent to PHNs and peak bodies, letters sent by the President or Vice-President of healthcare provider peak bodies, and a social media campaign targeted at women under the age of 25 (see adjacent table). Influencer Brittney-Lee Saunders Engagement 60 K views, 61 comments 607 K 176 K The Unrefined 53 K 1. 5 K Stella Badenoch 10. 5 K 209 likes, 8 comments wethe_wildones 105 K 3. 1 K likes, 24 comments ACCF Sjana Elise Earp 810 5. 4 K 1. 4 M 169 K Current statistics • Data collected by the National Cancer Screening Register (NCSR) shows that there has been a decrease in the rate of women under the age of 25 presenting for routine cervical screening, since the renewed program went live on 1 December 2017. • The adjacent graph depicts the ratio of routine cervical screening for women under the age of 25 for every 1, 000 cervical screening tests received by the NCSR. 5 125 K 979 likes, 22 comments 539 13 to 28 likes for 4 posts 30. 1 K likes, 172 comments

National Cervical Screening Program: Update Cervical screening for women under 25 years • Usability testing of communications activities and communications materials has been performed across participants of the NCSP, with a focus on women under the age of 25 and healthcare providers. • Usability testing identified specific areas where women under the age of 25 remain confused. For instance: • whether they should get the Pap test, the Cervical Screening Test, or both • how the two tests are different • what the Cervical Screening Test is for • timeframes about when to start testing • whether it is safe to be tested less frequently • whether they could choose to test before 25 • implications of being sexually active • how the HPV vaccination affects the timing and/or necessity of being tested • implications of previously having an abnormal test result, and • implications of pregnancy. • In order to address these gaps, further updates to the NCSP website (www. cancerscreening. gov. au/cervical) will be made in the upcoming weeks, and informed via the monthly NCSP newsletter. • We encourage healthcare providers to direct their patients to the NCSP website (www. cancerscreening. gov. au/cervical) for more information on the program. 6

National Cervical Screening Program: Update Cervical screening for women aged 70 years and older • Between the ages of 70 and 74, participants are encouraged to participate in routine cervical screening. • If a woman’s Cervical Screening Test result shows that no HPV infection is present, then she can safely stop screening and exit the National Cervical Screening Program. • If HPV is detected the woman should be followed up in accordance with the National Cervical Screening Program: Guidelines for the management of screen-detected abnormalities, screening in specific populations and investigation of abnormal vaginal bleeding (2016 Guidelines). • Women can choose to continue screening beyond 74 years of age, but will not receive reminders from the National Cancer Screening Register to screen. However, reminders for follow-up will be sent if an abnormal test result is received. • Women aged 75 and over are eligible for Medicare rebates if they are asymptomatic and have waited the appropriate timeframe between screening tests (i. e. four years and 9 months for clinician-collected Cervical Screening Tests and seven years for self-collected Cervical Screening Tests). Women with symptoms suggestive of cervical cancer can be tested at any age through Medicare. • Women aged 75 years or over who are in clinical management for cervical cancer and/or its precursors should complete clinical management and are eligible for Medicare rebates. 7

National Cervical Screening Program: Update Clarification of cervical cancer symptoms Background • Some healthcare providers are using low thresholds to determine whether a woman shows cervical cancer symptoms. • Pathology laboratories are getting requests for co-tests for women with symptoms that are usually not indicative of cervical cancer. • While women are encouraged to present to their doctor if they are experiencing symptoms suggestive of cervical cancer, the information previously provided to healthcare providers did not define the appropriate symptomatic threshold. • This has led to over-referral of women for co-tests, where a cervical screening test would have been appropriate, if due. • In order to address this gap, a set of more specific symptoms suggestive of cervical cancer has been developed and endorsed by the Steering Committee for the Renewal Implementation Project (SCRIP). Patient presents as Context What to write on the pathology request form Symptomatic For investigation of symptoms e. g. unusual or persistent vaginal bleeding (post coital, unexplained inter-menstrual or any post-menopausal), discharge (offensive and/or blood stained), or deep persistent dyspareunia. “Co-test” or “HPV & LBC”, “Symptomatic” (describe the symptoms) 8

National Cervical Screening Program: Update Pathology Test Guide for cervical and vaginal testing • When filling out a Pathology Test Request form, healthcare providers are required to describe the symptoms in more detail to help pathology laboratories understand why a co-test is requested. Symptoms Threshold Vaginal bleeding • • • post coital, unexplained inter-menstrual, or any post-menopausal Discharge • • offensive, and/or blood stained Pain • deep persistent dyspareunia • This information will be updated on the NCSP website (www. cancerscreening. gov. au/cervical) in the upcoming weeks. In addition to this, an item will be included in the May issue of the NCSP newsletter on this topic. Other communications strategies with pathology and healthcare provider peak bodies are being planned. 9

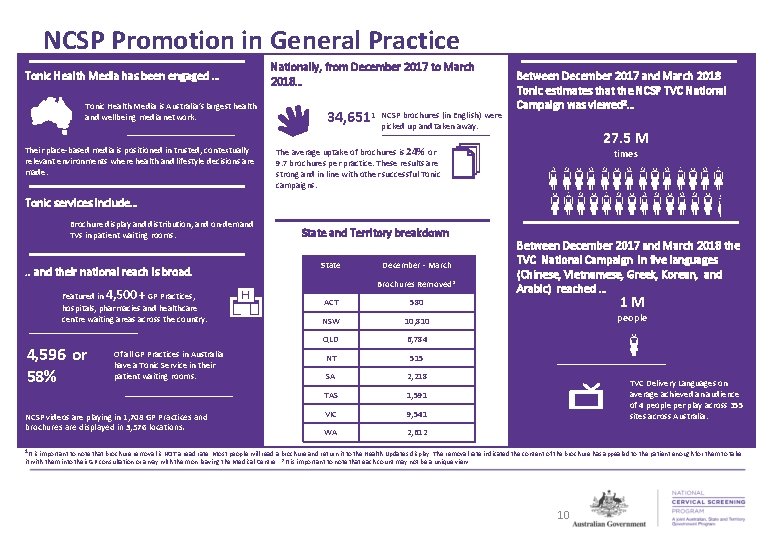
NCSP Promotion in General Practice Tonic Health Media has been engaged … Tonic Health Media is Australia’s largest health and wellbeing media network. Their place-based media is positioned in trusted, contextually relevant environments where health and lifestyle decisions are made. Nationally, from December 2017 to March 2018… 34, 6511 NCSP brochures (in English) were picked up and taken away. Between December 2017 and March 2018 Tonic estimates that the NCSP TVC National Campaign was viewed 2… 27. 5 M The average uptake of brochures is 24% or 9. 7 brochures per practice. These results are strong and in line with other successful Tonic campaigns. times Tonic services include… Brochure display and distribution, and on-demand TVs in patient waiting rooms. . . and their national reach is broad. Featured in 4, 500+ GP Practices, hospitals, pharmacies and healthcare centre waiting areas across the country. 4, 596 or 58% Of all GP Practices in Australia have a Tonic Service in their patient waiting rooms. NCSP videos are playing in 1, 708 GP Practices and brochures are displayed in 3, 576 locations. State and Territory breakdown State December - March Brochures Removed* ACT 580 NSW 10, 810 QLD 6, 784 NT 515 SA 2, 218 TAS 1, 591 VIC 9, 541 WA 2, 612 Between December 2017 and March 2018 the TVC National Campaign in five languages (Chinese, Vietnamese, Greek, Korean, and Arabic) reached … 1 M people TVC Delivery Languages on average achieved an audience of 4 people per play across 355 sites across Australia. 1 It is important to note that brochure removal is NOT a read rate. Most people will read a brochure and return it to the Health Updates display. The removal rate indicated the content of the brochure has appealed to the patient enough for them to take it with them into their GP consultation or away with them on leaving the Medical Centre. 2 It is important to note that each count may not be a unique view. 10

NCSP Promotion in General Practice Tonic Health Media is Australia’s largest health and wellbeing media network. Between 17 December 2017 and 18 March 2018 Tonic estimates that the NCSP TVC National Campaign reached… 27. 5 M Their place-based media is positioned in trusted, contextually relevant environments where health and lifestyle decisions are made. people Tonic services include… Brochure display and distribution, and on-demand TVs in patient waiting rooms . . and their national reach is broad. Featured in 4500+ GP Practices, hospitals, pharmacies and healthcare centre waiting areas across the country 4, 596 or 58% Between 17 December 2017 and 18 March 2018 the TVC National Campaign in multiple language reached … 1 M people Of all GP Practices in Australia have a Tonic Service in their patient waiting rooms NCSP videos are playing in 1, 708 GP Practices and brochures are displayed in 3, 576 locations 11

NCSP Promotion in General Practice Tonic Health Media is Australia’s largest health and wellbeing media network. Between 17 December 2017 and 18 March 2018 Tonic estimates that the NCSP TVC National Campaign reached… 27. 5 M Their place-based media is positioned in trusted, contextually relevant environments where health and lifestyle decisions are made. people Tonic services include… Brochure display and distribution, and on-demand TVs in patient waiting rooms . . and their national reach is broad. Featured in 4500+ GP Practices, hospitals, pharmacies and healthcare centre waiting areas across the country 4, 596 or 58% 1 M people Of all GP Practices in Australia have a Tonic Service in their patient waiting rooms TVC Delivery Languages on average achieved an audience of 4 people per play across 355 sites across Australia NCSP videos are playing in 1, 708 GP Practices and brochures are displayed in 3, 576 locations 12

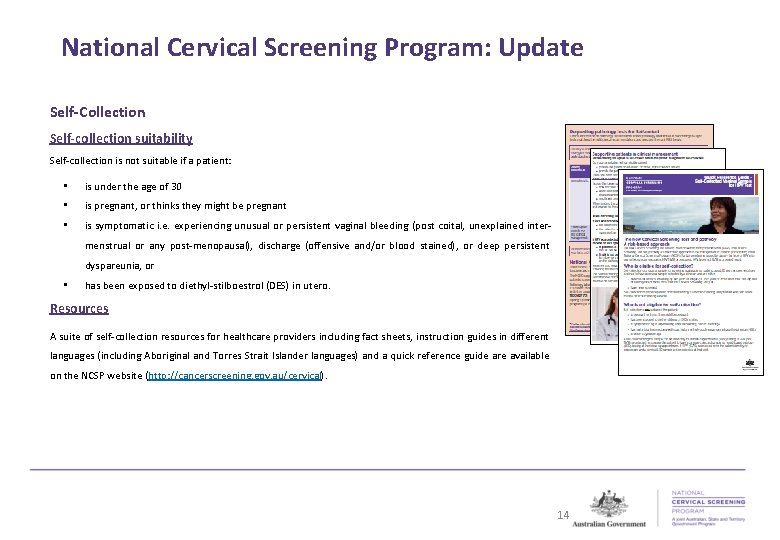
National Cervical Screening Program: Update Self-Collection Background • Self-collection of vaginal samples for HPV testing is now available under the NCSP for women aged 30 years or over, who have declined to have a cervical sample collected by a healthcare provider, and are either: • overdue for cervical screening by two years or longer (i. e. four years or more since their last Pap test, or seven years or more since their last Cervical Screening Test), or • have never screened. Current progress • Currently there is one large pathology laboratory accredited to process self-collected samples. • To assist pathology laboratories to attain accreditation from the National Pathology Accreditation Advisory Council (NPAAC) to perform tests on self-collected samples, a technical expert committee has been convened under the NPAAC to develop minimum validation standards for laboratories interested in providing this service. These standards are expected to be approved by NPAAC in the third quarter of 2018. • Prior to offering self-collection as an option to patients, healthcare providers are encouraged to check with their pathology laboratory on availability. 13

National Cervical Screening Program: Update Self-Collection Self-collection suitability Self-collection is not suitable if a patient: • is under the age of 30 • is pregnant, or thinks they might be pregnant • is symptomatic i. e. experiencing unusual or persistent vaginal bleeding (post coital, unexplained intermenstrual or any post-menopausal), discharge (offensive and/or blood stained), or deep persistent dyspareunia, or • has been exposed to diethyl-stilboestrol (DES) in utero. Resources A suite of self-collection resources for healthcare providers including fact sheets, instruction guides in different languages (including Aboriginal and Torres Strait Islander languages) and a quick reference guide are available on the NCSP website (http: //cancerscreening. gov. au/cervical). 14

National Cervical Screening Program: Update Toolkit for under-screened and never-screened women • This toolkit has been developed to assist healthcare providers to engage under-screened and never-screened women in cervical screening, and to support them should they choose to participate. The information is relevant to various healthcare providers including specialists, general practitioners, sexual health workers, disability support workers and Aboriginal health workers. The toolkit provides specific information about engaging with different groups of women, including: • Aboriginal and Torres Strait Islander women • women from culturally and linguistically diverse (CALD) backgrounds • women with disabilities • people who identify as lesbian, gay, bisexual, transgender and intersex (LGBTI) and have a cervix • women who have experienced sexual assault • women from remote areas • older women • women who experience socio-economic disadvantage, and • women who have not had the HPV vaccine. • The toolkit is on the NCSP website at http: //www. cancerscreening. gov. au/cervical-toolkit-under-and-never. 15

National Cancer Screening Register: Update Release 2 update • The implementation of Release 2 of the National Cancer Screening Register (NCSR) is on schedule to be completed by 29 June 2018. • This means that all pre 1 December 2017 results will have been migrated from the state and territory registers into the NCSR to provide national records for participants of the National Cervical Screening Program (NCSP). • To ensure a safety net is provided as part of the NCSP, participant follow-up and correspondence will continue to be delivered by the state and territory registers in conjunction with the NCSR until Release 2 is complete. There will be no gap in service for healthcare providers and participants of the NCSP. Accessing test results/screening histories • Healthcare providers should continue to speak to their pathology laboratory for their patient’s screening test result. • The NCSR can provide post 1 December 2017 cervical screening results if required (i. e. if the healthcare provider did not request the test, or cannot access the result from the pathology laboratory). • On completion of Release 2, healthcare providers will be able to access national cervical screening records through the NCSR contact centre. • Until Release 2 is complete, healthcare providers should continue to contact their state and territory register for screening histories up to 1 December 2017. 16

National Cancer Screening Register: Update Follow-up • Participant follow-up is the primary responsibility of the healthcare provider requesting the test. • The NCSR and state and territory registers will jointly deliver a participant follow-up safety net function until Release 2 is complete. • Participant follow-up of all results received prior to 1 December 2017 will continue to be provided by the state and territory registers. • The NCSR will follow up all results received post 1 December 2017. • At Release 2, the NCSR will provide safety net functions for the program. This includes invitations to screen, routine reminders, and follow-up correspondence to participants and healthcare providers; and follow up phone calls to healthcare providers (where required). Update on screening correspondence • To ensure the clinical safety of participants of the NCSP, during the transition to Release 2, both the state and territory registers and the NCSR will send screening correspondence (letters) to healthcare providers and their patients. • This means that healthcare providers or their patients may receive more than one letter. For example, a patient may receive a routine reminder letter when they have had a test since 1 December, and also receive a follow-up letter for an abnormal result. • If this occurs, healthcare providers should act on the advice of the most recent cervical screening result. In this example, healthcare providers would act on the follow-up letter. • Healthcare providers are encouraged to continue to actively manage their patient’s cervical screening in accordance with the 2016 Guidelines, to update their recall and reminder systems to align with the new cervical screening program intervals, and to send correspondence from their clinical practice systems. 17

Further information and contact National Cervical Screening Program • For further information on the National Cervical Screening Program, visit our website at www. cancerscreening. gov. au/cervical. • Feedback and enquiries can be directed to your local state/territory government cancer screening program area, or to cervicalrenewal@health. gov. au. National Cancer Screening Register • The telephone number for the NCSR contact centre is 1800 627 701. • For further information, please refer to the Transition Quick Start Guide for Healthcare Providers available on the NCSR website. 18