National 5 Physics Waves Radiation True or False




























- Slides: 75

National 5 Physics Waves & Radiation

True or False: Can energy be transferred by waves? Answer TRUE

What is meant by the frequency of a wave? Answer Frequency is the number of waves passing a point per second.

What is meant by the period of a wave? Answer Period is the time taken for one wave to pass a point.

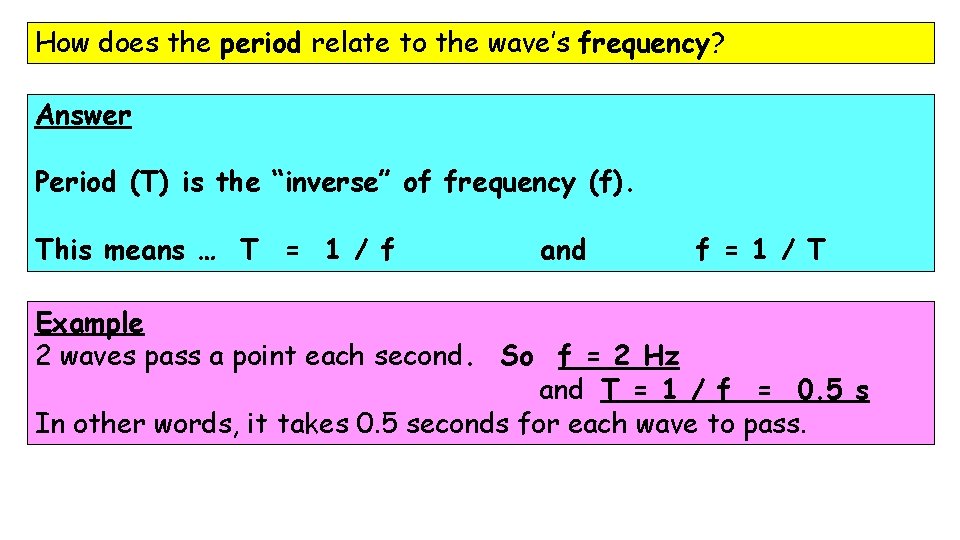
How does the period relate to the wave’s frequency? Answer Period (T) is the “inverse” of frequency (f). This means … T = 1 / f and f = 1 / T Example 2 waves pass a point each second. So f = 2 Hz and T = 1 / f = 0. 5 s In other words, it takes 0. 5 seconds for each wave to pass.

What is meant by the wavelength of a wave? Answer The length of 1 wave which is the distance between identical points on 2 waves next to each other. Eg) the distance from one wave crest to the next wave crest.

What is meant by the amplitude of a wave? Answer The height of the wave from the centre line (called the “line of zero disturbance”).

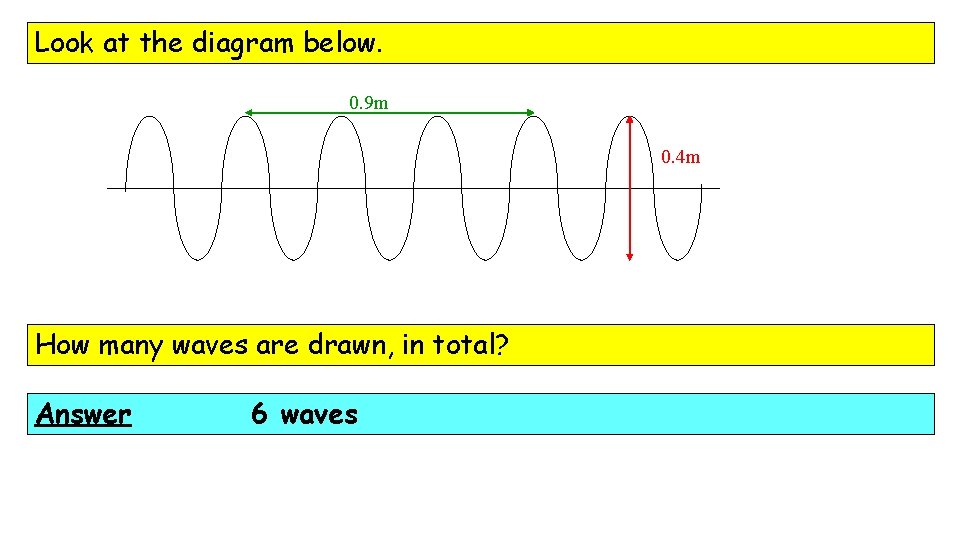
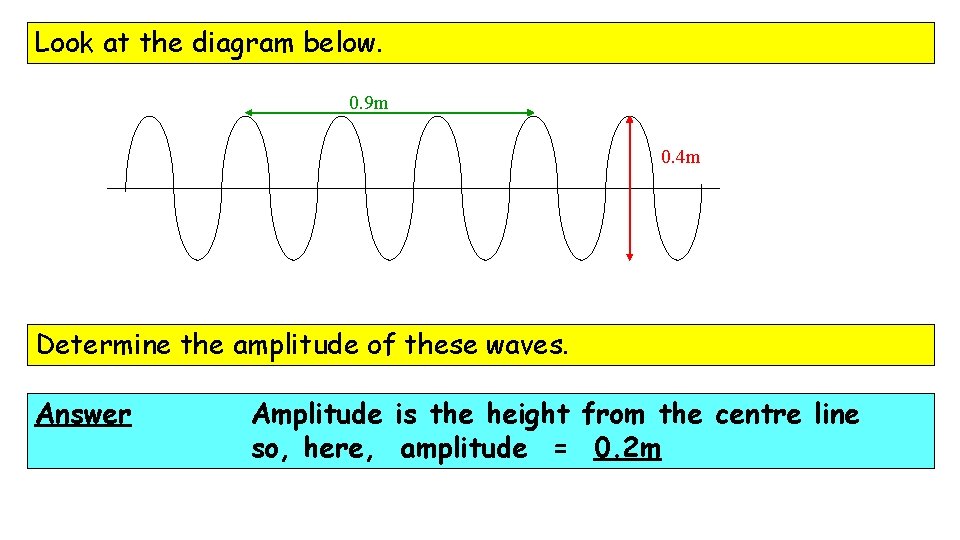
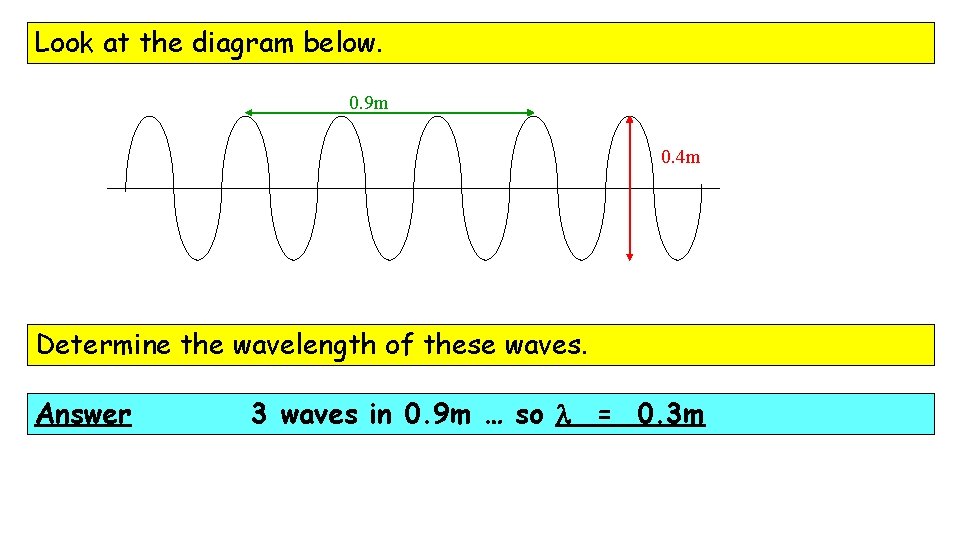
Look at the diagram below. 0. 9 m 0. 4 m How many waves are drawn, in total? Answer 6 waves

Look at the diagram below. 0. 9 m 0. 4 m Determine the amplitude of these waves. Answer Amplitude is the height from the centre line so, here, amplitude = 0. 2 m

Look at the diagram below. 0. 9 m 0. 4 m Determine the wavelength of these waves. Answer 3 waves in 0. 9 m … so l = 0. 3 m

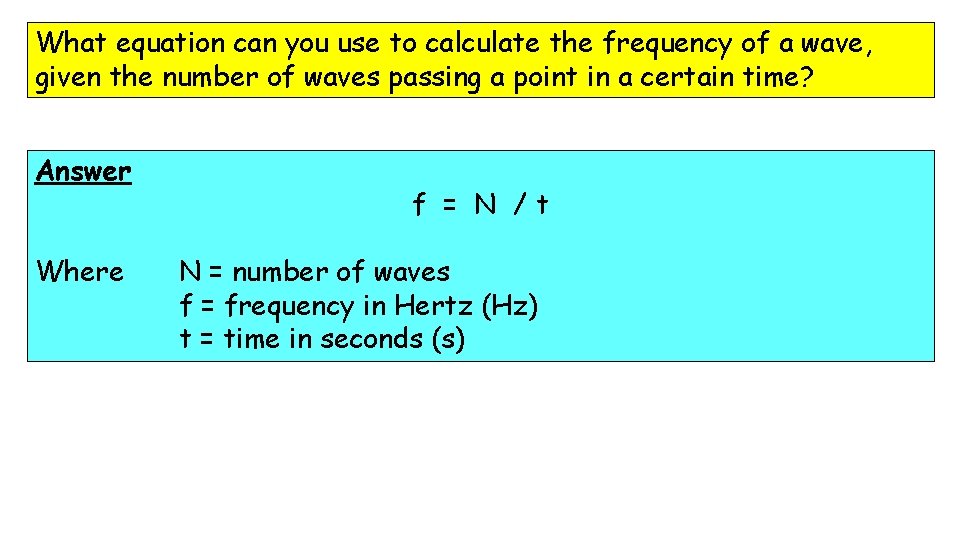
What equation can you use to calculate the frequency of a wave, given the number of waves passing a point in a certain time? Answer Where f = N /t N = number of waves f = frequency in Hertz (Hz) t = time in seconds (s)

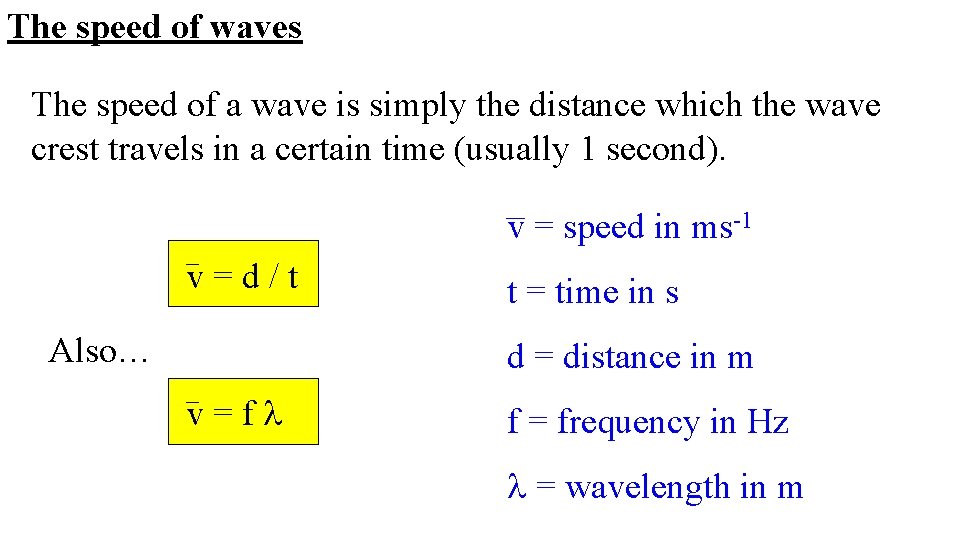
What is meant by the speed of a wave? Answer The distance covered by a wave per unit of time. OR “the distance covered per second”.

The speed of waves The speed of a wave is simply the distance which the wave crest travels in a certain time (usually 1 second). v = speed in ms-1 v=d/t Also… t = time in s d = distance in m v=fl f = frequency in Hz l = wavelength in m

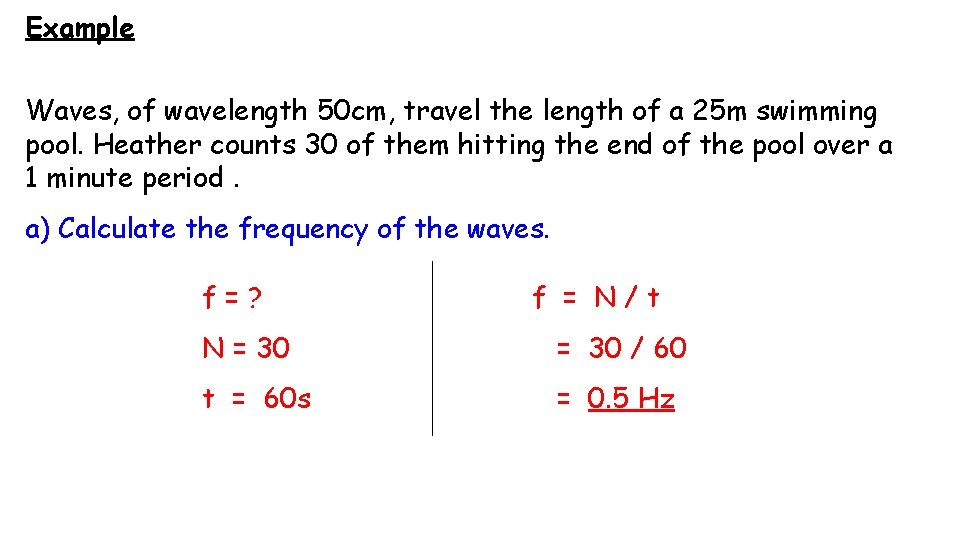
Example Waves, of wavelength 50 cm, travel the length of a 25 m swimming pool. Heather counts 30 of them hitting the end of the pool over a 1 minute period. a) Calculate the frequency of the waves. f=? f = N/t N = 30 / 60 t = 60 s = 0. 5 Hz

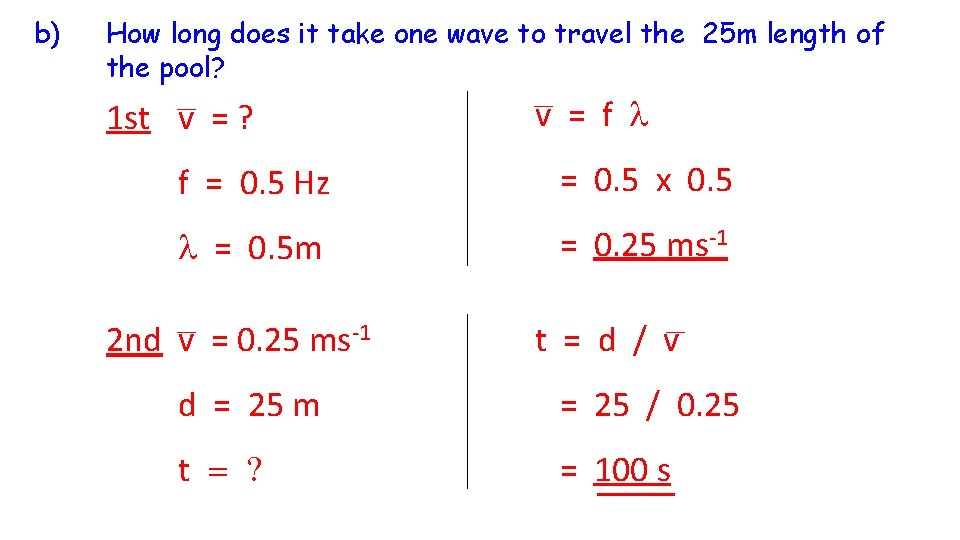
b) How long does it take one wave to travel the 25 m length of the pool? 1 st v = ? v = f l f = 0. 5 Hz = 0. 5 x 0. 5 l = 0. 5 m = 0. 25 ms-1 2 nd v = 0. 25 ms-1 t = d / v d = 25 m = 25 / 0. 25 t = ? = 100 s

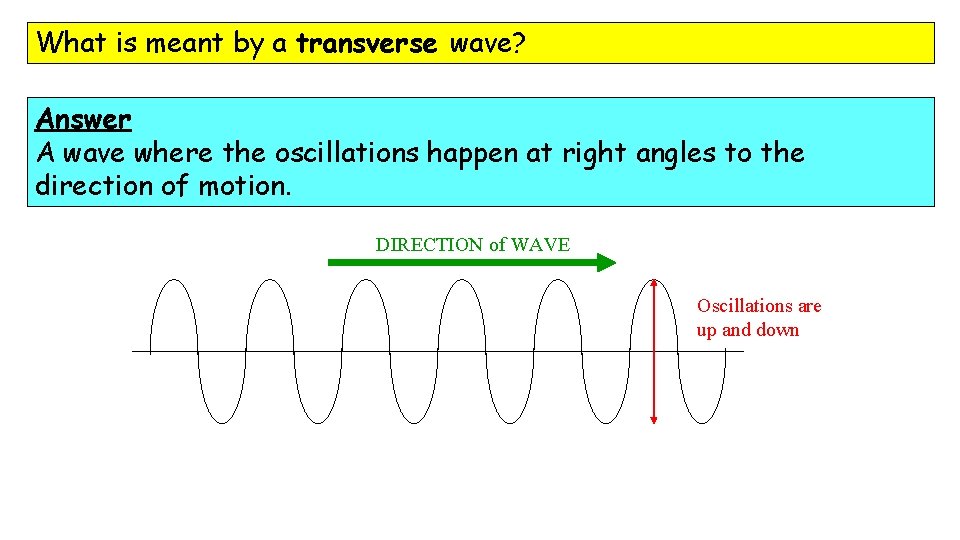
What is meant by a transverse wave? Answer A wave where the oscillations happen at right angles to the direction of motion. DIRECTION of WAVE Oscillations are up and down

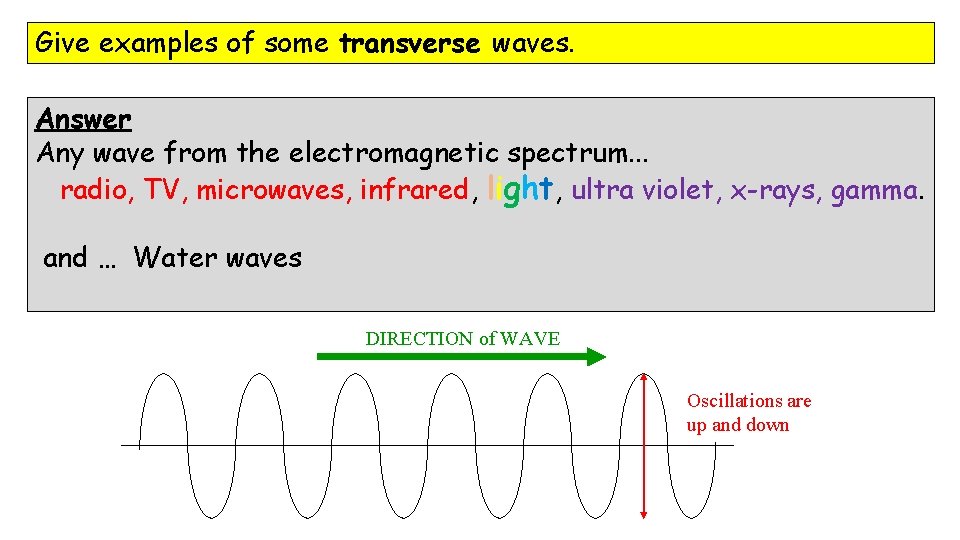
Give examples of some transverse waves. Answer Any wave from the electromagnetic spectrum. . . radio, TV, microwaves, infrared, light, ultra violet, x-rays, gamma. and … Water waves DIRECTION of WAVE Oscillations are up and down

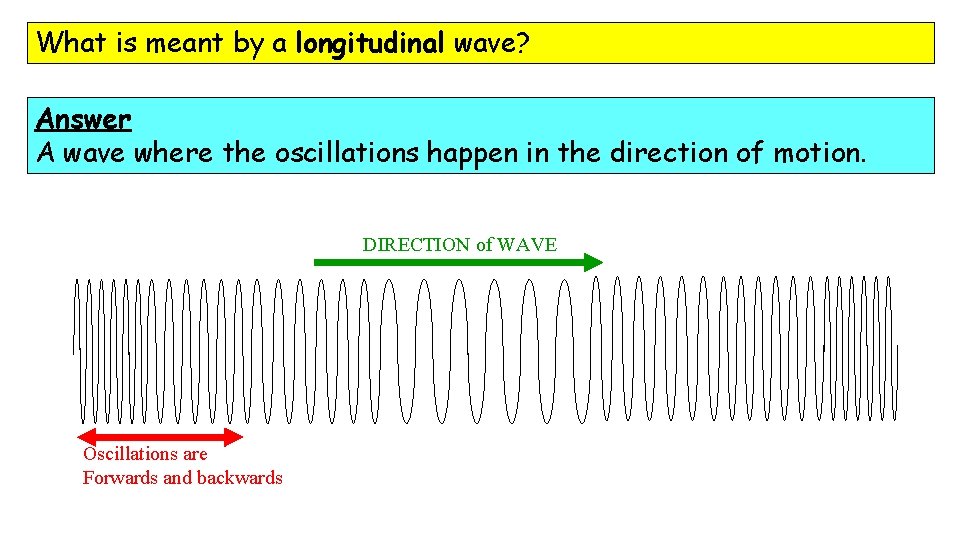
What is meant by a longitudinal wave? Answer A wave where the oscillations happen in the direction of motion. DIRECTION of WAVE Oscillations are Forwards and backwards

Give an example of longitudinal waves. Answer Sound waves … (including ultrasound). DIRECTION of WAVE Oscillations are Forwards and backwards

What is meant by diffraction of waves? Answer Waves bending around obstacles.

What wavelengths are better at diffracting; long or short? Answer Long waves diffract better than short waves.

Explain the differences in Radio and TV reception in terms of wave diffraction? Answer Long waves diffract better than short waves. Radio waves have longer wavelengths than TV waves. So, it is easier to pick up radio signals than T. V. signals because radio waves diffract around obstacles better (due to their longer wavelength. )

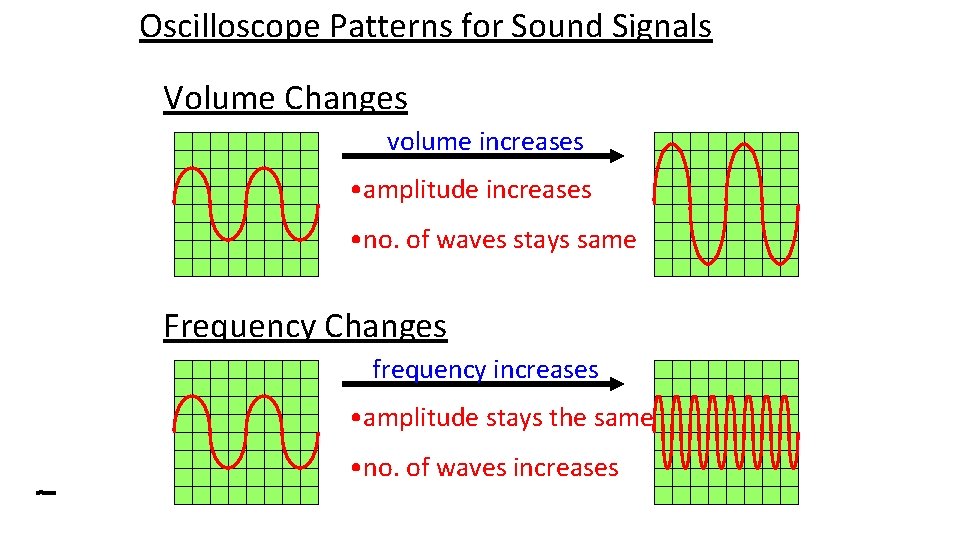
Sound Signals as Waves on an Oscilloscope What are the missing words below: The pitch of a sound is determined by the _____ frequency of the sound waves. The volume of a sound is determined by the amplitude _____ of the sound waves.

Oscilloscope Patterns for Sound Signals Volume Changes volume increases • amplitude increases • no. of waves stays same Frequency Changes frequency increases • amplitude stays the same • no. of waves increases

Ultrasounds are high frequency sound vibrations beyond the range of human hearing. What is the range of human hearing? 20 Hz - 20 000 Hz State one use of ultrasound in medicine To scan and monitor a baby in the womb. Describe how this works Jelly is put on the mum to reduce sound reflections from skin. Ultrasound is transmitted into the womb. The ultrasound reflects from different parts of the baby. The reflected ultrasound travels back to the receiver. The computer uses the different ‘echo times’ from the different parts of the baby to build up an image of the baby.

Noise Levels and Noise Pollution Two examples of noise pollution are: 1. Pneumatic drills 2. Aeroplanes … loud discos, heavy traffic, Kirsten, Heather, Shannon & Lucy hearing Excessive noise can damage ______. Sound levels are measured in Decibels ______ (d. B).

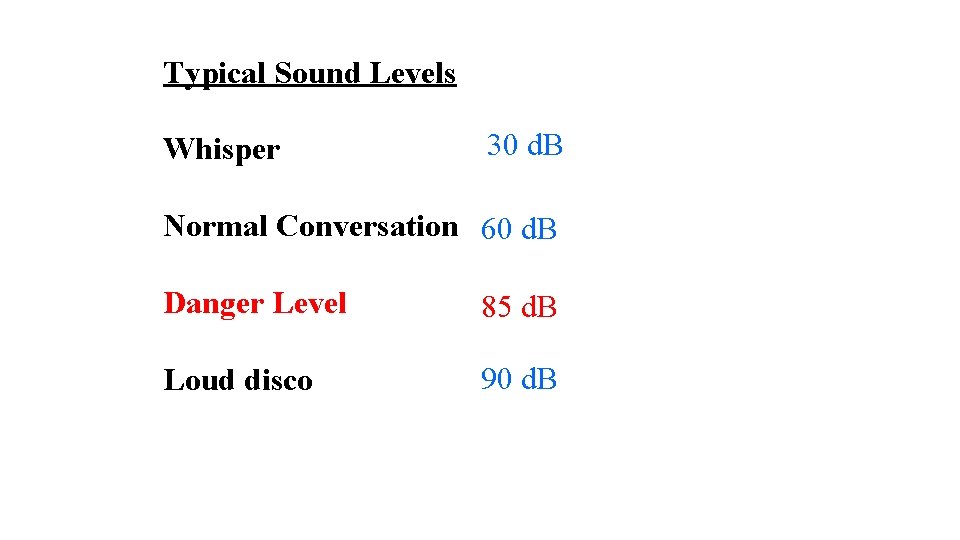
Typical Sound Levels Whisper 30 d. B Normal Conversation 60 d. B Danger Level 85 d. B Loud disco 90 d. B

State the sections of the ELECTROMAGNETIC SPECTRUM starting with the lowest frequency. Answer radio, TV, microwaves, infrared, light, ultra violet, x-rays, gamma.

State the sections of the ELECTROMAGNETIC SPECTRUM starting with the shortest wavelength. Answer Gamma, x-rays, ultra violet, light, infrared, microwaves, TV, radio.

State the sections of the ELECTROMAGNETIC SPECTRUM starting with the lowest energy. Answer radio, TV, microwaves, infrared, light, ultra violet, x-rays, gamma.

What do all waves in the electromagnetic spectrum have in common. Answer They all travel at the speed of light in air, 3 x 10 8 ms-1. .

The Electromagnetic Spectrum 1. State one application of lasers in medicine. Eye surgery, removing tatoos etc…, 2. State one application of ultra violet. Treatment of skin disorders (acne), sterilising surgical aparatus, …

3. State one application of infra red. Heat treatment for muscles, thermograms (as tumours are warmer than surrounding tissue so they give out more infra red which can be shown on thermograms. ) 4. State one application of x-rays. Detecting broken bones. 5. What is used to detect x-rays? Photographic film. X-rays blacken the film.

5. Excessive exposure to ultra violet radiation can be harmful. Why? It can cause skin cancer. 6. “Tomography” is the process of taking a series of x-ray slices to build up an image. A “CT” scan is computerised tomography. What’s the advantage of this? 1. A more detailed 3 -D image is made so problems can’t be hidden. 2. Less risk of damage to healthy cells.

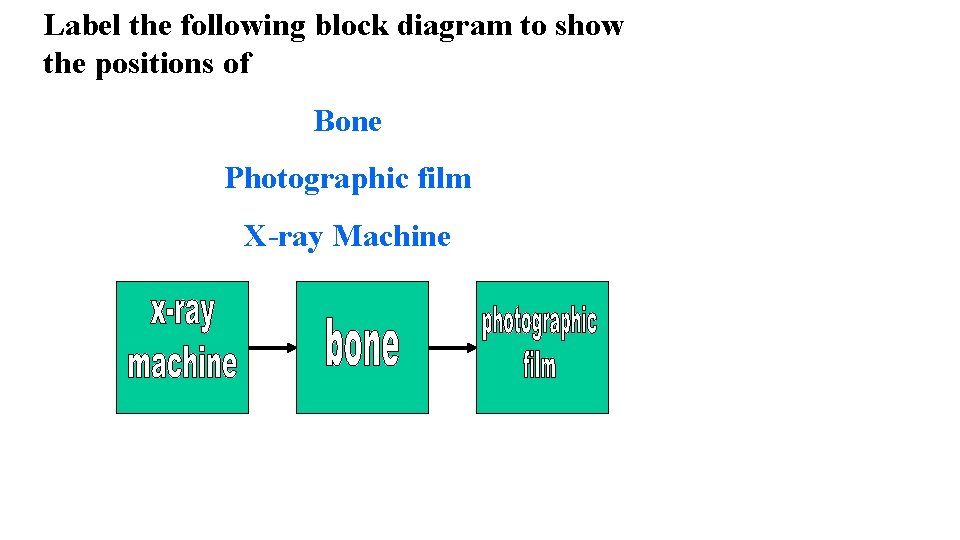
Label the following block diagram to show the positions of Bone Photographic film X-ray Machine

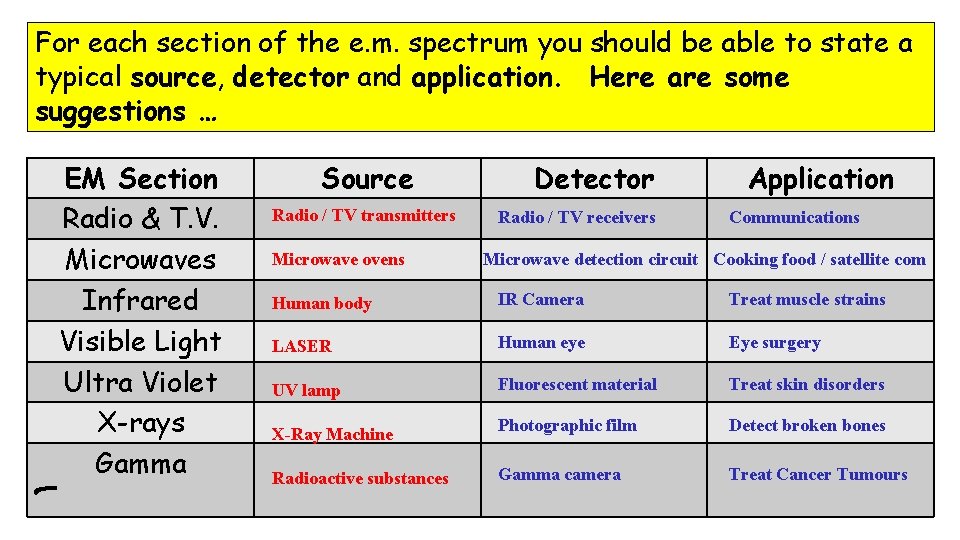
For each section of the e. m. spectrum you should be able to state a typical source, detector and application. Here are some suggestions … EM Section Radio & T. V. Microwaves Infrared Visible Light Ultra Violet X-rays Gamma Source Radio / TV transmitters Microwave ovens Detector Radio / TV receivers Application Communications Microwave detection circuit Cooking food / satellite com Human body IR Camera Treat muscle strains LASER Human eye Eye surgery UV lamp Fluorescent material Treat skin disorders Photographic film Detect broken bones Gamma camera Treat Cancer Tumours X-Ray Machine Radioactive substances

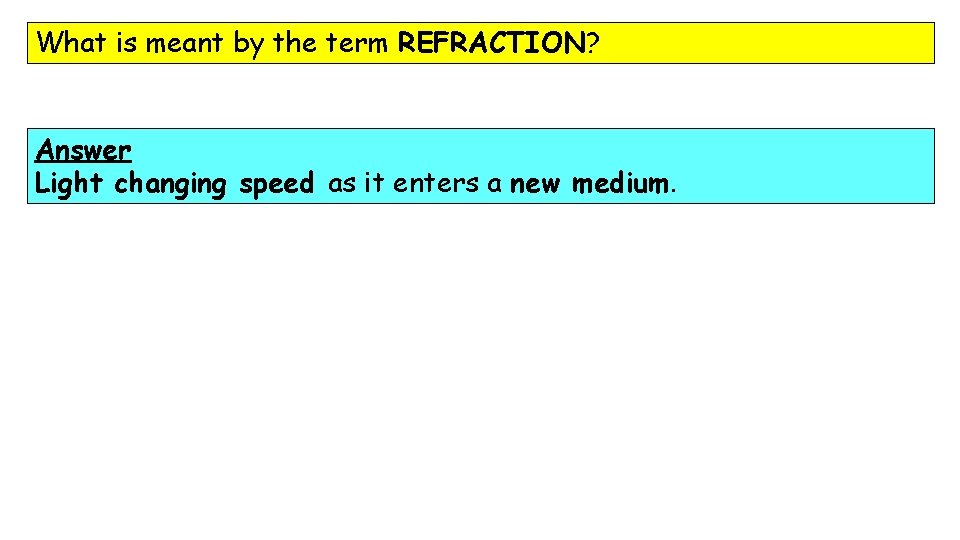
What is meant by the term REFRACTION? Answer Light changing speed as it enters a new medium.

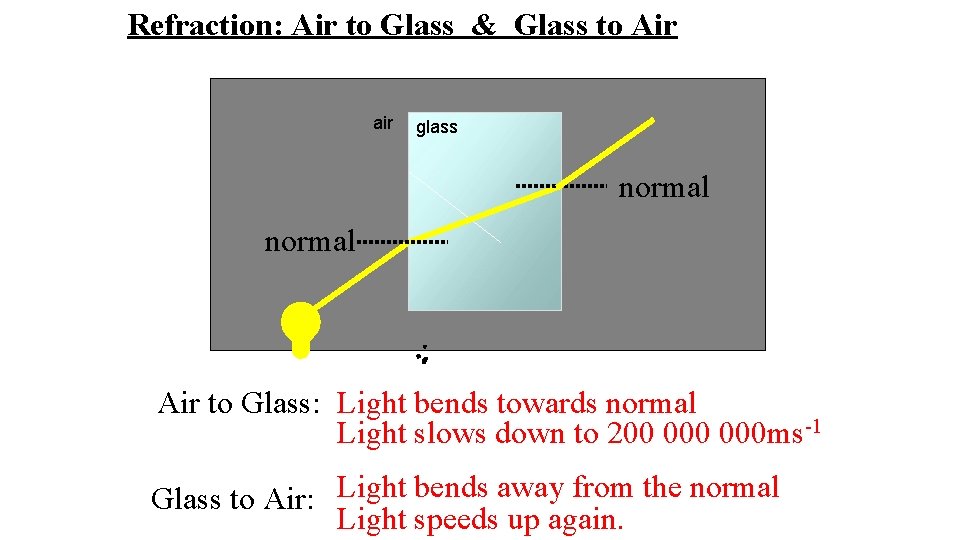
Refraction: Air to Glass & Glass to Air air glass normal Air to Glass: Light bends towards normal Light slows down to 200 000 ms-1 Glass to Air: Light bends away from the normal Light speeds up again.

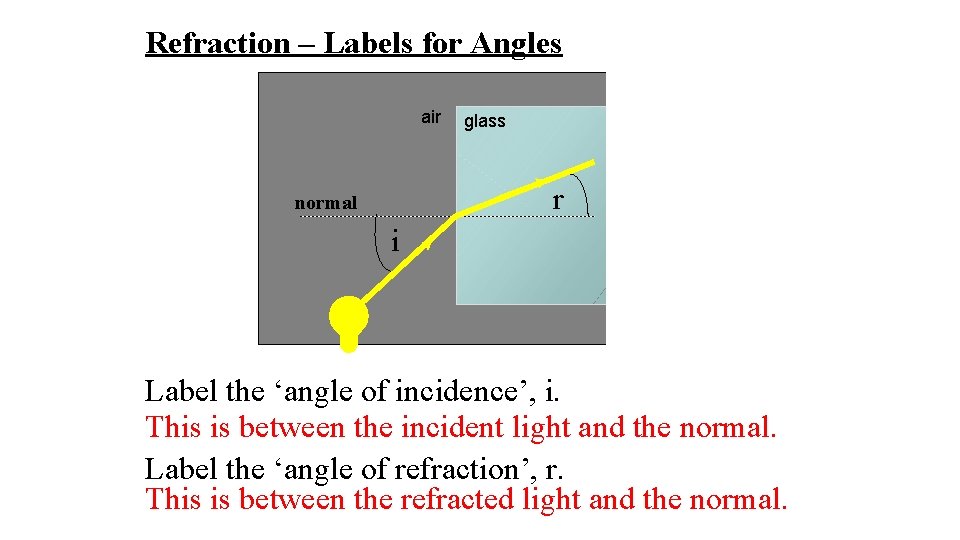
Refraction – Labels for Angles air glass r normal i Label the ‘angle of incidence’, i. This is between the incident light and the normal. Label the ‘angle of refraction’, r. This is between the refracted light and the normal.

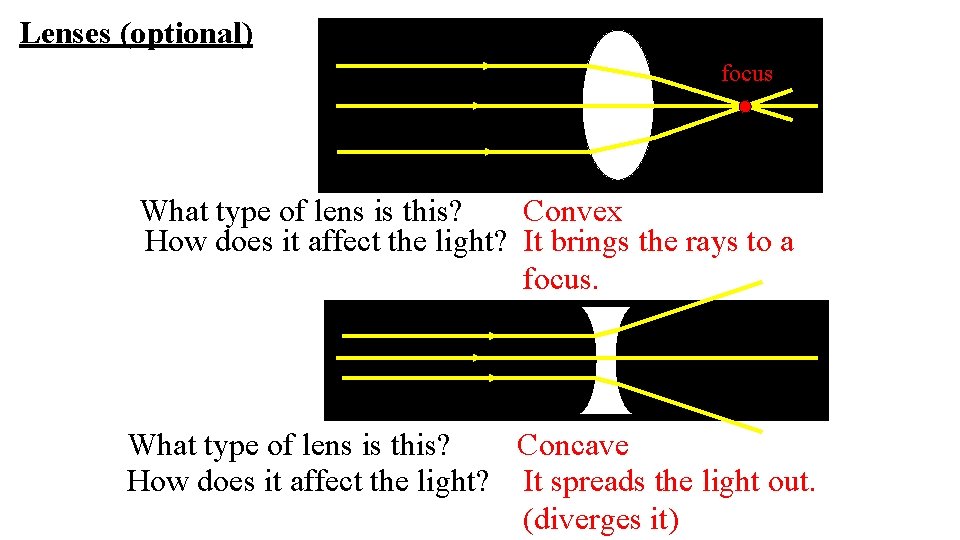
Lenses (optional) focus What type of lens is this? Convex How does it affect the light? It brings the rays to a focus. What type of lens is this? Concave How does it affect the light? It spreads the light out. (diverges it)

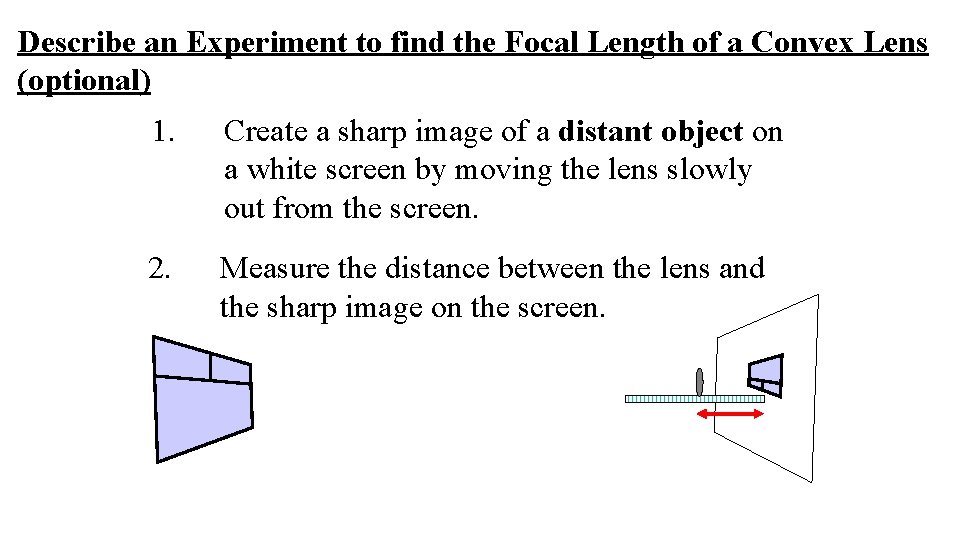
Describe an Experiment to find the Focal Length of a Convex Lens (optional) 1. Create a sharp image of a distant object on a white screen by moving the lens slowly out from the screen. 2. Measure the distance between the lens and the sharp image on the screen.

Nuclear radiation Radiation can kill living cells (or change them). Describe one medical application of this. Treatment of Cancer Sterilisation of surgical instruments

Radiation is easy to detect. Describe one medical application of this. Tracers…. Radioactive materials are put into the body and the radiation they emit is ‘followed’ around the body by a gamma camera. This is good for locating blockages. The half life of tracers must be carefully chosen. It can’t be too short as the activity might decrease too much during the examination. It can’t be too long as the patient would then be ‘radioactive’ for a time after the examination.

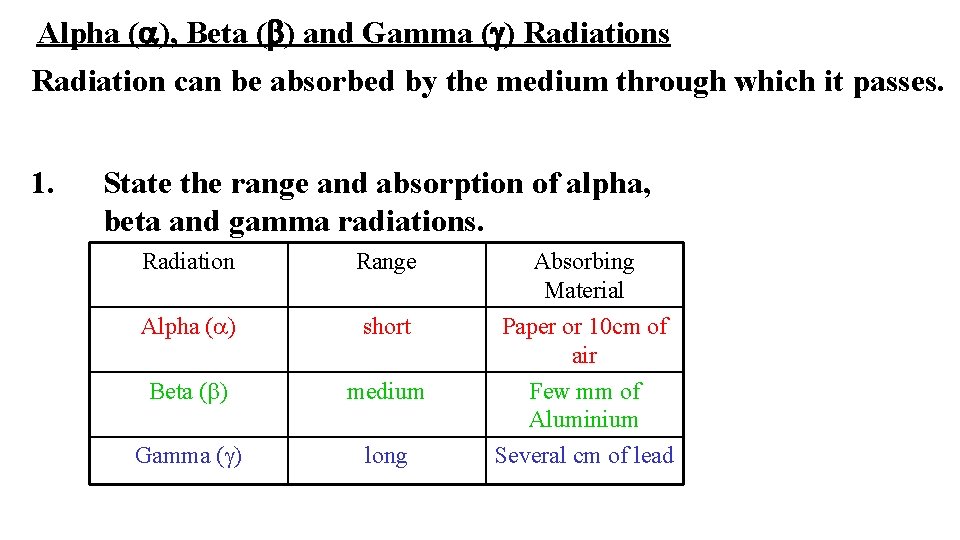
Alpha (a), Beta (b) and Gamma (g) Radiations Radiation can be absorbed by the medium through which it passes. 1. State the range and absorption of alpha, beta and gamma radiations. Radiation Range Absorbing Material Alpha (a) short Paper or 10 cm of air Beta (b) medium Gamma (g) long Few mm of Aluminium Several cm of lead

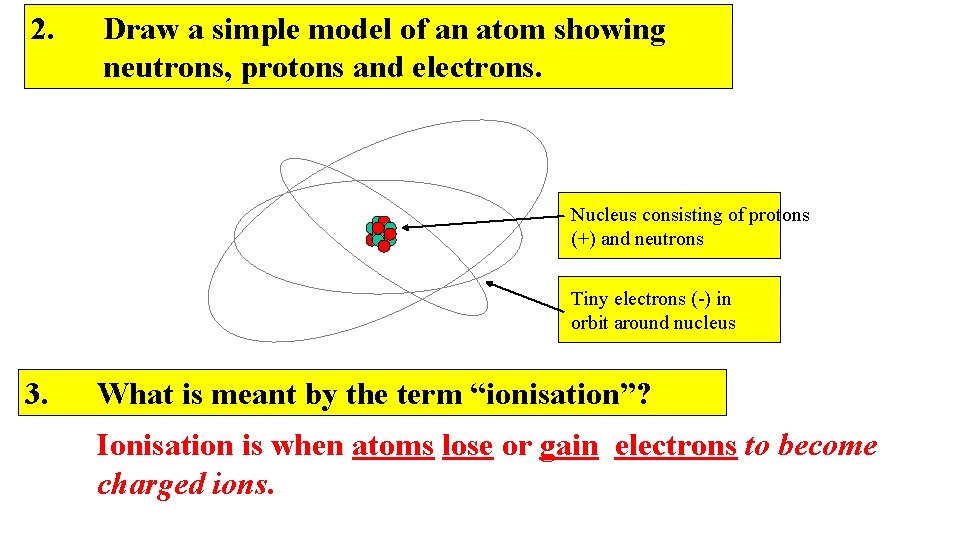
2. Draw a simple model of an atom showing neutrons, protons and electrons. Nucleus consisting of protons (+) and neutrons Tiny electrons (-) in orbit around nucleus 3. What is meant by the term “ionisation”? Ionisation is when atoms lose or gain electrons to become charged ions.

4. Which type of nuclear radiation is the most dangerous because it can cause greatest ionisation. (i. e. it can ‘rip’ electrons away from atoms of living things, mutating cells) Alpha. This is because alpha particles are big and positive so they act like a magnet to the electrons (-) orbiting atoms. 5. Describe how an effect of radiation (on non living things) is used in a detector of radiation. Film badges in hospitals. These are worn by staff to check exposure levels to radiation. The radiation ‘fogs’ the film eventually. This is seen when the films are developed.

Activity and Half Life The “activity” of a radioactive source tells us how many radioactive ‘particles’ it emits per second. What is the unit for activity? Becquerels (Bq) What happens to the activity of a radioactive source as time passes? It decreases. What is meant by the term “half life”. “Half life” is the time taken for the activity of a radioactive source to halve.

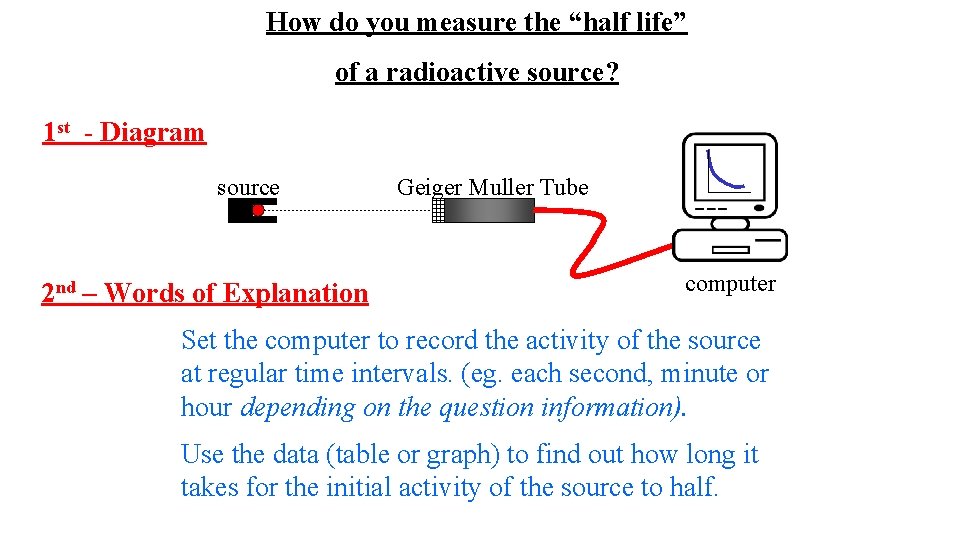
How do you measure the “half life” of a radioactive source? 1 st - Diagram source 2 nd – Words of Explanation Geiger Muller Tube computer Set the computer to record the activity of the source at regular time intervals. (eg. each second, minute or hour depending on the question information). Use the data (table or graph) to find out how long it takes for the initial activity of the source to half.

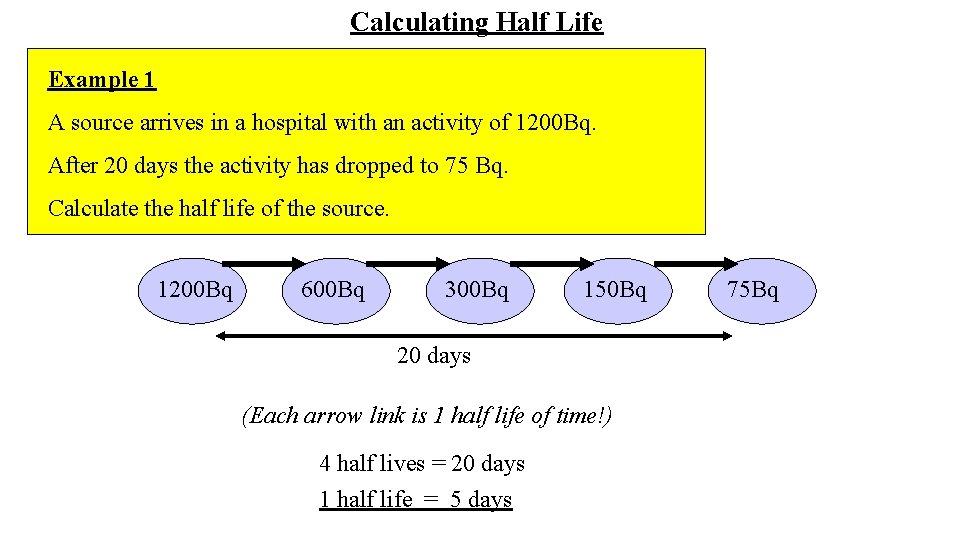
Calculating Half Life Example 1 A source arrives in a hospital with an activity of 1200 Bq. After 20 days the activity has dropped to 75 Bq. Calculate the half life of the source. 1200 Bq 600 Bq 300 Bq 150 Bq 20 days (Each arrow link is 1 half life of time!) 4 half lives = 20 days 1 half life = 5 days 75 Bq

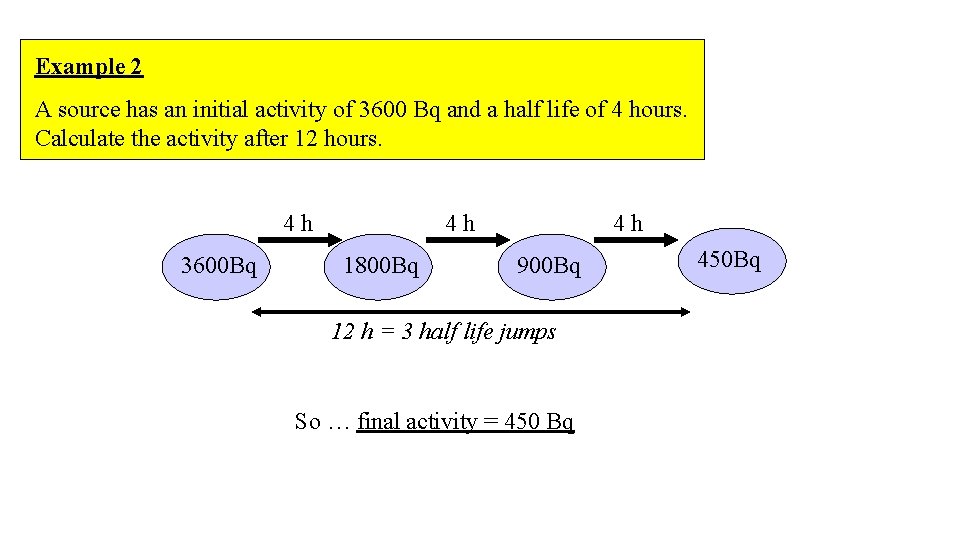
Example 2 A source has an initial activity of 3600 Bq and a half life of 4 hours. Calculate the activity after 12 hours. 4 h 3600 Bq 4 h 1800 Bq 4 h 900 Bq 12 h = 3 half life jumps So … final activity = 450 Bq 450 Bq

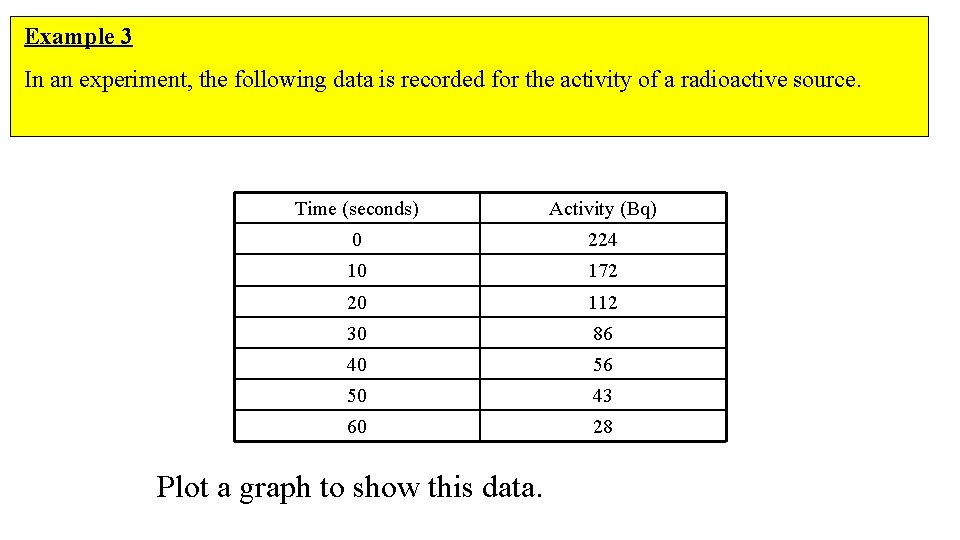
Example 3 In an experiment, the following data is recorded for the activity of a radioactive source. Time (seconds) Activity (Bq) 0 224 10 172 20 112 30 86 40 56 50 43 60 28 Plot a graph to show this data.

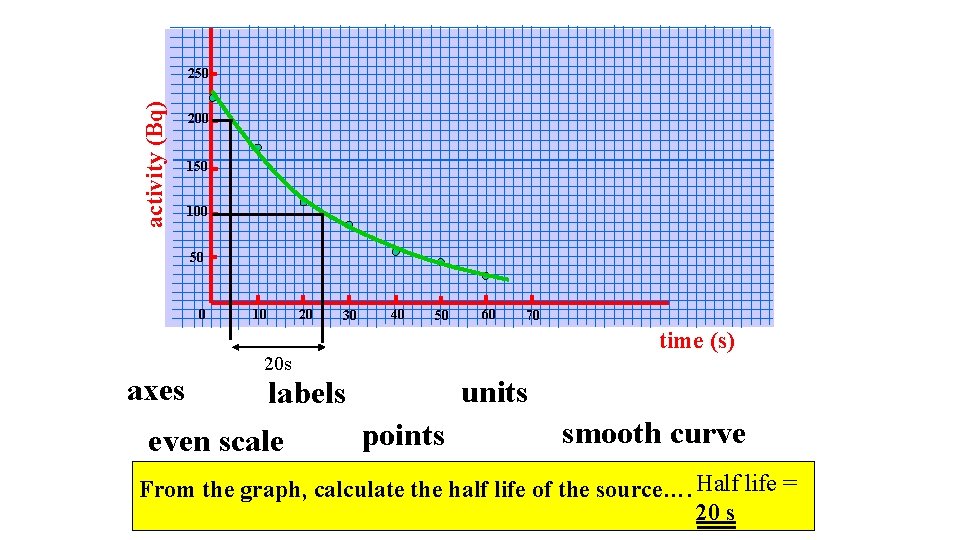
activity (Bq) 250 200 150 100 50 0 axes 10 20 30 40 50 70 time (s) 20 s units labels even scale 60 points smooth curve From the graph, calculate the half life of the source…. Half life = 20 s

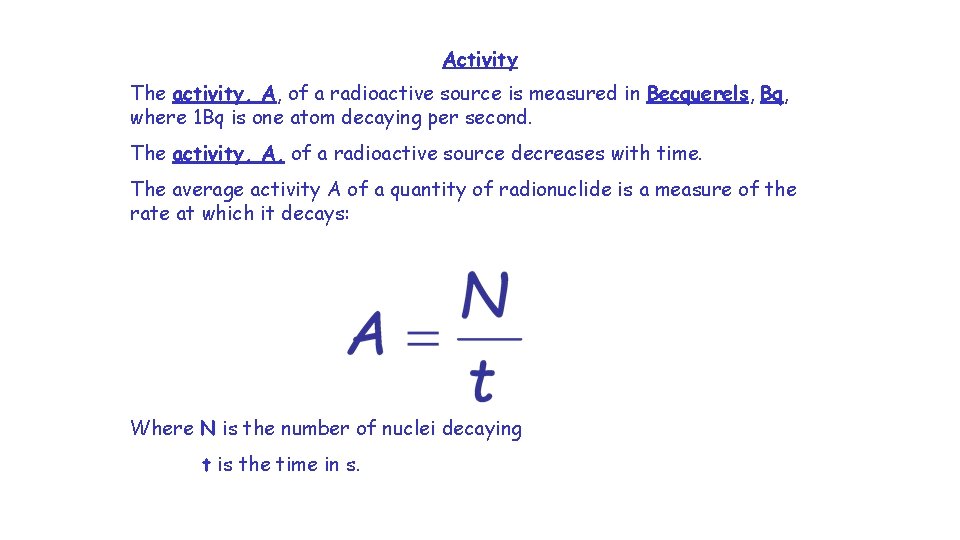
Activity The activity, A, of a radioactive source is measured in Becquerels, Bq, where 1 Bq is one atom decaying per second. The activity, A, of a radioactive source decreases with time. The average activity A of a quantity of radionuclide is a measure of the rate at which it decays: Where N is the number of nuclei decaying t is the time in s.

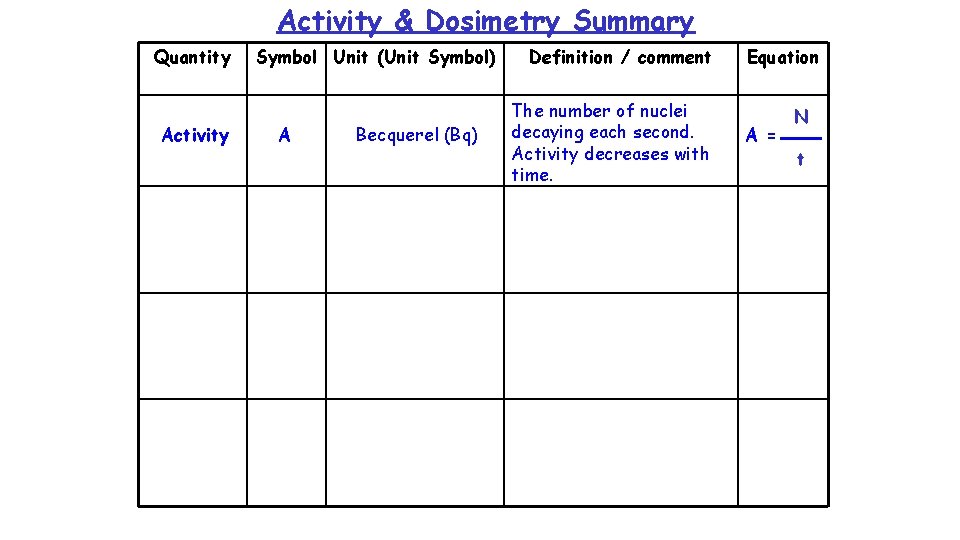
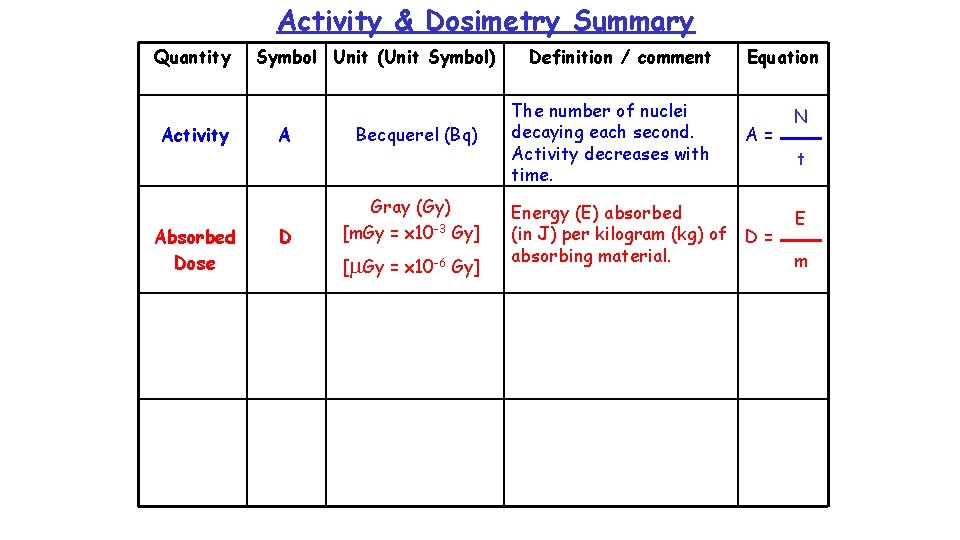
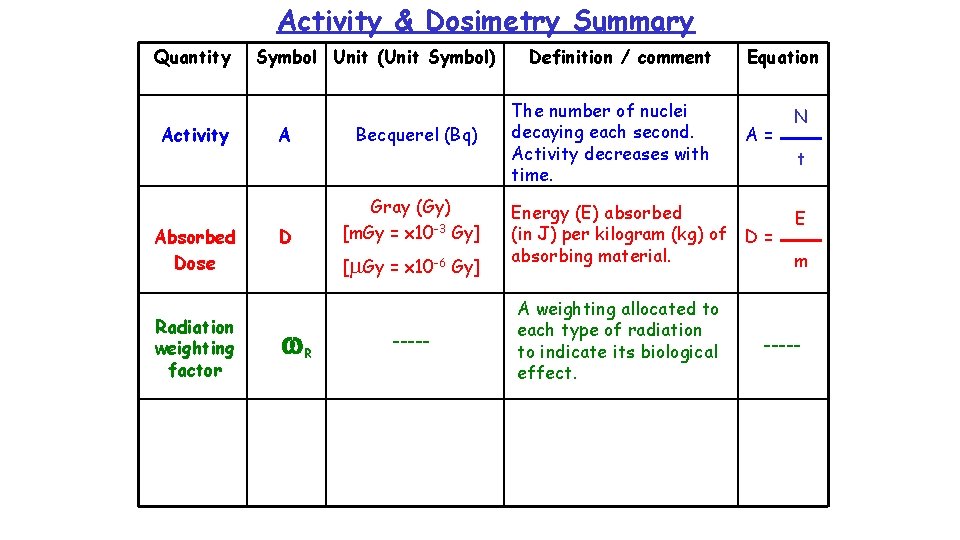
Activity & Dosimetry Summary Quantity Activity Symbol Unit (Unit Symbol) A Becquerel (Bq) Definition / comment The number of nuclei decaying each second. Activity decreases with time. Equation A = N t

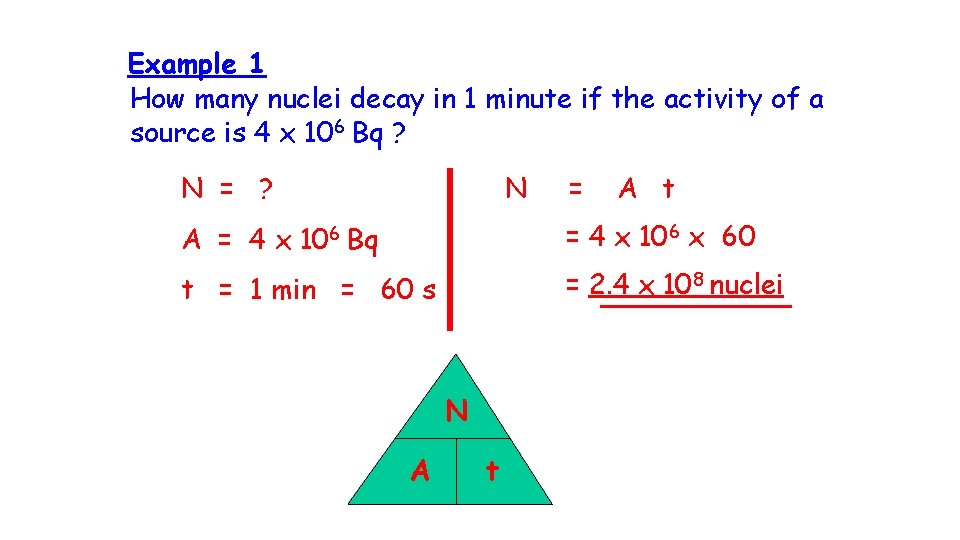
Example 1 How many nuclei decay in 1 minute if the activity of a source is 4 x 106 Bq ? N = ? N = A t A = 4 x 106 Bq = 4 x 106 x 60 t = 1 min = 60 s = 2. 4 x 108 nuclei N A t

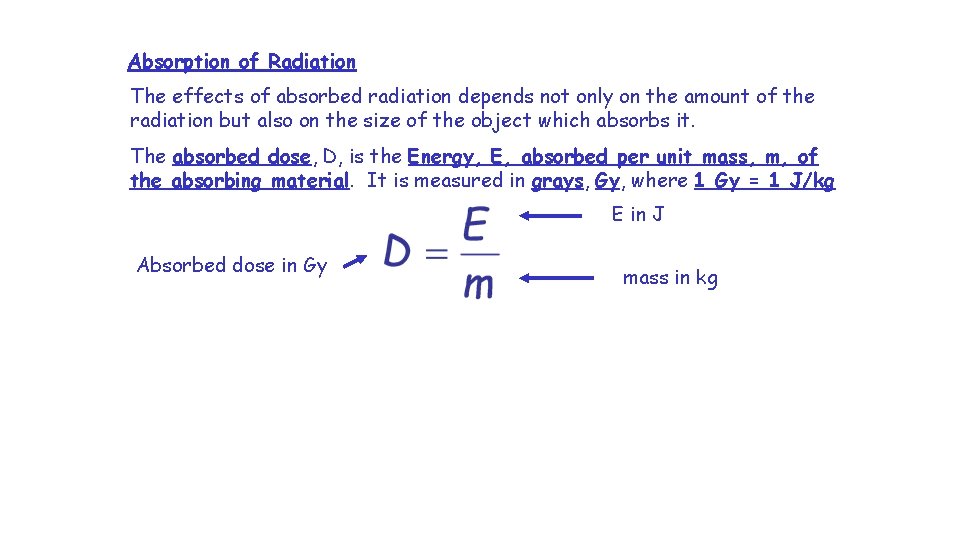
Absorption of Radiation The effects of absorbed radiation depends not only on the amount of the radiation but also on the size of the object which absorbs it. The absorbed dose, D, is the Energy, E, absorbed per unit mass, m, of the absorbing material. It is measured in grays, Gy, where 1 Gy = 1 J/kg E in J Absorbed dose in Gy mass in kg

Activity & Dosimetry Summary Quantity Activity Absorbed Dose Symbol Unit (Unit Symbol) A Becquerel (Bq) D Gray (Gy) [m. Gy = x 10 -3 Gy] [m. Gy = x 10 -6 Gy] Definition / comment The number of nuclei decaying each second. Activity decreases with time. Equation A= Energy (E) absorbed (in J) per kilogram (kg) of D = absorbing material. N t E m

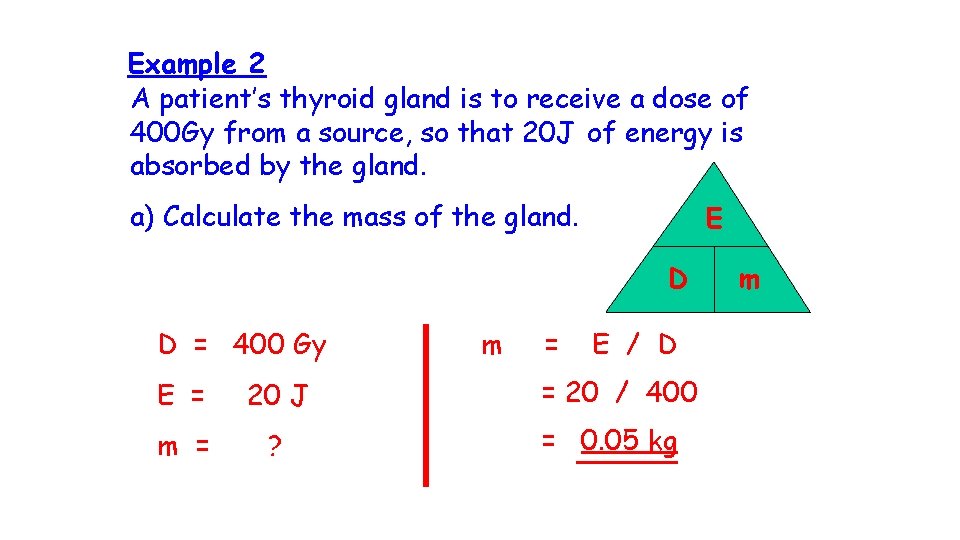
Example 2 A patient’s thyroid gland is to receive a dose of 400 Gy from a source, so that 20 J of energy is absorbed by the gland. a) Calculate the mass of the gland. E D D = 400 Gy E = 20 J m = ? m = E / D = 20 / 400 = 0. 05 kg m

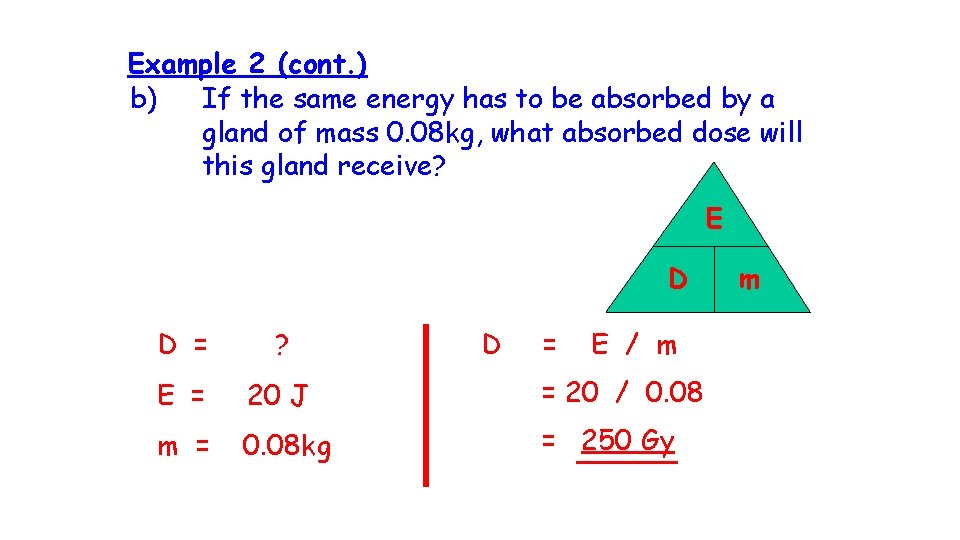
Example 2 (cont. ) b) If the same energy has to be absorbed by a gland of mass 0. 08 kg, what absorbed dose will this gland receive? E D D = ? D = E / m E = 20 J = 20 / 0. 08 m = 0. 08 kg = 250 Gy m

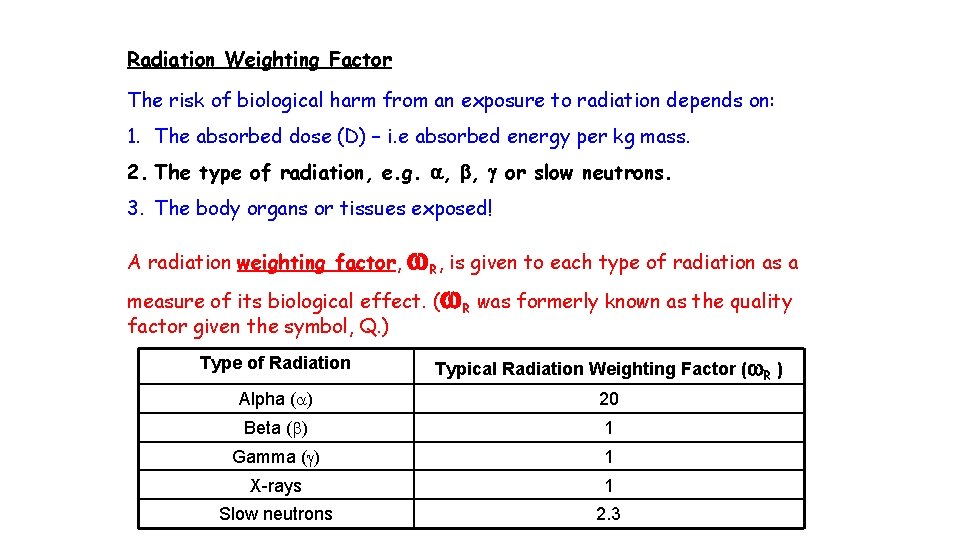
Radiation Weighting Factor The risk of biological harm from an exposure to radiation depends on: 1. The absorbed dose (D) – i. e absorbed energy per kg mass. 2. The type of radiation, e. g. a, b, g or slow neutrons. 3. The body organs or tissues exposed! w. R, is given to each type of radiation as a measure of its biological effect. (w. R was formerly known as the quality A radiation weighting factor, factor given the symbol, Q. ) Type of Radiation Typical Radiation Weighting Factor (w. R ) Alpha (a) 20 Beta (b) 1 Gamma (g) 1 X-rays 1 Slow neutrons 2. 3

Activity & Dosimetry Summary Quantity Activity Absorbed Dose Radiation weighting factor Symbol Unit (Unit Symbol) A Becquerel (Bq) D Gray (Gy) [m. Gy = x 10 -3 Gy] [m. Gy = x 10 -6 Gy] w. R ----- Definition / comment The number of nuclei decaying each second. Activity decreases with time. Equation A= Energy (E) absorbed (in J) per kilogram (kg) of D = absorbing material. A weighting allocated to each type of radiation to indicate its biological effect. N t E m -----

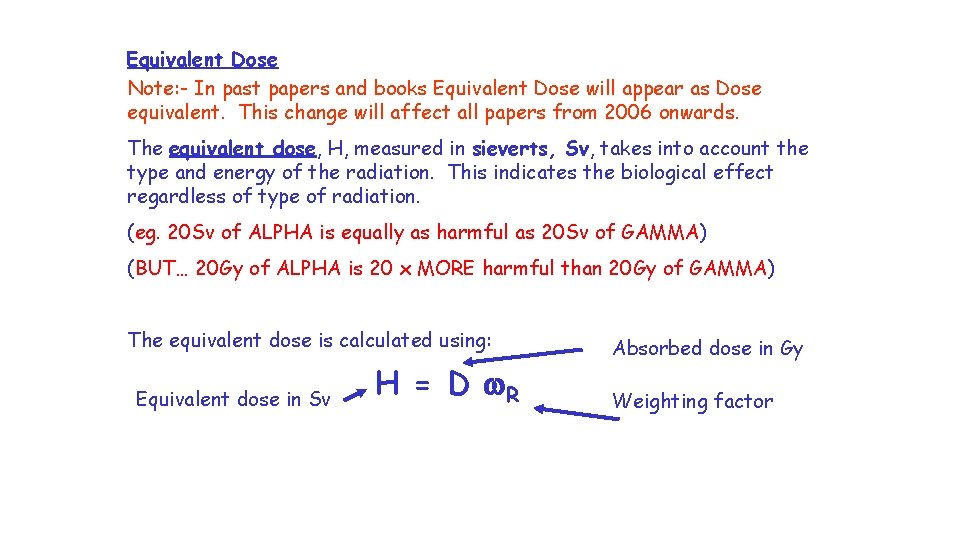
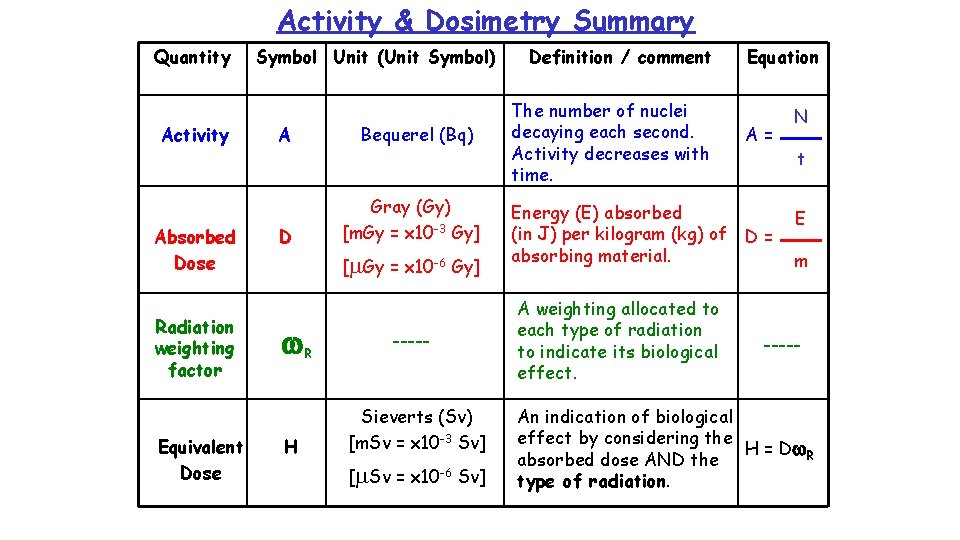
Equivalent Dose Note: - In past papers and books Equivalent Dose will appear as Dose equivalent. This change will affect all papers from 2006 onwards. The equivalent dose, H, measured in sieverts, Sv, takes into account the type and energy of the radiation. This indicates the biological effect regardless of type of radiation. (eg. 20 Sv of ALPHA is equally as harmful as 20 Sv of GAMMA) (BUT… 20 Gy of ALPHA is 20 x MORE harmful than 20 Gy of GAMMA) The equivalent dose is calculated using: Equivalent dose in Sv H = D w. R Absorbed dose in Gy Weighting factor

Activity & Dosimetry Summary Quantity Activity Absorbed Dose Radiation weighting factor Equivalent Dose Symbol Unit (Unit Symbol) A D Bequerel (Bq) Gray (Gy) [m. Gy = x 10 -3 Gy] [m. Gy = x 10 -6 Gy] w. R H ----- Sieverts (Sv) [m. Sv = x 10 -3 Sv] [m. Sv = x 10 -6 Sv] Definition / comment The number of nuclei decaying each second. Activity decreases with time. Equation A= Energy (E) absorbed (in J) per kilogram (kg) of D = absorbing material. A weighting allocated to each type of radiation to indicate its biological effect. N t E m ----- An indication of biological effect by considering the H = D w. R absorbed dose AND the type of radiation.

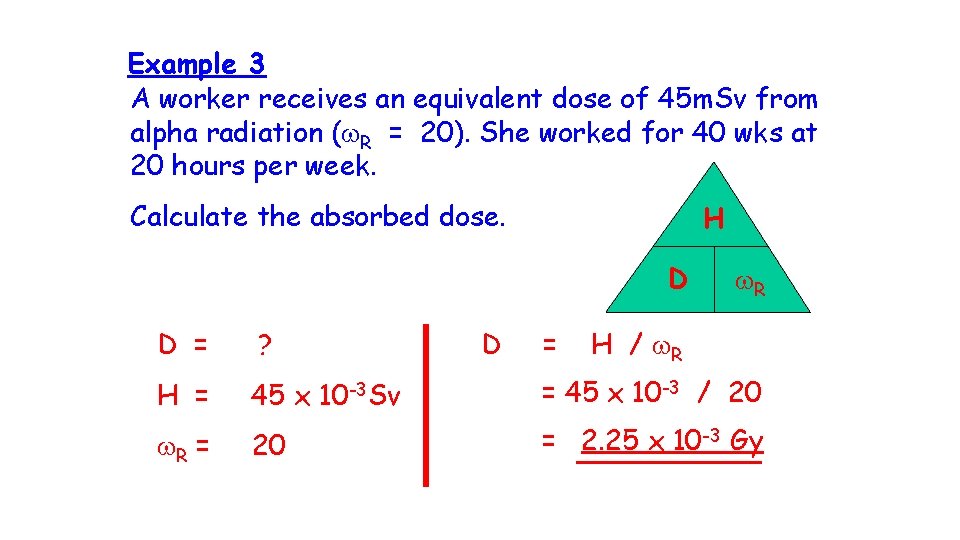
Example 3 A worker receives an equivalent dose of 45 m. Sv from alpha radiation (w. R = 20). She worked for 40 wks at 20 hours per week. Calculate the absorbed dose. H D D = w. R D = ? H / w. R H = 45 x 10 -3 Sv = 45 x 10 -3 / 20 w. R = 20 = 2. 25 x 10 -3 Gy

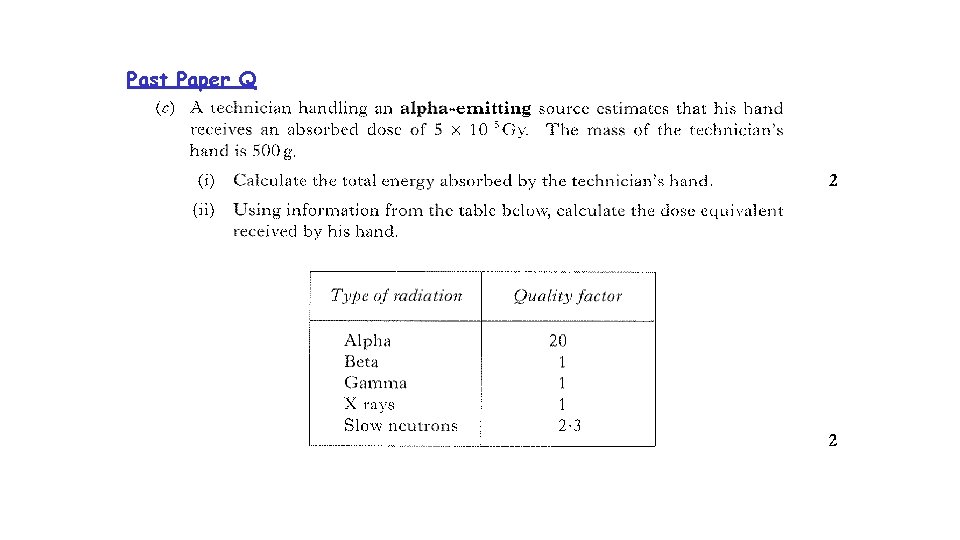
Past Paper Q Weighting Factor

Past Paper Q

Past Paper Q Wr

Background Radiation We are exposed to continual background radiation. The average annual effective equivalent dose for a person in the UK from natural sources is about 2 m. SV. Exposure limits have been set to: • General public – 5 m. Sv in addition to background count in any one year. • Radiation workers – 50 m. Sv in addition to background count in any one year. (Workers may include –pilots, medical workers, physics teachers, as well as those that work in nuclear power plants. ) Sources of background radiation include • Cosmic radiation • Inhaled radon and daughter products • Radioactivity from rocks, soil and buildings • Radioactivity present in the human body

Safety & Equivalent Dose Suggest some safety precautions necessary when dealing with radioactive substances. Use tongs (don’t touch the source), never point source at people, store in a lead box, etc… What is the unit for “equivalent dose”? Sieverts (Sv) What 2 main factors determine the biological effect of radiation on living things? The absorbing tissue and the radiation itself. (The equivalent dose takes account of the type of radiation and the energy it carries. )

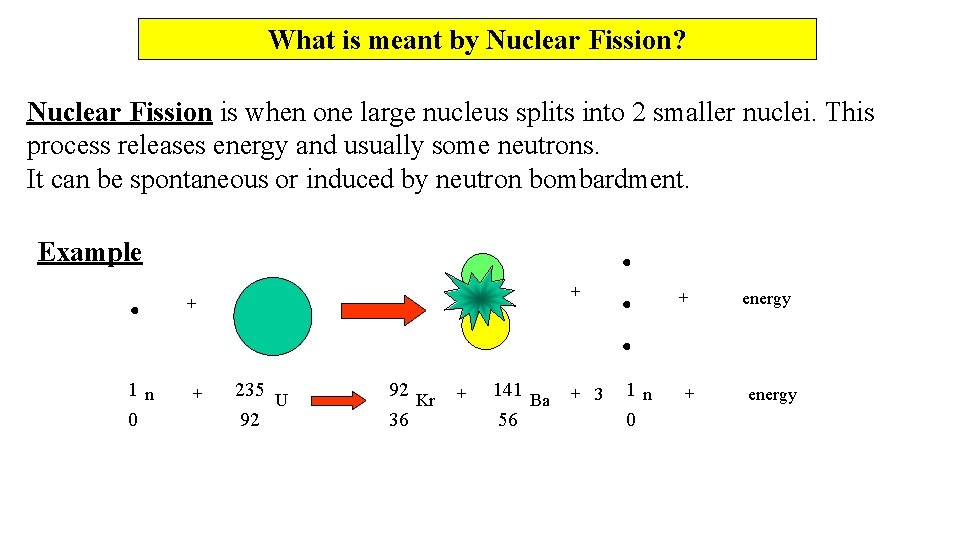
What is meant by Nuclear Fission? Nuclear Fission is when one large nucleus splits into 2 smaller nuclei. This process releases energy and usually some neutrons. It can be spontaneous or induced by neutron bombardment. Example + + 1 n 0 + 235 U 92 92 Kr 36 + 141 Ba 56 + 3 + 1 n 0 + energy

State an Application of Nuclear Fission? • In nuclear reactors, fission processes are used to generate huge amounts of heat. • The heat turns water to steam which, in turn, turns turbines. • The turbines connect to the generator to make electricity.

State disadvantage of using nuclear fission to generate electricity? Large amounts of radioactive waste are generated. Safe storage of these materials for a very long term is very important.

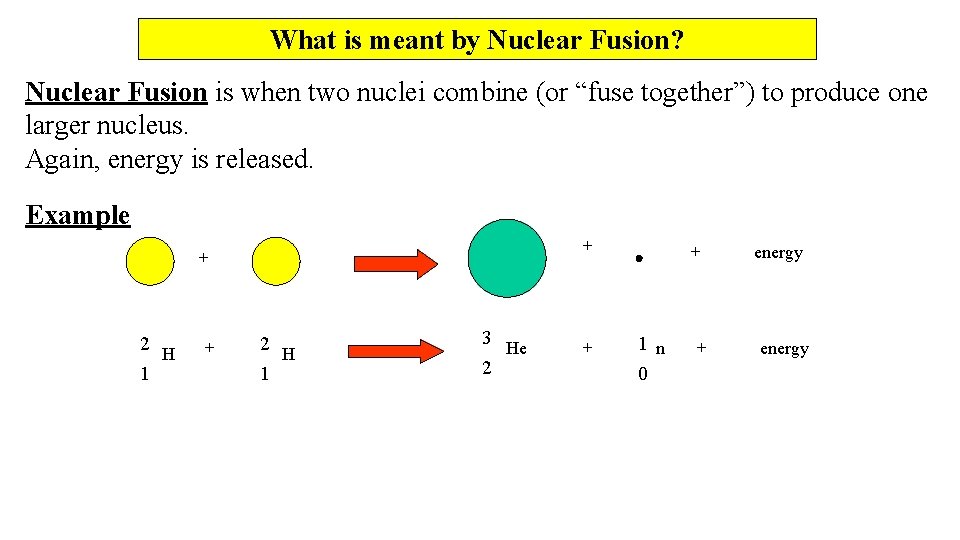
What is meant by Nuclear Fusion? Nuclear Fusion is when two nuclei combine (or “fuse together”) to produce one larger nucleus. Again, energy is released. Example + + 2 H 1 3 He 2 + + 1 n 0 + energy

State an Application of Nuclear Fusion? • Nuclear fusion is the reaction which happens in stars to create elements. • It requires very high temperatures and pressures.

State why nuclear fusion is a much preferred way to generate electricity from nuclear sources. • Nuclear fusion is clean – it generates no radioactive waste materials. • Nuclear fusion can be made to happen using readily available material – water!! So why don’t we use fusion instead of fission to generate our electricity in power stations? Fusion requires huge temperatures and pressures. Once we find ways of making fusion happen at low temperatures, we will use fusion instead of fission! Lots of research already underway … with some success starting.