Naming Ionic Compounds Ionic compounds form between a


Naming Ionic Compounds • Ionic compounds form between a metal and a nonmetal. • The metal forms a cation (+ ion) and the nonmetal forms an anion (-ion). • The cation is always named first and is the element name. • The anion is always named second – the root of the element name with an –ide ending.

Examples: • Li. Cl • Be. F 2 • Al 2 O 3 • Ca 3 P 2 • Na. Br • K 2 S

Writing Formulas of Ionic Compounds • Determine the charge on the cation and anion • Balance the charges (so that the net charge on the compound is neutral) to write the formula.

Examples: • • • Sodium chloride Lithium oxide Beryllium fluoride Aluminum sellenide Strontium arsenide

Naming Covalent Compounds • Covalent compounds form between two nonmetals. • Numerical prefixes tell how many atoms of each element there are. • The first nonmetal gets the prefix and name of the element • The second nonmetal gets the prefix and an –ide ending.

Numerical Prefixes 1 = mono 2 = di 3 = tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca • If there is only one atom of the first element, the name does not get a prefix
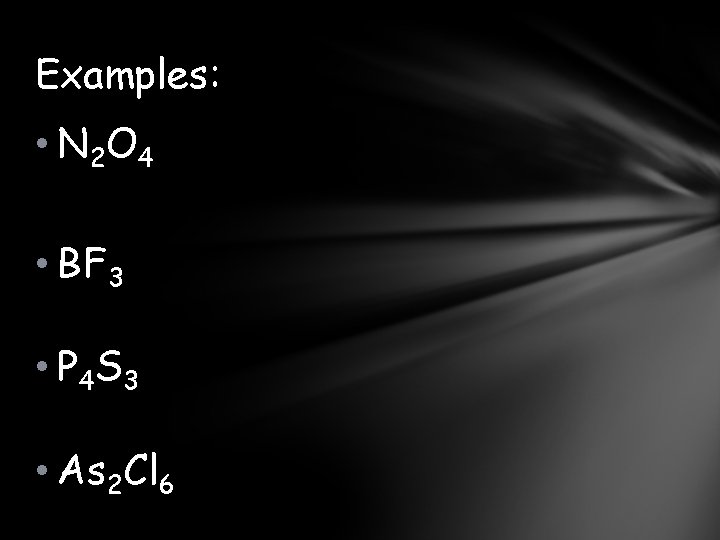
Examples: • N 2 O 4 • BF 3 • P 4 S 3 • As 2 Cl 6

Writing Formulas Covalent Compounds • Write the element symbols for each nonmetal • Put a subscript after each symbol, based on the prefix

Examples: • Carbon dioxide • Trisilicon heptacarbide • Pentaiodine octafluroide • Nonabromine monophosphate
- Slides: 10