Molecular Modeling and Informatics Characteristics of Molecular Modeling

















- Slides: 36

Molecular Modeling and Informatics

Characteristics of Molecular Modeling n Representing behavior of molecular systems Visual (tinker toys – LCDs) rendering of molecules n Mathematical rendering (differential equations, matrix algebra) of molecular interactions n Time dependent and time independent realms n

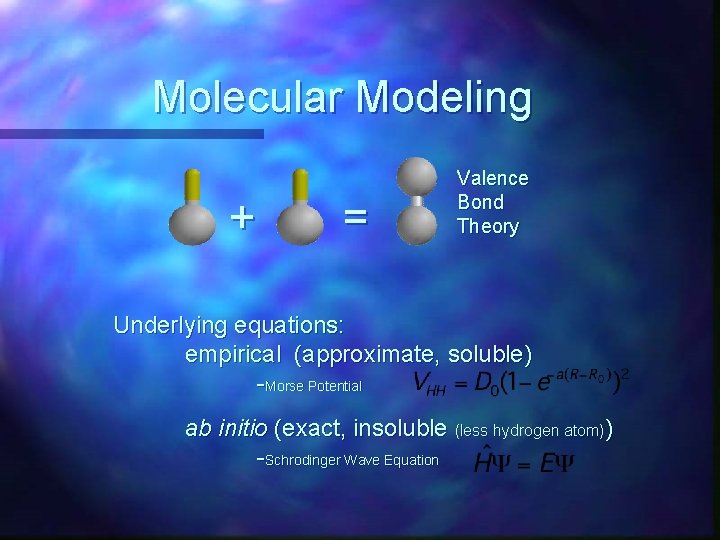
Molecular Modeling + = Valence Bond Theory Underlying equations: empirical (approximate, soluble) -Morse Potential ab initio (exact, insoluble (less hydrogen atom)) -Schrodinger Wave Equation


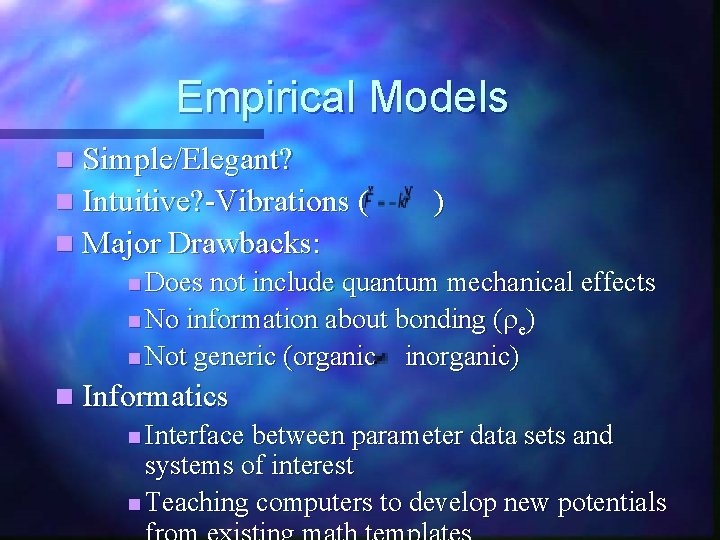
Empirical Models n Simple/Elegant? n Intuitive? -Vibrations ( ) n Major Drawbacks: n Does not include quantum mechanical effects n No information about bonding (re) n Not generic (organic inorganic) n Informatics n Interface between parameter data sets and systems of interest n Teaching computers to develop new potentials

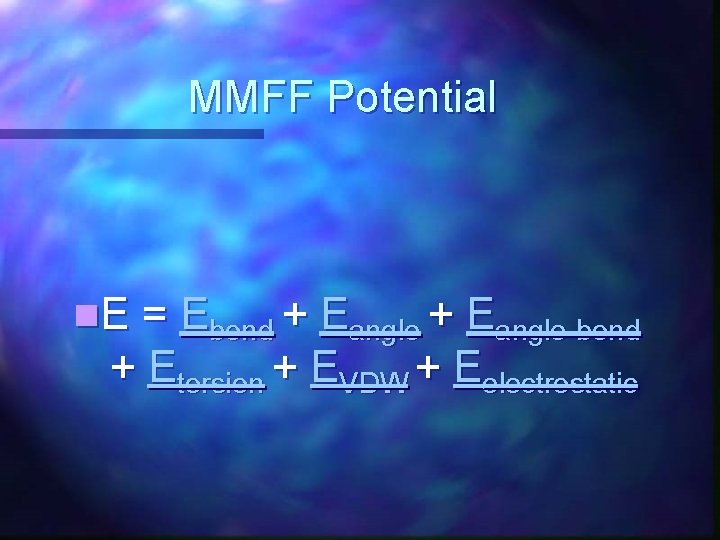
MMFF Potential n. E = Ebond + Eangle-bond + Etorsion + EVDW + Eelectrostatic

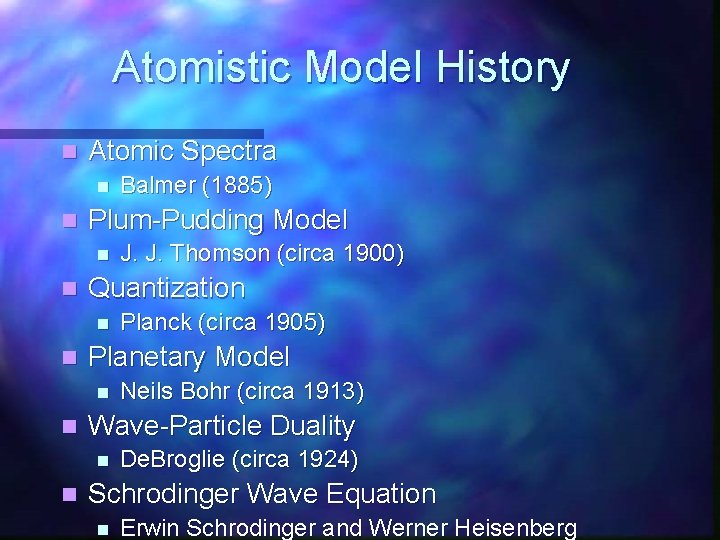
Atomistic Model History n Atomic Spectra n n Plum-Pudding Model n n Neils Bohr (circa 1913) Wave-Particle Duality n n Planck (circa 1905) Planetary Model n n J. J. Thomson (circa 1900) Quantization n n Balmer (1885) De. Broglie (circa 1924) Schrodinger Wave Equation n Erwin Schrodinger and Werner Heisenberg

Classical vs. Quantum Trajectory n Real numbers n n Deterministic (“The value is ___”) Variables n Continuous energy spectrum n Wavefunction n Complex (Real and Imaginary components) n Probabilistic (“The average value is __ ” n Operators n Discrete/Quantized energy n Tunneling n Zero-point energy n

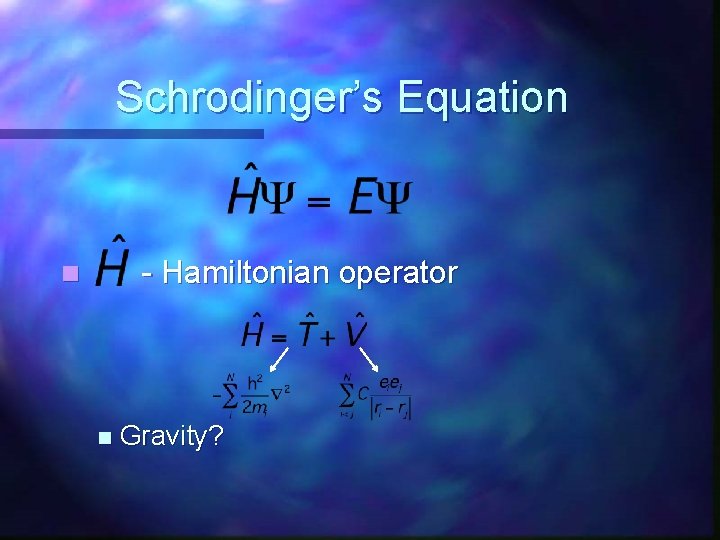
Schrodinger’s Equation - Hamiltonian operator n n Gravity?


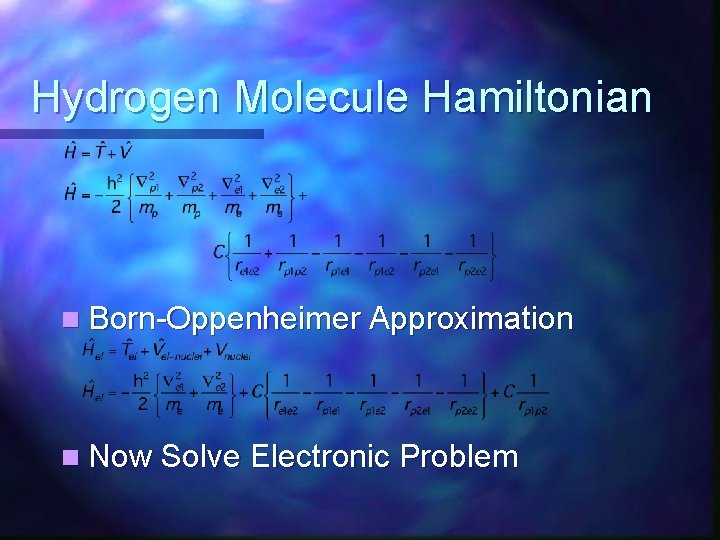
Hydrogen Molecule Hamiltonian n Born-Oppenheimer Approximation n Now Solve Electronic Problem

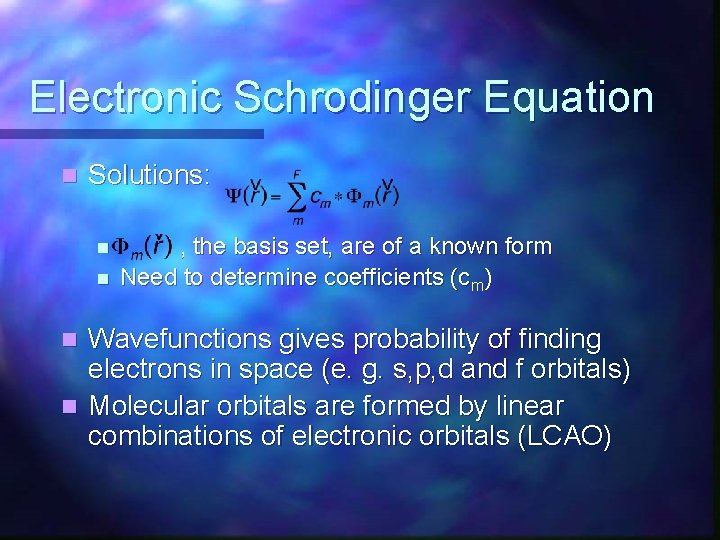
Electronic Schrodinger Equation n Solutions: n n , the basis set, are of a known form Need to determine coefficients (cm) Wavefunctions gives probability of finding electrons in space (e. g. s, p, d and f orbitals) n Molecular orbitals are formed by linear combinations of electronic orbitals (LCAO) n

Hydrogen Molecule n HOMO n LUMO

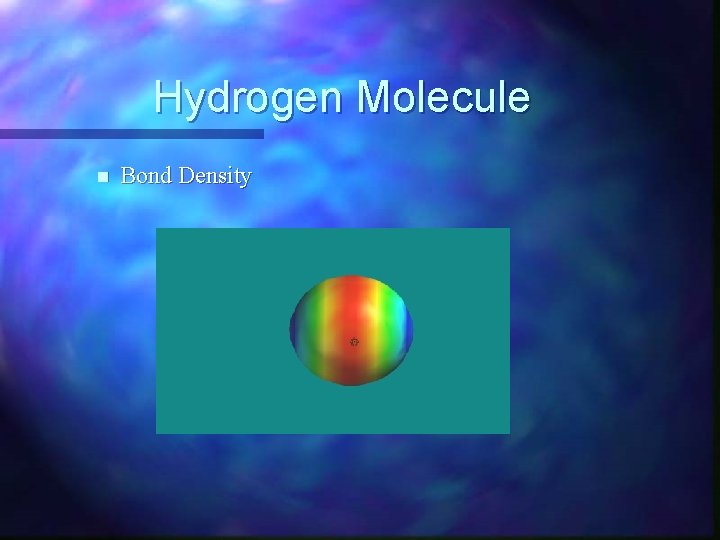
Hydrogen Molecule n Bond Density

Ab Initio/DFT n n n Complete Description! Generic! Major Drawbacks: n n n Mathematics can be cumbersome Exact solution only for hydrogen Informatics n Approximate solution time and storage intensive – Acquisition, manipulation and dissemination problems

Approximate Methods n SCF (Self Consistent Field) Method (a. ka. Mean Field or Hartree Fock) n n n Pick single electron and average influence of remaining electrons as a single force field (V 0 external) Then solve Schrodinger equation for single electron in presence of field (e. g. H-atom problem with extra force field) Perform for all electrons in system Combine to give system wavefunction and energy (E) (E Repeat to error tolerance (Ei+1 -Ei)

Correcting Approximations n Accounting for Electron Correlations DFT(Density Functional Theory) n Moller-Plesset (Perturbation Theory) n Configuration Interaction (Coupling single electron problems) n

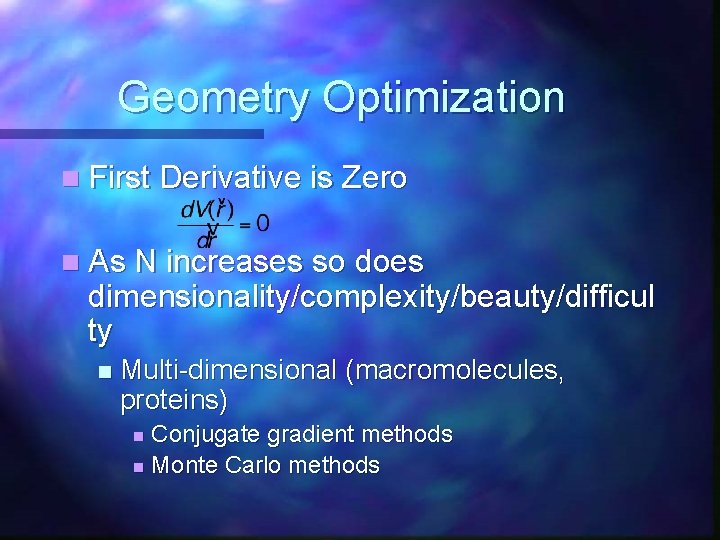
Geometry Optimization n First Derivative is Zero n As N increases so does dimensionality/complexity/beauty/difficul ty n Multi-dimensional (macromolecules, proteins) Conjugate gradient methods n Monte Carlo methods n

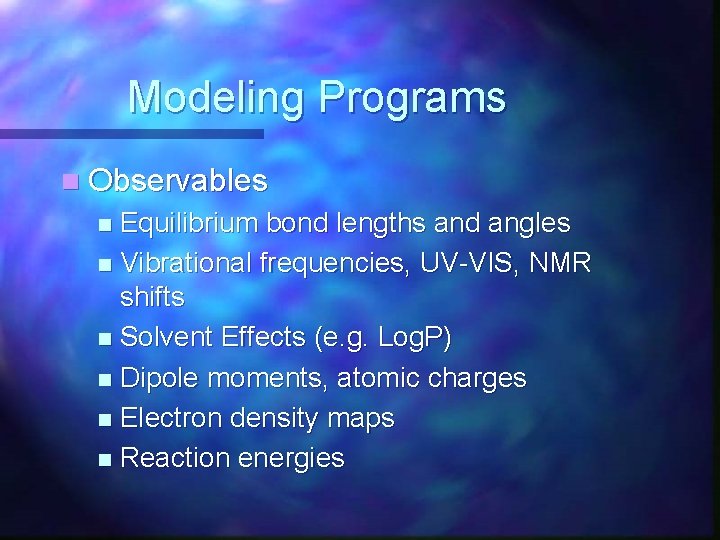
Modeling Programs n Observables Equilibrium bond lengths and angles n Vibrational frequencies, UV-VIS, NMR shifts n Solvent Effects (e. g. Log. P) n Dipole moments, atomic charges n Electron density maps n Reaction energies n

Comparison to Experiments n Electronic Schrodinger Equation gives bonding energies for non-vibrating molecules (nuclei fixed at equilibrium geometry) at 0 K n n Can estimate G= H - TS using frequencies Eout NOT DHf! n Bond separation reactions (simplest 2 -heavy atom components) provide path to heats of formation

Ab Initio Modeling Limits Function of basis and method used n Accuracy n n ~. 02 angstroms ~2 -4 kcal N n n HF - 50 -100 atoms DFT - 500 -1000 atoms

Semi-Empirical Methods n Neglect Inner Core Electrons n Neglect of Diatomic Differential Overlap (NDDO) Atomic orbitals on two different atomic centers do not overlap n Reduces computation time dramatically n

Other Methods n Energetics n n Monte Carlo Genetic Algorithms Maximum Entropy Methods Simulated Annealing n Dynamics n n n Finite Difference Monte Carlo Fourier Analysis

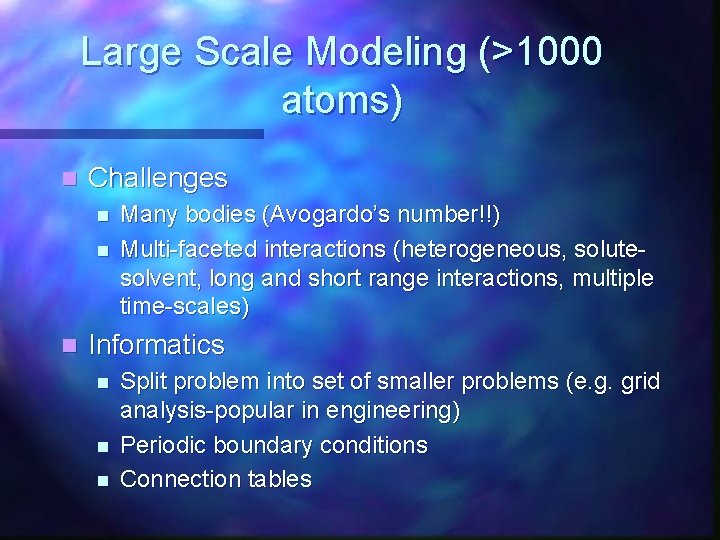
Large Scale Modeling (>1000 atoms) n Challenges n n n Many bodies (Avogardo’s number!!) Multi-faceted interactions (heterogeneous, solutesolvent, long and short range interactions, multiple time-scales) Informatics n n n Split problem into set of smaller problems (e. g. grid analysis-popular in engineering) Periodic boundary conditions Connection tables

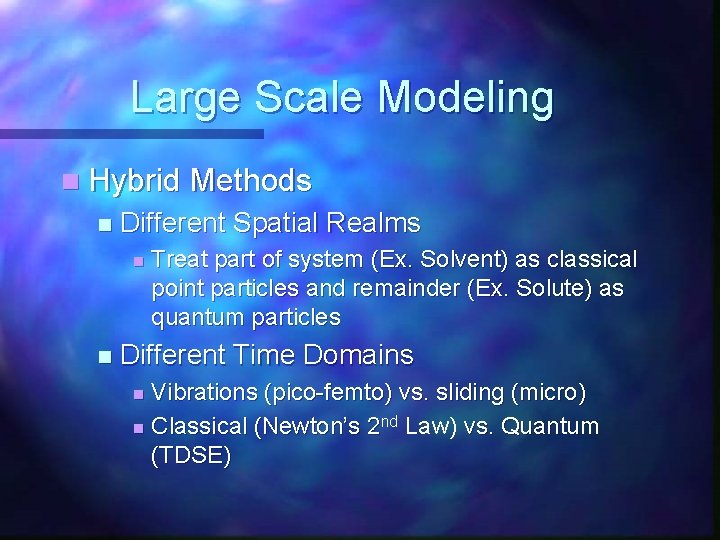
Large Scale Modeling n Hybrid Methods n Different Spatial Realms n n Treat part of system (Ex. Solvent) as classical point particles and remainder (Ex. Solute) as quantum particles Different Time Domains Vibrations (pico-femto) vs. sliding (micro) n Classical (Newton’s 2 nd Law) vs. Quantum (TDSE) n

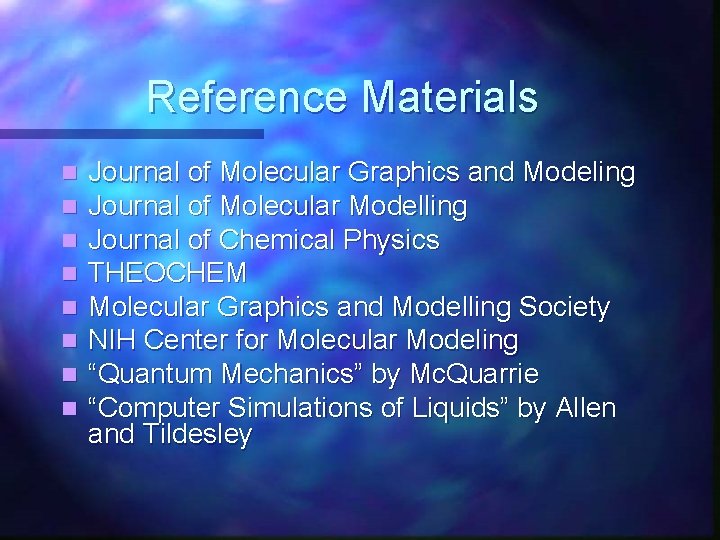
Reference Materials n n n n Journal of Molecular Graphics and Modeling Journal of Molecular Modelling Journal of Chemical Physics THEOCHEM Molecular Graphics and Modelling Society NIH Center for Molecular Modeling “Quantum Mechanics” by Mc. Quarrie “Computer Simulations of Liquids” by Allen and Tildesley

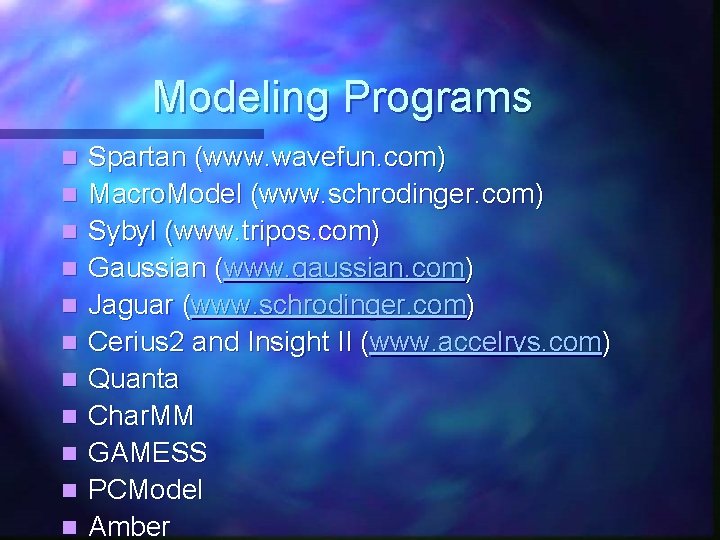
Modeling Programs n n n Spartan (www. wavefun. com) Macro. Model (www. schrodinger. com) Sybyl (www. tripos. com) Gaussian (www. gaussian. com) Jaguar (www. schrodinger. com) Cerius 2 and Insight II (www. accelrys. com) Quanta Char. MM GAMESS PCModel Amber

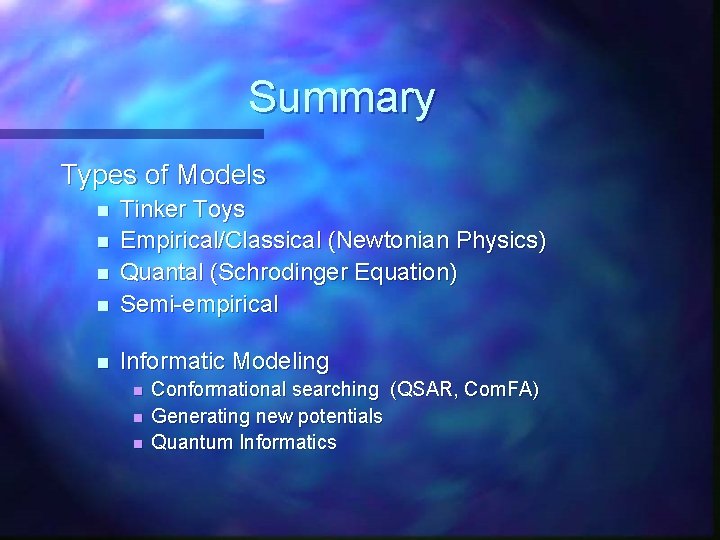
Summary Types of Models n Tinker Toys Empirical/Classical (Newtonian Physics) Quantal (Schrodinger Equation) Semi-empirical n Informatic Modeling n n n Conformational searching (QSAR, Com. FA) Generating new potentials Quantum Informatics

Next Time n QSAR (Read Chapter 4)

MMFF Energy n Stretching

MMFF Energy n Bending

MMFF Energy n Stretch-Bend Interactions

MMFF Energy n Torsion (4 -atom bending)

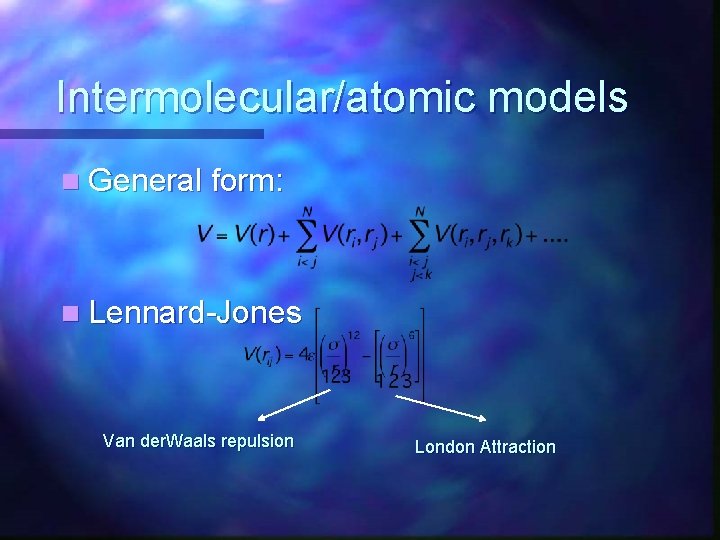
MMFF Energy n Analogous to Lennard-Jones 6 -12 potential London Dispersion Forces n Van der Waals Repulsions n

Intermolecular/atomic models n General form: n Lennard-Jones Van der. Waals repulsion London Attraction

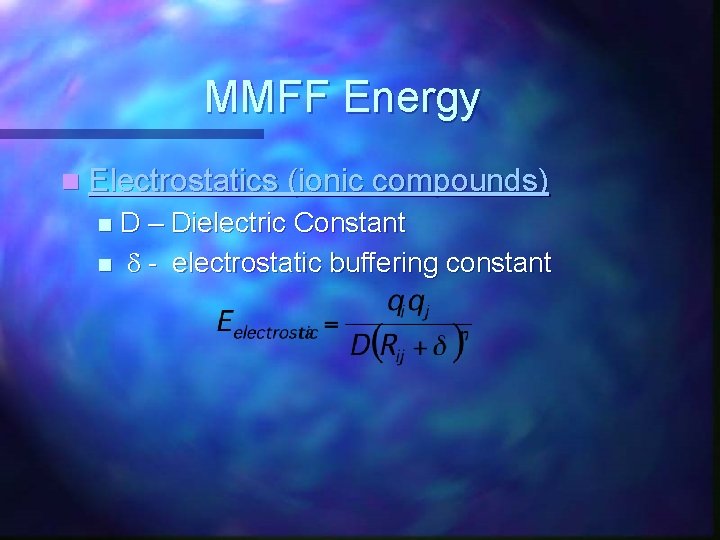
MMFF Energy n Electrostatics (ionic compounds) D – Dielectric Constant n d - electrostatic buffering constant n
 Erickson nursing theory
Erickson nursing theory Relational modeling vs dimensional modeling
Relational modeling vs dimensional modeling Covalent bond boiling point
Covalent bond boiling point Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Observational health data sciences and informatics
Observational health data sciences and informatics Nursing informatics and healthcare policy
Nursing informatics and healthcare policy Nursing informatics theories, models and frameworks
Nursing informatics theories, models and frameworks Belarusian university of informatics and radioelectronics
Belarusian university of informatics and radioelectronics Cybernetics statistics and economic informatics
Cybernetics statistics and economic informatics Chapter 26 informatics and documentation
Chapter 26 informatics and documentation School of computing and informatics
School of computing and informatics School of computing and informatics
School of computing and informatics Department of land surveying and geo-informatics
Department of land surveying and geo-informatics Introduction to medical informatics
Introduction to medical informatics Informatics 43 uci
Informatics 43 uci In4matx 43 uci
In4matx 43 uci Supply chain informatics
Supply chain informatics Python for informatics: exploring information
Python for informatics: exploring information Metastructures of nursing informatics
Metastructures of nursing informatics Supply chain informatics
Supply chain informatics Python for informatics
Python for informatics Banaprint
Banaprint Python for informatics
Python for informatics Health informatics questions
Health informatics questions Health informatics
Health informatics Poc informatics systems
Poc informatics systems Python for informatics
Python for informatics Pharmacy informatics definition
Pharmacy informatics definition Social informatics definition
Social informatics definition Personal traits for health informatics services workers
Personal traits for health informatics services workers History of pharmacy informatics
History of pharmacy informatics History of pharmacy informatics
History of pharmacy informatics Biomedical informatics definition
Biomedical informatics definition Va office of health informatics
Va office of health informatics Pitt health informatics
Pitt health informatics Olympiad in informatics
Olympiad in informatics