Molecular Geometry VSEPR Theory Valence shell electronpair repulsion
- Slides: 18

Molecular Geometry • VSEPR Theory- “Valence- shell, electron-pair repulsion” • states that repulsion between the sets of valence-level electrons surrounding an atom cause these sets to be oriented as far apart as possible.

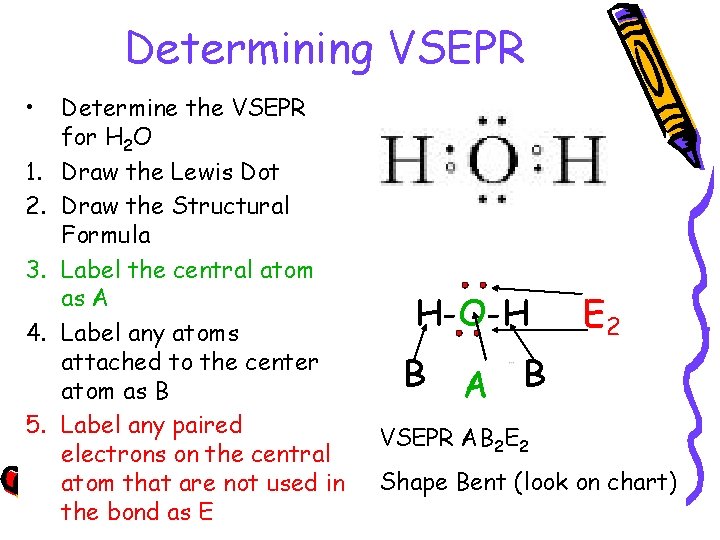
Determining VSEPR • 1. 2. 3. 4. 5. Determine the VSEPR for H 2 O Draw the Lewis Dot Draw the Structural Formula Label the central atom as A Label any atoms attached to the center atom as B Label any paired electrons on the central atom that are not used in the bond as E H-O-H B E 2 A B VSEPR AB 2 E 2 Shape Bent (look on chart)

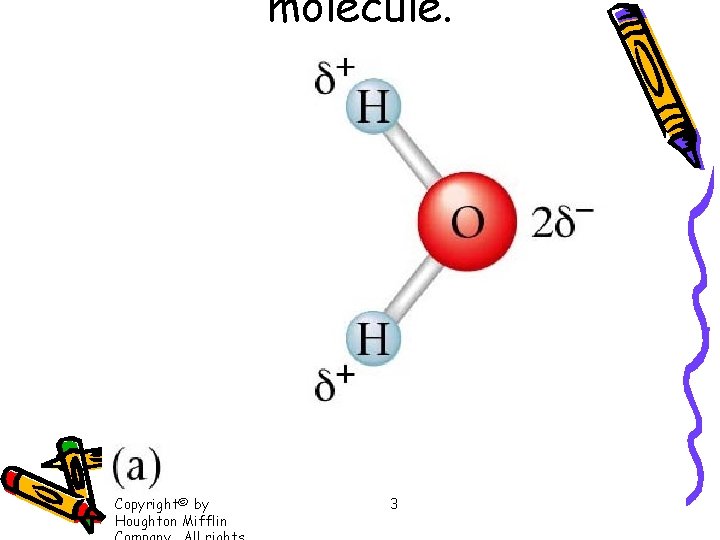
molecule. Copyright© by Houghton Mifflin 3

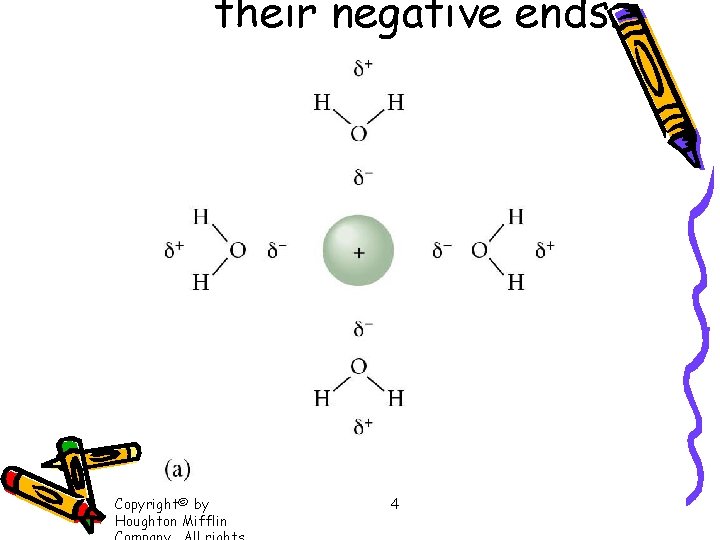
their negative ends. Copyright© by Houghton Mifflin 4

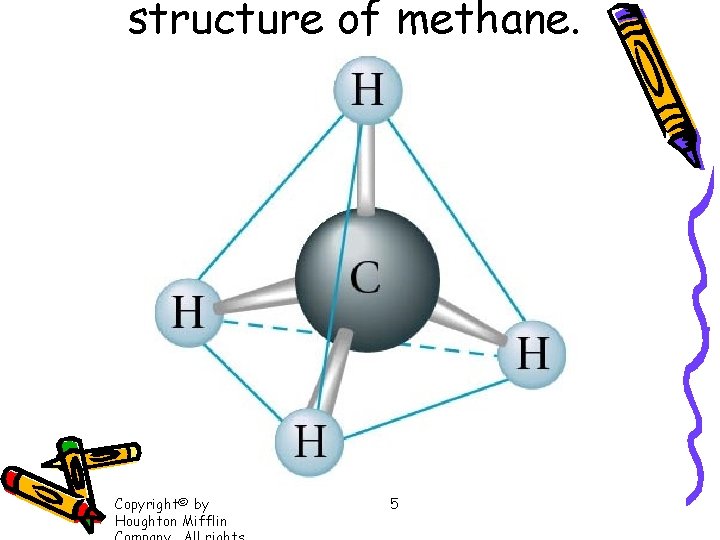
structure of methane. Copyright© by Houghton Mifflin 5

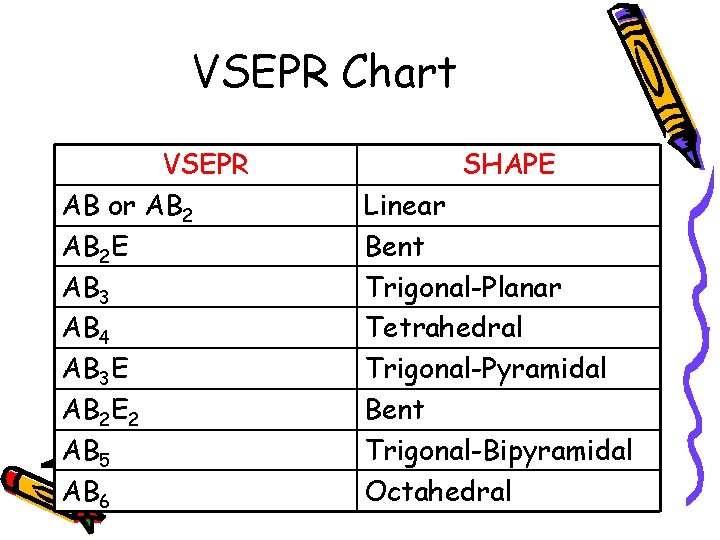
VSEPR Chart VSEPR AB or AB 2 E AB 3 AB 4 AB 3 E AB 2 E 2 AB 5 AB 6 SHAPE Linear Bent Trigonal-Planar Tetrahedral Trigonal-Pyramidal Bent Trigonal-Bipyramidal Octahedral

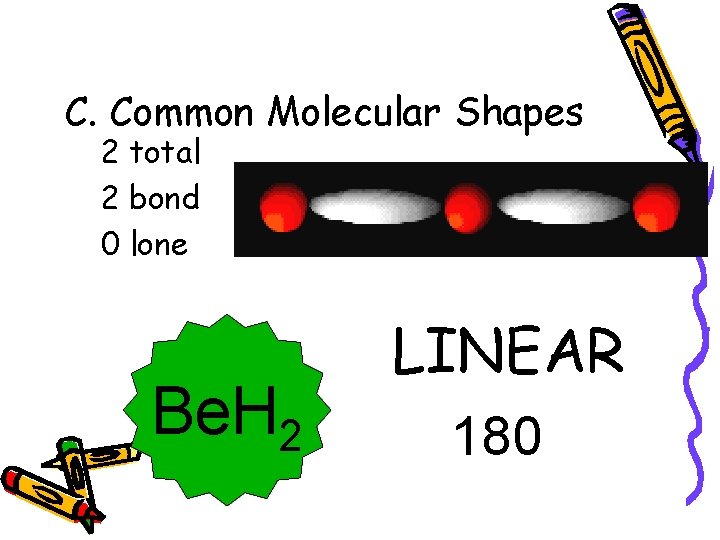
C. Common Molecular Shapes 2 total 2 bond 0 lone Be. H 2 LINEAR 180°

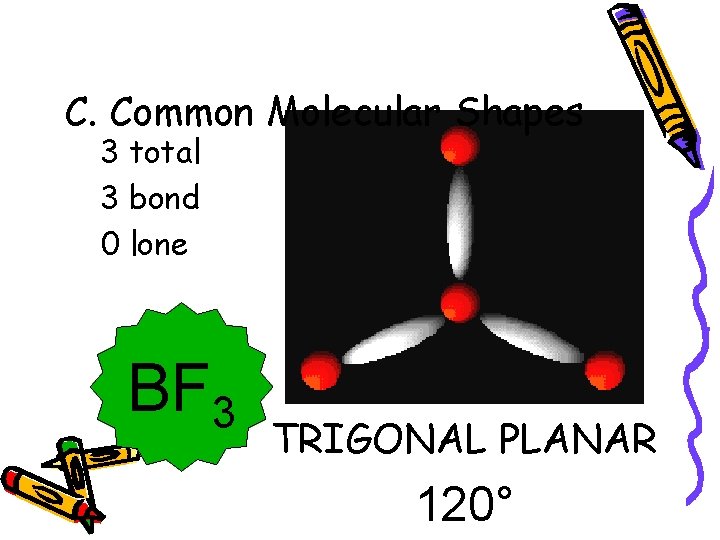
C. Common Molecular Shapes 3 total 3 bond 0 lone BF 3 TRIGONAL PLANAR 120°

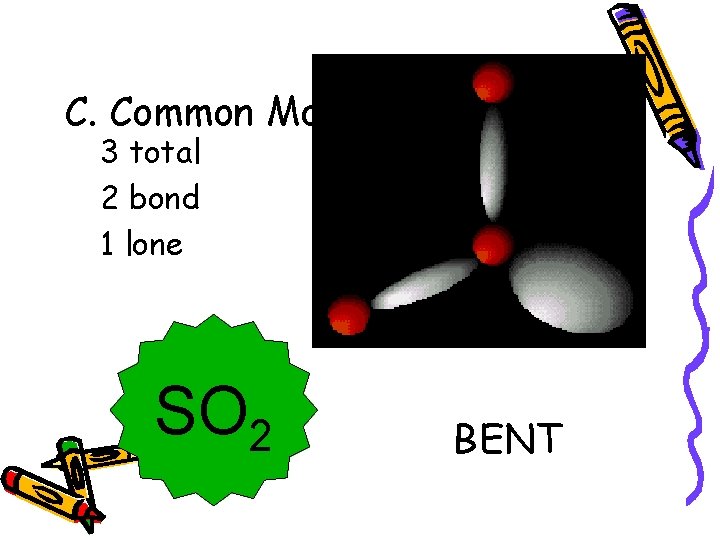
C. Common Molecular Shapes 3 total 2 bond 1 lone SO 2 BENT <120°

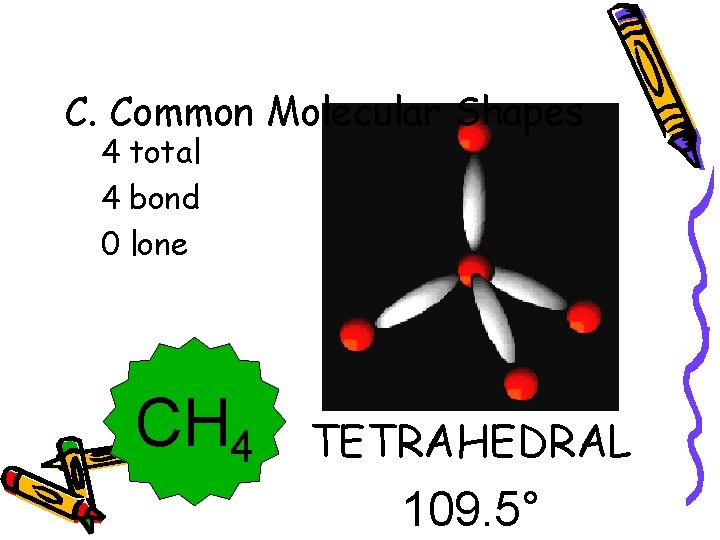
C. Common Molecular Shapes 4 total 4 bond 0 lone CH 4 TETRAHEDRAL 109. 5°

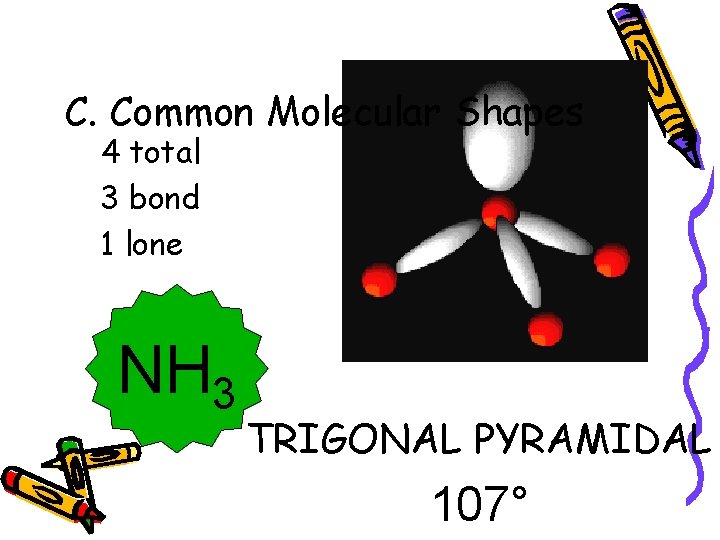
C. Common Molecular Shapes 4 total 3 bond 1 lone NH 3 TRIGONAL PYRAMIDAL 107°

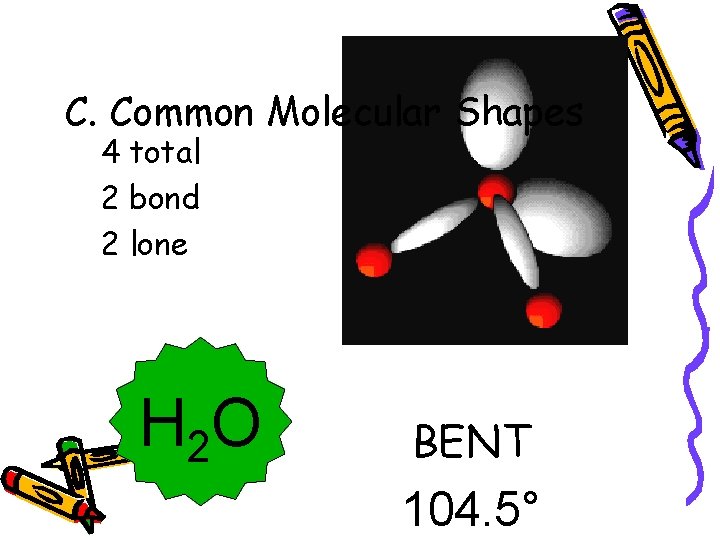
C. Common Molecular Shapes 4 total 2 bond 2 lone H 2 O BENT 104. 5°

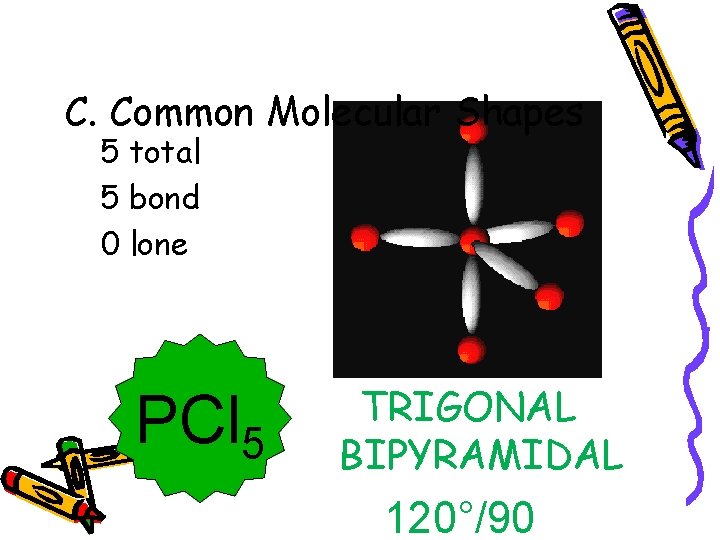
C. Common Molecular Shapes 5 total 5 bond 0 lone PCl 5 TRIGONAL BIPYRAMIDAL 120°/90°

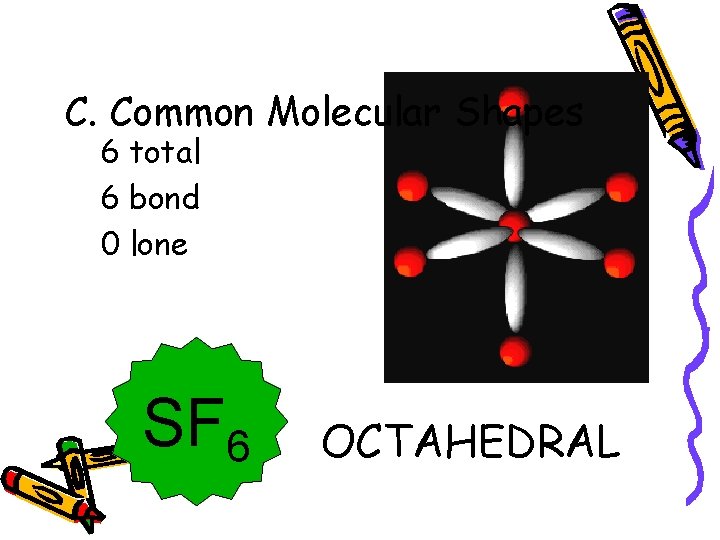
C. Common Molecular Shapes 6 total 6 bond 0 lone SF 6 OCTAHEDRAL 90°

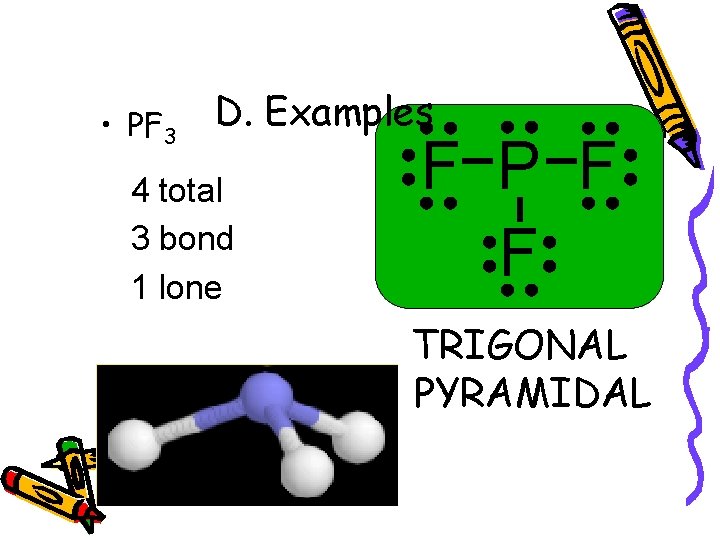
• PF 3 D. Examples 4 total 3 bond 1 lone F P F F TRIGONAL PYRAMIDAL 107°

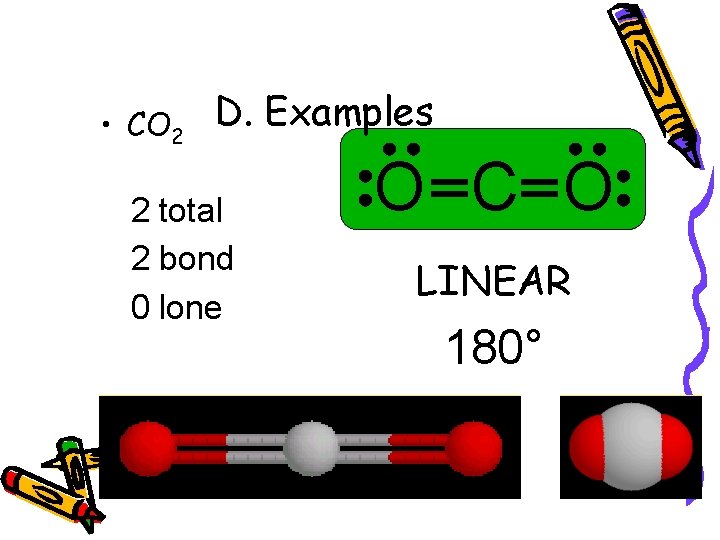
• CO 2 D. Examples 2 total 2 bond 0 lone O C O LINEAR 180°

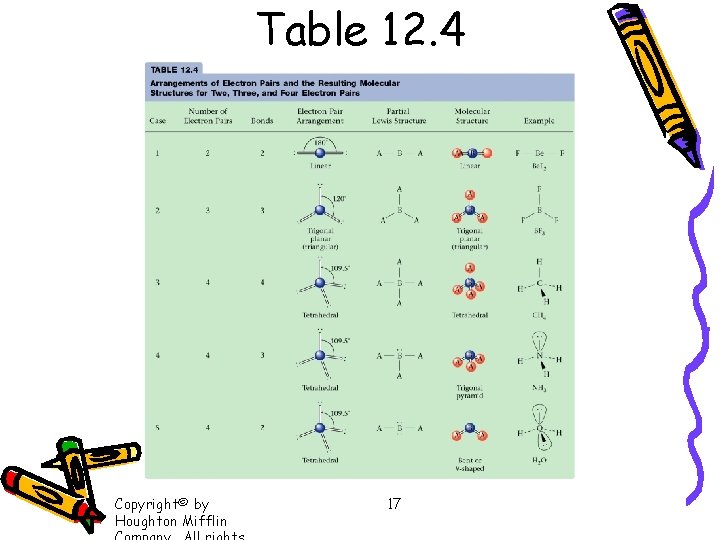
Table 12. 4 Copyright© by Houghton Mifflin 17

• Hybridization-The Blending of Orbitals. • Dipole- is created by equal but opposite charges that are separated by a short distance. • Dipole-Dipole Attractions-Attraction between Attractions oppositely charged regions of neighboring molecules. • Hydrogen Bonding- Bonding between hydrogen and more electronegative neighboring atoms such as oxygen and nitrogen. Hydrogen bonding in Kevlar, a strong polymer used in bullet-proof vests. • London Dispersion Forces- The temporary separations of charge that lead to the London force attractions are what attract one nonpolar molecule to its neighbors. London forces increase with the size of the molecules.