Minerals Characteristics and Properties 1 Mineral Characteristics Forms





























- Slides: 35

Minerals Characteristics and Properties 1

Mineral Characteristics • • Forms in nature (naturally occurring) Is an inorganic solid Has a specific chemical composition Has a crystal structure 2

Rocks and minerals are not the same • Minerals must have the four characteristics listed in the previous slides. • A rock only has to have the two characteristics listed below: • It is a solid • It forms naturally *A rock usually contains two or more types of minerals and may vary in the amount it contains. 3

What does it mean to be naturally occurring? • This simply means it was formed by natural processes…. thus man made or synthetically made substances (ex. diamonds, rubies and emeralds) are NOT considered minerals. 4

What does it mean to be inorganic? • Inorganic means they are not alive and never were alive during any part of their existence. • So, salt is a mineral but sugar is not since it is harvested from plants. 5

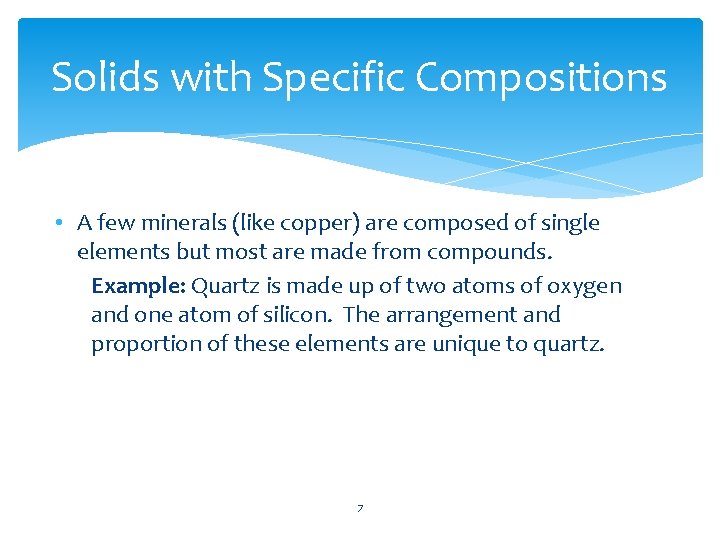
Solids with Specific Compositions • Solids have definite shapes and volumes • Specific composition means that each type of mineral has a chemical composition unique to that mineral. 6

Solids with Specific Compositions • A few minerals (like copper) are composed of single elements but most are made from compounds. Example: Quartz is made up of two atoms of oxygen and one atom of silicon. The arrangement and proportion of these elements are unique to quartz. 7

Element A substance that contains only one type of atom. • An atom is the smallest particle an element can be divided into. • Examples: Copper, Silver, Oxygen 8

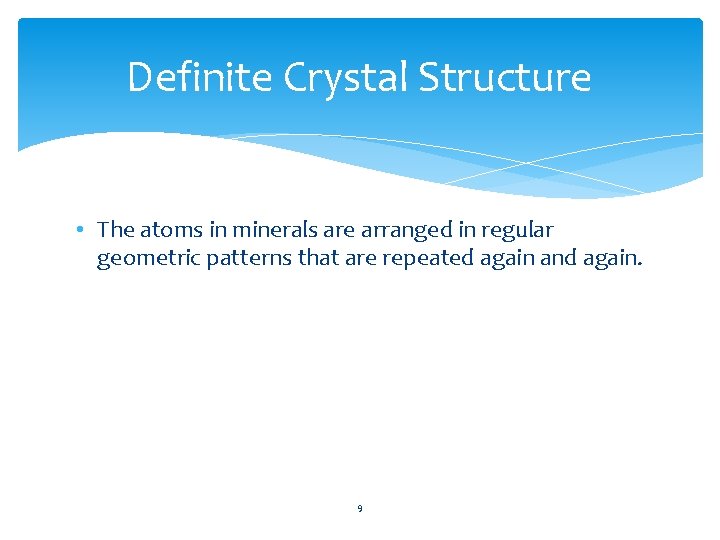
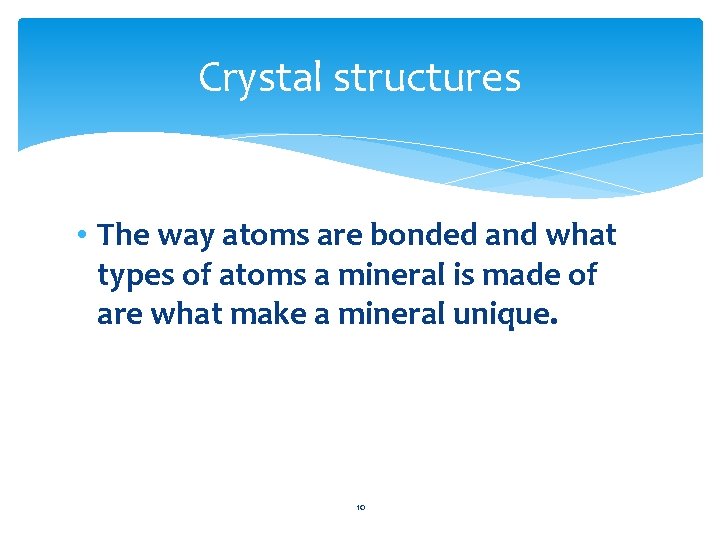
Definite Crystal Structure • The atoms in minerals are arranged in regular geometric patterns that are repeated again and again. 9

Crystal structures • The way atoms are bonded and what types of atoms a mineral is made of are what make a mineral unique. 10

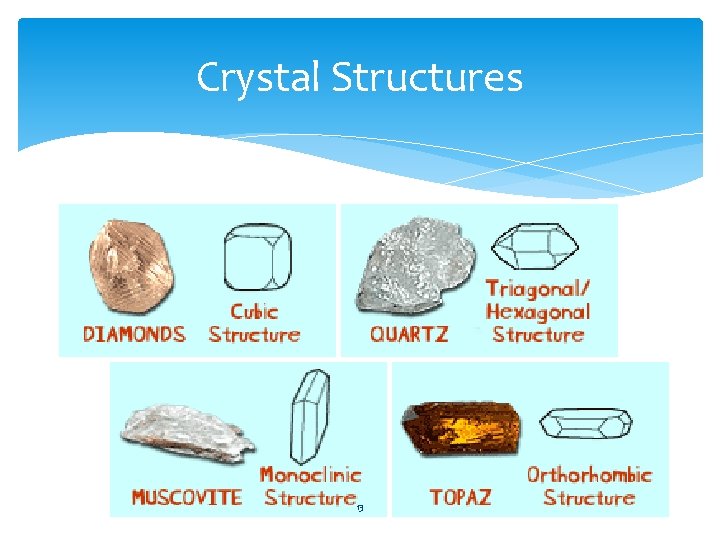
Crystal structures • Crystal- a solid in which the atoms are arranged in an orderly, repeating threedimensional pattern • Each mineral has its own type of crystal structure. In some cases two minerals have the same chemical composition but different crystal structures. 11

Atoms arranged in crystal structure 12

Crystal Structures 13

Minerals from Magma • Minerals can form from cooling magma. • The type and amount of elements present in the magma determine which minerals will form • The rate the magma cools determines the size of the mineral crystals. • Slow cooling forms large crystals • Fast cooling forms small crystals 14

Minerals from Solution • Supersaturated solutions (solution overfilled with another substance) can have mineral crystals begin to drop out of this solution (think rock candy) • Minerals can also form when elements dissolve in a supersaturated solution—when the liquid part of the solution evaporates, the minerals remain and can begin to arrange into crystals. (gypsum) 15

Mineral Groups • The most common minerals are referred to as rockforming minerals because they make up most of the rocks found in Earth’s crust. • 8 elements make up the majority of minerals. 16

Most Common Mineral Groups • Silicates: Minerals that contain oxygen and silicon • make up ~96% of the minerals found in Earth’s crust • Feldspar and quartz are some common silicates 17

Most Common Mineral Groups • Carbonates: Minerals composed one or more metallic elements with the carbonate compound (CO 3 ) • Limestone and marble are common carbonate minerals 18

Most Common Mineral Groups • Oxides: Minerals composed one oxygen and a metal • Hematite and magnetite are common iron oxides 19

IDENTIFYING MINERALS Color Streak Luster Cleavage Fracture 20

Color and Streak Three main factors cause minerals to vary in color. 1. 2. 3. Tiny amounts of an element that is not part of its normal chemical make-up Coming in contact with the Earth’s atmosphere or water Defects in their crystal structures *One of the least reliable clues to identify a mineral 21

Streak • Streak – the color of the powder left behind when the mineral is scraped across a surface. • Streak is a better clue to a mineral’s identity than surface color is. • Ceramic plates called streak plates are used in this process. *a mineral has to be softer than ceramic in order for this method to be able to be used 22

Luster • Luster- the way in which light reflects from a mineral’s surface. There are two major types of luster. • Metallic- looks as if it were made of metal. • Example: Pyrite, silver, gold • Nonmetallic- luster can be shiny but does not appear to be metallic. Can be described as dull, pearly, waxy or silky. • Example: Garnet, sulfur 23

Metallic Luster-Pyrite 24

Nonmetallic Luster-Garnet 25

Cleavage- the tendency of a mineral to break along flat surfaces. This determines how it’s atoms are bonded, or joined together. The bonds in the crystal structure are weaker in the directions in which the mineral breaks. 26

Cleavage 27

Fracture- the tendency of a mineral to break into irregular pieces. The bonds that join the atoms are fairly equal in strength in all directions. 28

Fracture-Quartz 29

Density- the amount of mass in a given volume of the substance. D= M/V • Very helpful in identifying minerals. 30

Hardness- a mineral’s resistance to being scratched. This is determined by its crystal structure and the strength of the bonds between its atoms. Example: Harder minerals have stronger bonds. 31

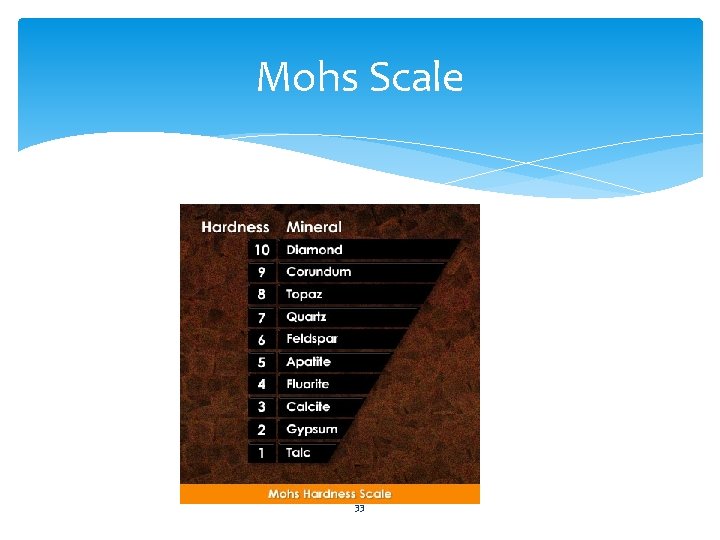
Mohs Scale Mohs scale is used to describe a mineral’s hardness. • Based on the fact that a harder mineral will scratch a softer mineral. • a mineral can only be scratched by other minerals that have the same hardness or are harder. 32

Mohs Scale 33

Special Properties • Some minerals have special properties such as reacting with acid, having fluorescence, magnetism and radioactivity. • Minerals in the carbonate group react with acid • Fluorite has fluorescence • Magnetite is magnetic • Unstable elements such as plutonium are radioactive. 34

Mineral Uses • Minerals are virtually everywhere • They make computers, jewelry, desks, cars, paints and medicines to name just a few • Ores are minerals that contain a useful substance that can be mined for a profit • Examples of ores are: hematite (for iron) and rutile (for titanium) • Gems are valuable minerals that are prized for their natural beauty. • Examples of gems are: rubies, emeralds and diamonds 35
 Properties of mineral
Properties of mineral Properties of minerals
Properties of minerals Fracture mineral properties
Fracture mineral properties Directions of cleavage
Directions of cleavage Properties of minerals
Properties of minerals Optical properties of minerals
Optical properties of minerals Amphibole thin section ppl
Amphibole thin section ppl Cleavage minerals
Cleavage minerals The uses of minerals
The uses of minerals Physical properties of minerals graphic organizer
Physical properties of minerals graphic organizer 5 mineral characteristics
5 mineral characteristics 5 characteristics of minerals
5 characteristics of minerals Calcite vs gypsum
Calcite vs gypsum Minerals characteristics
Minerals characteristics Contracted form of am not
Contracted form of am not Weak and strong forms
Weak and strong forms Intensive and extensive properties
Intensive and extensive properties Chemical property of matter
Chemical property of matter Minerals and their functions sources and deficiency chart
Minerals and their functions sources and deficiency chart Why are related forms more agreeable than unrelated forms
Why are related forms more agreeable than unrelated forms Why are related forms more agreeable than unrelated forms
Why are related forms more agreeable than unrelated forms Why are related forms more agreeable than unrelated forms?
Why are related forms more agreeable than unrelated forms? Mineral resources and petroleum authority of mongolia
Mineral resources and petroleum authority of mongolia Mineral assemblage of metamorphic facies
Mineral assemblage of metamorphic facies Strip mining before and after
Strip mining before and after Creaming is a.......... process
Creaming is a.......... process Mineral exploration and mining active reading
Mineral exploration and mining active reading Portfolio committee on mineral resources and energy
Portfolio committee on mineral resources and energy Types of rocks song
Types of rocks song Granite and basalt difference
Granite and basalt difference Chapter 8 vitamins and minerals
Chapter 8 vitamins and minerals Major elements
Major elements Difference between rocks and minerals
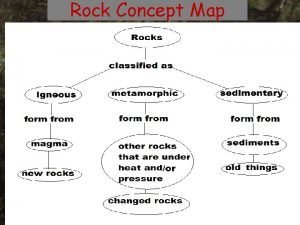
Difference between rocks and minerals Rock the concept map
Rock the concept map Suzanna socked me sunday poem
Suzanna socked me sunday poem Absorbs water and minerals
Absorbs water and minerals