Miejsce i rola porwnania poredniego w HTA The

- Slides: 15

Miejsce i rola porównania pośredniego w HTA The place and role of indirect treatment comparison in HTA Przemysław Ryś 9 październik 2018 r. ; Kraków hta. pl

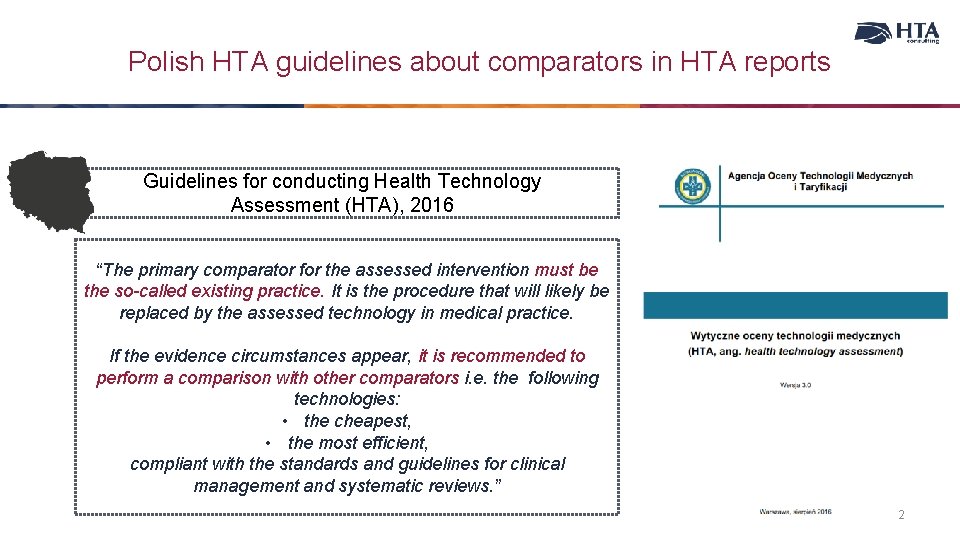
Polish HTA guidelines about comparators in HTA reports Guidelines for conducting Health Technology Assessment (HTA), 2016 “The primary comparator for the assessed intervention must be the so-called existing practice. It is the procedure that will likely be replaced by the assessed technology in medical practice. If the evidence circumstances appear, it is recommended to perform a comparison with other comparators i. e. the following technologies: • the cheapest, • the most efficient, compliant with the standards and guidelines for clinical management and systematic reviews. ” 2

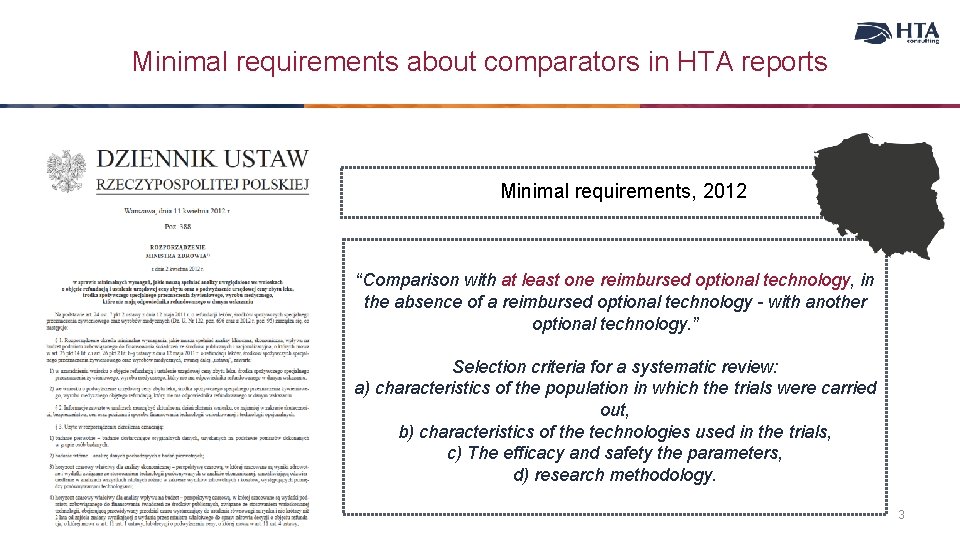
Minimal requirements about comparators in HTA reports Minimal requirements, 2012 “Comparison with at least one reimbursed optional technology, in the absence of a reimbursed optional technology - with another optional technology. ” Selection criteria for a systematic review: a) characteristics of the population in which the trials were carried out, b) characteristics of the technologies used in the trials, c) The efficacy and safety the parameters, d) research methodology. 3

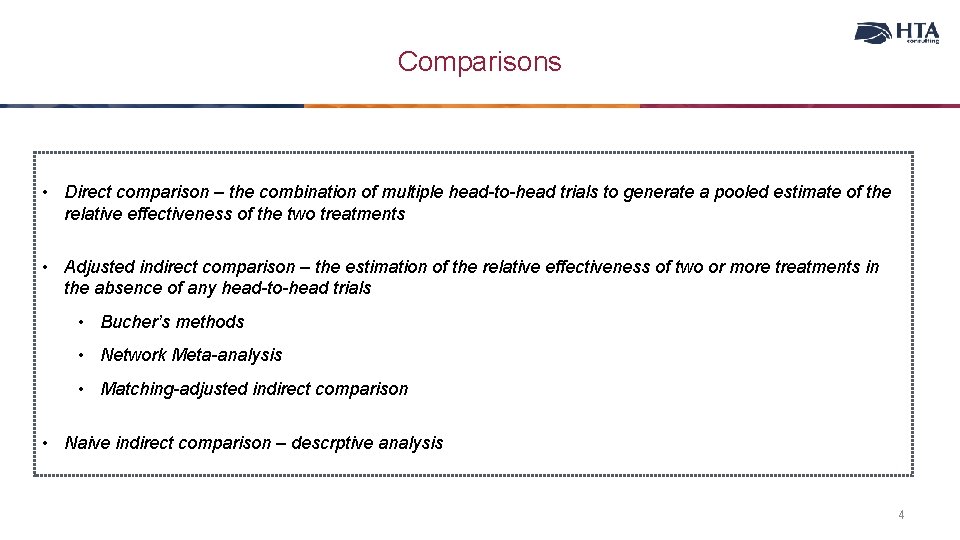
Comparisons • Direct comparison – the combination of multiple head-to-head trials to generate a pooled estimate of the relative effectiveness of the two treatments • Adjusted indirect comparison – the estimation of the relative effectiveness of two or more treatments in the absence of any head-to-head trials • Bucher’s methods • Network Meta-analysis • Matching-adjusted indirect comparison • Naive indirect comparison – descrptive analysis 4

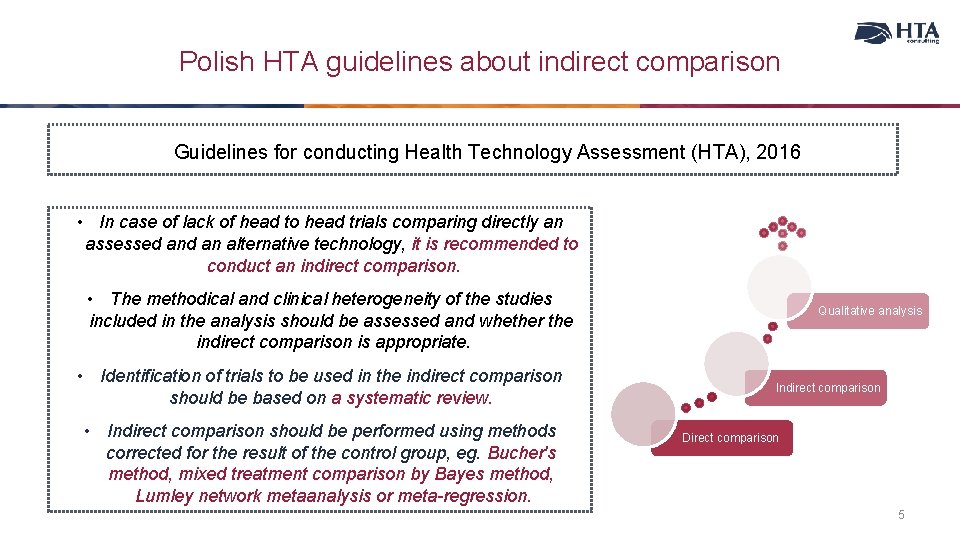
Polish HTA guidelines about indirect comparison Guidelines for conducting Health Technology Assessment (HTA), 2016 • In case of lack of head to head trials comparing directly an assessed an alternative technology, it is recommended to conduct an indirect comparison. • The methodical and clinical heterogeneity of the studies included in the analysis should be assessed and whether the indirect comparison is appropriate. • Identification of trials to be used in the indirect comparison should be based on a systematic review. • Indirect comparison should be performed using methods corrected for the result of the control group, eg. Bucher's method, mixed treatment comparison by Bayes method, Lumley network metaanalysis or meta-regression. Qualitative analysis Indirect comparison Direct comparison 5

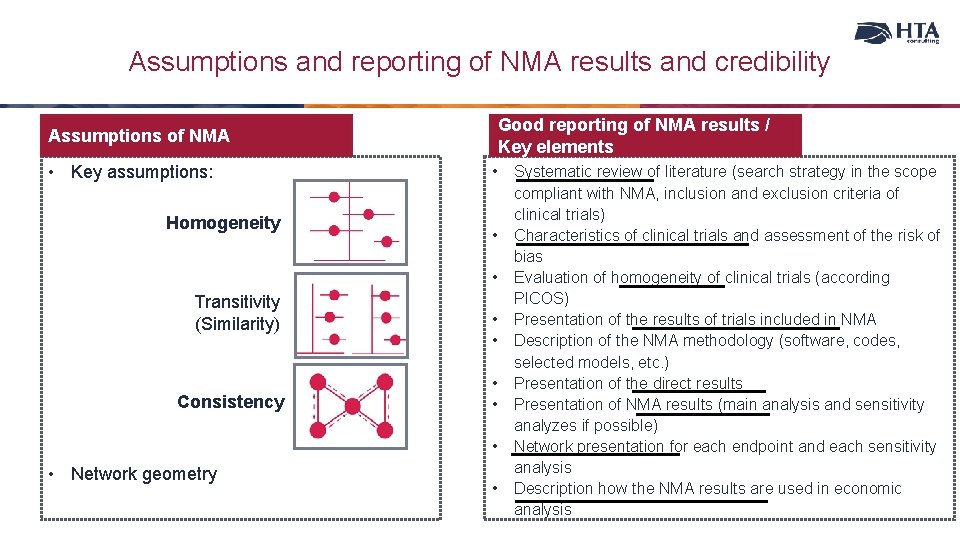
Assumptions and reporting of NMA results and credibility Good reporting of NMA results / Key elements Assumptions of NMA • Key assumptions: Homogeneity • • • Transitivity (Similarity) Consistency • • • Network geometry • Systematic review of literature (search strategy in the scope compliant with NMA, inclusion and exclusion criteria of clinical trials) Characteristics of clinical trials and assessment of the risk of bias Evaluation of homogeneity of clinical trials (according PICOS) Presentation of the results of trials included in NMA Description of the NMA methodology (software, codes, selected models, etc. ) Presentation of the direct results Presentation of NMA results (main analysis and sensitivity analyzes if possible) Network presentation for each endpoint and each sensitivity analysis Description how the NMA results are used in economic analysis

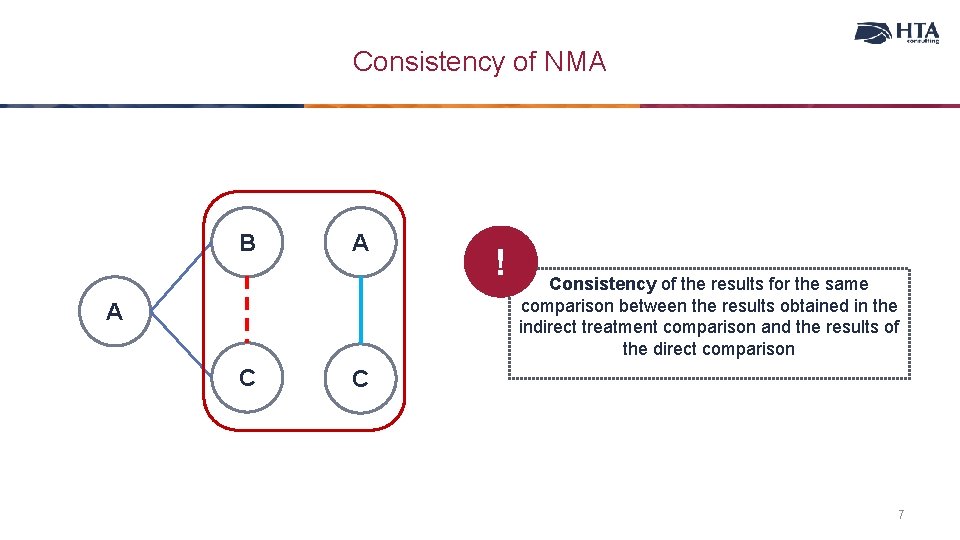
Consistency of NMA B A A C ! Consistency of the results for the same comparison between the results obtained in the indirect treatment comparison and the results of the direct comparison C 7

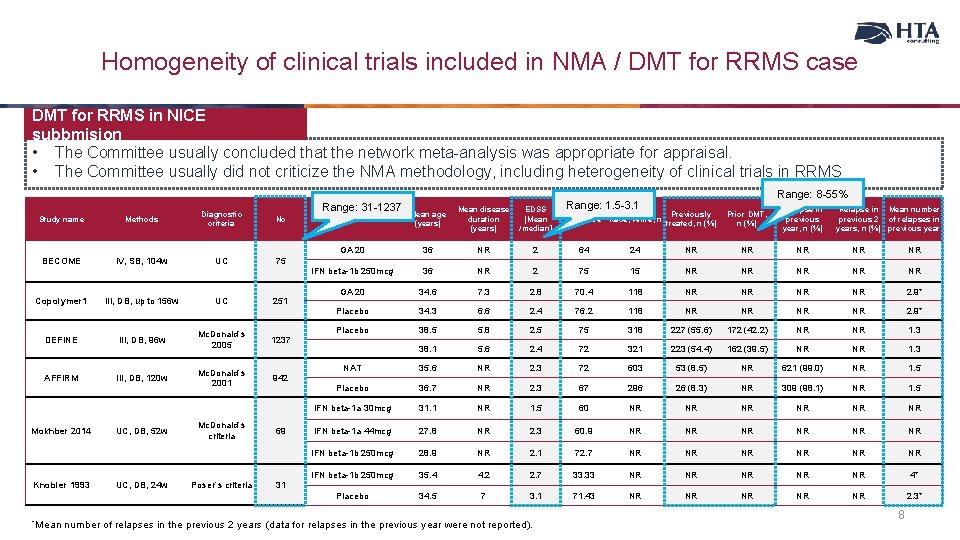
Homogeneity of clinical trials included in NMA / DMT for RRMS case DMT for RRMS in NICE subbmision • The Committee usually concluded that the network meta-analysis was appropriate for appraisal. • The Committee usually did not criticize the NMA methodology, including heterogeneity of clinical trials in RRMS Range: 31 -1237 Study name Methods Diagnostic criteria No BECOME IV, SB, 104 w UC 75 Copolymer 1 III, DB, up to 156 w UC 251 DEFINE III, DB, 96 w Mc. Donald`s 2005 1237 AFFIRM III, DB, 120 w Mc. Donald`s 2001 942 Mokhber 2014 Knobler 1993 *Mean UC, DB, 52 w UC, DB, 24 w Mc. Donald`s criteria Poser`s criteria 69 31 EDSS [Mean /median] Range: 1. 5 -3. 1 Treatment Mean age (years) Mean disease duration (years) GA 20 36 NR 2 64 IFN beta-1 b 250 mcg 36 NR 2 GA 20 34. 6 7. 3 Placebo 34. 3 Placebo Range: 8 -55% Previously treated, n (%) Prior DMT, n (%) Relapse in previous year, n (%) 24 NR NR NR 75 15 NR NR NR 2. 8 70. 4 118 NR NR 2. 9* 6. 6 2. 4 76. 2 118 NR NR 2. 9* 38. 5 5. 8 2. 5 75 318 227 (55. 6) 172 (42. 2) NR NR 1. 3 38. 1 5. 6 2. 4 72 321 223 (54. 4) 162 (39. 5) NR NR 1. 3 NAT 35. 6 NR 2. 3 72 603 53 (8. 5) NR 621 (99. 0) NR 1. 5 Placebo 36. 7 NR 2. 3 67 296 26 (8. 3) NR 309 (98. 1) NR 1. 5 IFN beta-1 a 30 mcg 31. 1 NR 1. 5 60 NR NR NR IFN beta-1 a 44 mcg 27. 8 NR 2. 3 60. 9 NR NR NR IFN beta-1 b 250 mcg 28. 9 NR 2. 1 72. 7 NR NR NR IFN beta-1 b 250 mcg 35. 4 4. 2 2. 7 33. 33 NR NR NR 4* Placebo 34. 5 7 3. 1 71. 43 NR NR NR 2. 3* number of relapses in the previous 2 years (data for relapses in the previous year were not reported). % female Race, white, n Relapse in Mean number previous 2 of relapses in years, n (%) previous year 8

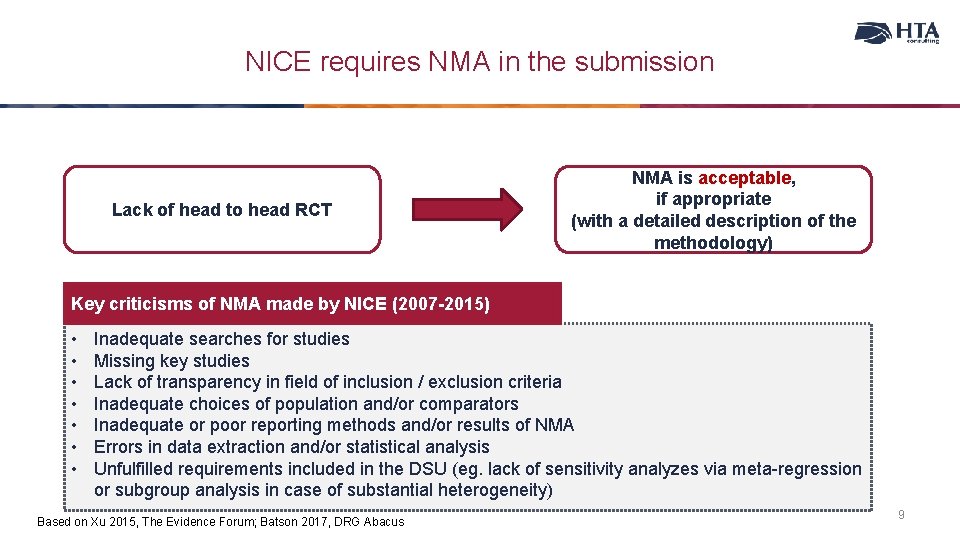
NICE requires NMA in the submission Lack of head to head RCT NMA is acceptable, if appropriate (with a detailed description of the methodology) Key criticisms of NMA made by NICE (2007 -2015) • • Inadequate searches for studies Missing key studies Lack of transparency in field of inclusion / exclusion criteria Inadequate choices of population and/or comparators Inadequate or poor reporting methods and/or results of NMA Errors in data extraction and/or statistical analysis Unfulfilled requirements included in the DSU (eg. lack of sensitivity analyzes via meta-regression or subgroup analysis in case of substantial heterogeneity) Based on Xu 2015, The Evidence Forum; Batson 2017, DRG Abacus 9

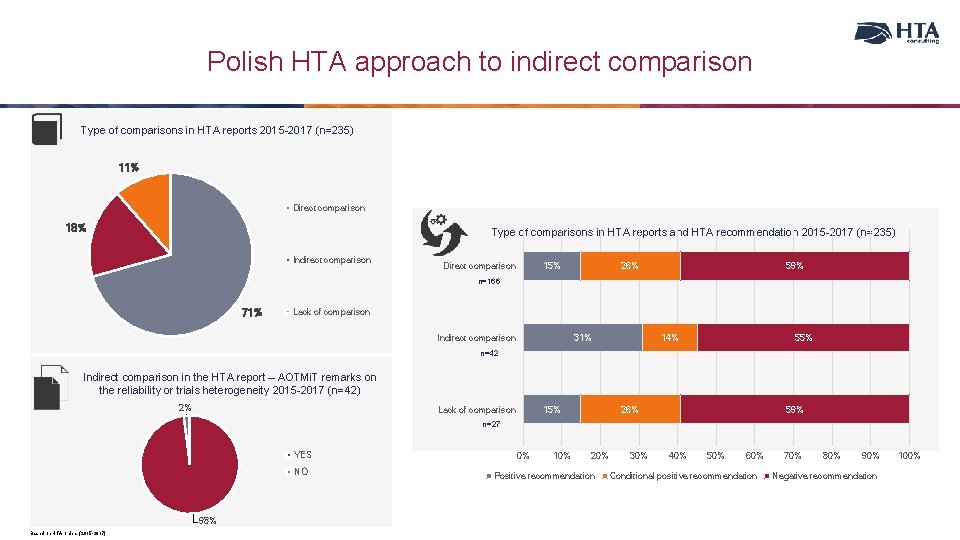
Polish HTA approach to indirect comparison Type of comparisons in HTA reports 2015 -2017 (n=235) 11% Direct comparison 18% Type of comparisons in HTA reports and HTA recommendation 2015 -2017 (n=235) Indirect comparison 15% Direct comparison 26% 59% n=166 71% Lack of comparison Indirect comparison 31% 14% 55% n=42 Indirect comparison in the HTA report – AOTMi. T remarks on the reliability or trials heterogeneity 2015 -2017 (n=42) 2% Lack of comparison 15% 26% 59% n=27 YES NO 98% Based on HTA orders (2015 -2017) 0% 10% 20% Positive recommendation 30% 40% 50% 60% Conditional positive recommendation 70% 80% 90% Negative recommendation 100%

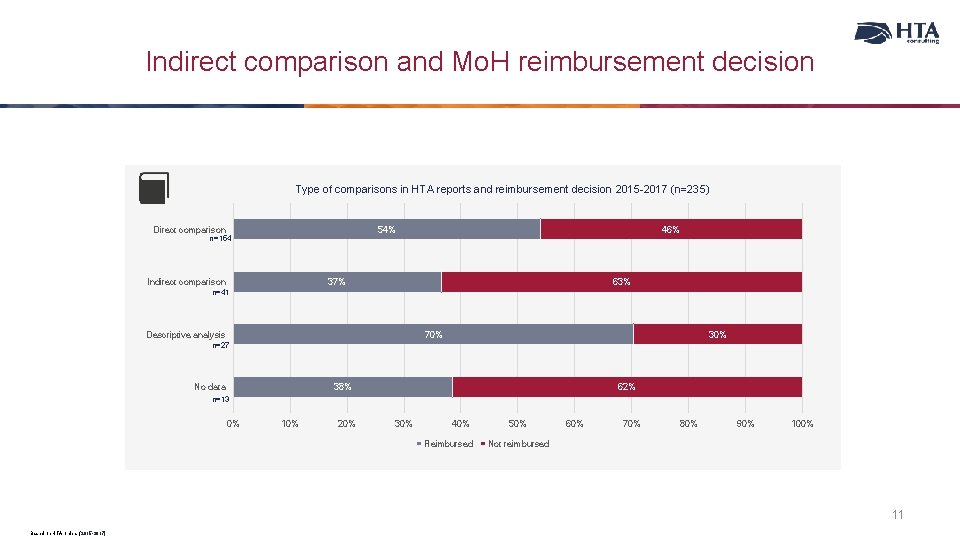
Indirect comparison and Mo. H reimbursement decision Type of comparisons in HTA reports and reimbursement decision 2015 -2017 (n=235) Direct comparison 54% n=154 Indirect comparison 46% 37% 63% n=41 Descriptive analysis 70% 30% n=27 No data 38% 62% n=13 0% 10% 20% 30% 40% Reimbursed 50% 60% 70% 80% 90% 100% Not reimbursed 11 Based on HTA orders (2015 -2017)

Matching-adjusted indirect comparison What is the MAIC method? • The MAIC method is a new tool for comparative effectiveness, relies on the use of individual patient data (patient-level data) from trial(s) for the assessed intervention and adjusting it to the data for the comparator (usually aggregated). • The MAIC method relies on minimizing the differences between studies in the field of: differences in the initial patients’ characteristics, differences in the definition of endpoints, differences in the dosage range, etc. Advantages of the MAIC method • It allows estimation of the relative effect of therapeutic options also based on studies for which there is no common reference group or only single arm studies are available. Limitations of the MAIC method • It is not possible when significant discrepancies (impossible to remove) between trials, eg in relation to the design of the study or the patients’ initial characteristics

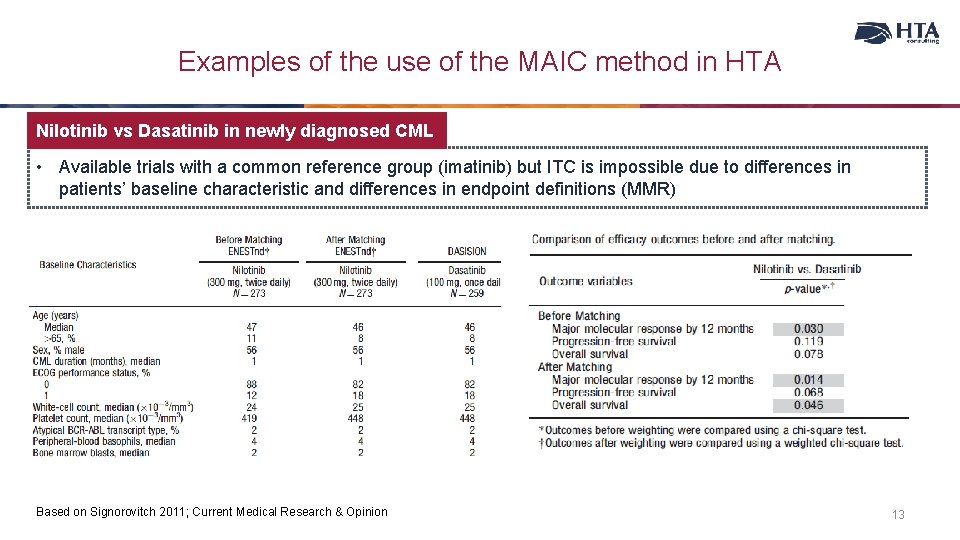
Examples of the use of the MAIC method in HTA Nilotinib vs Dasatinib in newly diagnosed CML • Available trials with a common reference group (imatinib) but ITC is impossible due to differences in patients’ baseline characteristic and differences in endpoint definitions (MMR) Based on Signorovitch 2011; Current Medical Research & Opinion 13

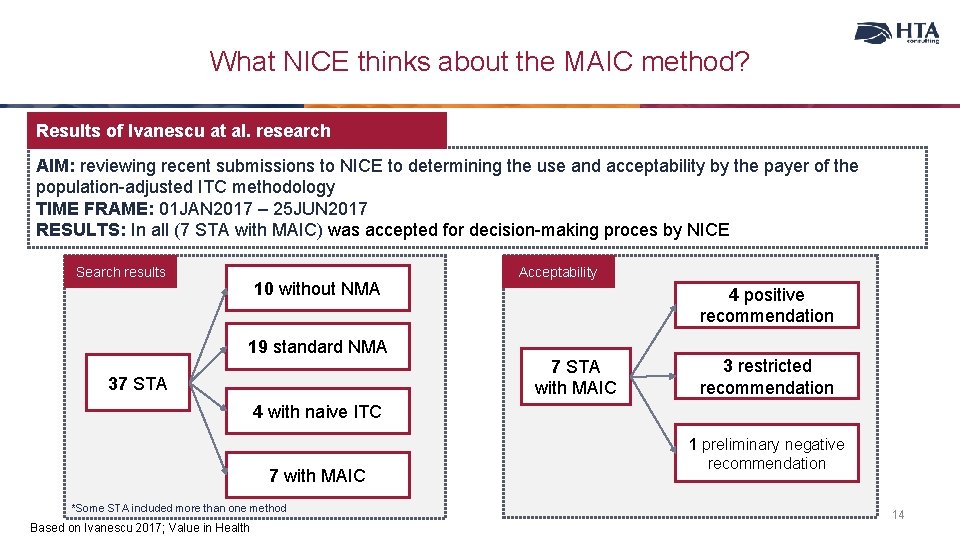
What NICE thinks about the MAIC method? Results of Ivanescu at al. research AIM: reviewing recent submissions to NICE to determining the use and acceptability by the payer of the population-adjusted ITC methodology TIME FRAME: 01 JAN 2017 – 25 JUN 2017 RESULTS: In all (7 STA with MAIC) was accepted for decision-making proces by NICE Search results 10 without NMA 19 standard NMA 37 STA Acceptability 4 positive recommendation 7 STA with MAIC 3 restricted recommendation 4 with naive ITC 7 with MAIC *Some STA included more than one method Based on Ivanescu 2017; Value in Health 1 preliminary negative recommendation 14

Kontakt HTA Consulting ul. Starowislna 17/3 31 -038 Krakow, POLAND e-mail: office@hta. pl Phone mobile fax (+48) 12 421 88 32 (+48) 12 421 88 33 (+48) 508 180 859 (+48) 12 395 38 32 15
 Pogoda oymyakon
Pogoda oymyakon Jezioro lśniących wód co to było
Jezioro lśniących wód co to było Skodowska
Skodowska Czas i miejsce powstania ewangelii mateusza
Czas i miejsce powstania ewangelii mateusza Miejsce kultu islamu
Miejsce kultu islamu Jakim wierszem napisany jest pan tadeusz
Jakim wierszem napisany jest pan tadeusz Kamienie na szaniec czas i miejsce akcji
Kamienie na szaniec czas i miejsce akcji Ocena pinokia
Ocena pinokia Miejsce zerowe funkci
Miejsce zerowe funkci święte miejsce buddyzmu
święte miejsce buddyzmu Jan iii sobieski data i miejsce urodzenia
Jan iii sobieski data i miejsce urodzenia Oznakowanie regałów magazynowych
Oznakowanie regałów magazynowych Monotoniczność funkcji
Monotoniczność funkcji Sylwia grzeszczak data i miejsce urodzenia
Sylwia grzeszczak data i miejsce urodzenia Fryderyk chopin data urodzenia
Fryderyk chopin data urodzenia Laikat i jego rola w kościele
Laikat i jego rola w kościele