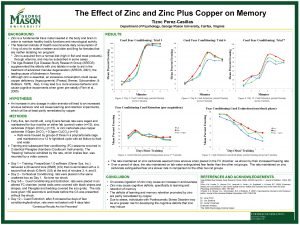
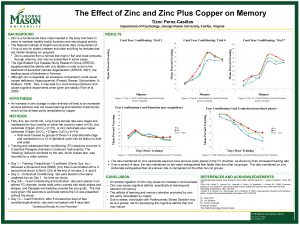
MetalContaining Pesticides Metals Essential Zinc Copper Iron Chromium

















- Slides: 24

Metal-Containing Pesticides

Metals • Essential* – Zinc – Copper – Iron – Chromium – Phosphorus – Selenium – Cobalt • *All metals are toxic in excess. • Neutral or detrimental – Tin – Bismuth – Boron – Barium – Antimony – *Arsenic – *Thallium – *Lead – *Mercury • * Designates invariably harmful metals

General Considerations • • • Elements do not degrade Environmental accumulation is a significant risk of continuing use – As in soil of old orchards? Bioaccumulation may occur if metal is in a form that can be taken up by plants – Most metals are excluded by most plants – Cadmium is taken up by some plants Environmental presence in soil implies risk of transport into water Movement from soil to air, air to soil also occurs Environmental transformations may occur – Hg ---> Me. Hg • Toxicology – For most metal-containing pesticides, the toxicity is due to the metal – Example: • As-containing herbicide, fungicide will have similar toxicities despite very different structures – Exception: • Dithiocarbamate fungicides

Barium • Barium carbonate: Ba. CO 3 – Rodenticide • 20 -25% active ingredient in baits • Neurotoxicant – Human poisoning? • 1 case of mass poisoning • Substituted for flour in pastry

Chromium • Sodium dichromate: Na 2 Cr 2 O 7 • 2 H 2 O – Cotton defoliant – Wood preservative • Toxicity: – Bronchiogenic cancer suspected – May require inhalation • Chromium is also an essential element

Copper • Copper sulfate: Cu. SO 4 – Also known as blue vitriol – Fungicide, algicide • Bordeaux mixture: Cu. SO 4 and Ca(OH)2 – Fungicide and seed treatment • Very alkaline • Toxicology – Acute: • Renal failure – Chronic: • Fatty degeneration of kidneys, liver • Pneumoconiosis from occupational exposure to Bordeaux mixture • Granulomas of lung, liver , kidney

Zinc • Zinc chloride: Zn. Cl 2 – Wood preservative – LD 50 : • po, rat: 2, 200 to 19, 000 mg/kg • Ip, mouse: 31 mg/kg • Zinc compounds tend to be emetic • : Zinc phosphide: Zn 3 P 2 – Rodenticide – Toxicity is due to production of phosphine (PH 3) by stomach acid – Phosphine causes pulmonary and cerebral edema

Cadmium • • • No known biological function Cadmium chloride: Cd. Cl 2 – Turf fungicide Toxicity – Acute • Salivation, diarrhea, vomiting – Chronic • Poor growth, anemia, kidney damage • Itai-itai from dietary exposure – May be aggravated by Ca and/or vitamin D deficiencies • High blood pressure? • Accumulates in kidneys – Human poisoning with Cd. Cl 2 has not been reported

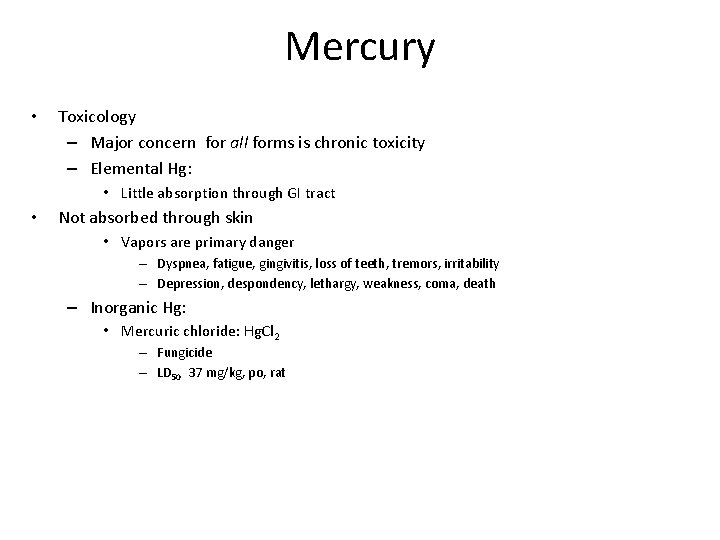
Mercury • Toxicology – Major concern for all forms is chronic toxicity – Elemental Hg: • Little absorption through GI tract • Not absorbed through skin • Vapors are primary danger – Dyspnea, fatigue, gingivitis, loss of teeth, tremors, irritability – Depression, despondency, lethargy, weakness, coma, death – Inorganic Hg: • Mercuric chloride: Hg. Cl 2 – Fungicide – LD 50 37 mg/kg, po, rat

Organic Mercury Compounds • 1915: – Phenylmercury salts • Hg. C 6 H 6 • 1929: – Alkylmercury salts • Methylmercury, Hg. CH 3 • LD 50 ca 30 mg/kg • NOAEL = 0. 5 mg/kg/day for 150 days • 1960 s-1970 s – Hg-containing pesticides banned by most countries

Organic Mercury Compounds • Toxicology – Absorbed through skin, GI tract – Crosses blood/brain barrier – Little excretion from CNS – Neurological damage is 1 st symptom • • CNS Tremors, loss of peripheral vision, irritability progressing to dementia Some improvement if exposure ends Most damage is permanent – Pregnant women excrete Hg to fetus • Congenital Minamata disease • No repair possible • Arylmercury compounds – Acrodynia or “pink disease”

Thallium • Rodenticide – Home uses banned in US in 1975 • Toxicology – Crosses skin, GI tract – Mechanism of action not well understood • • Interferes with intracellular K? Thallium sulfate, Tl 2 SO 4 LD 50 10. 6 mg/kg, rat po 1/50 of LD 50 per day: – Depilation in 4 months – Death in 6 months

• Arsenic Insecticides – Paris Green • Copper acetoarsenite – Cu(C 2 H 3 OO)2 • 3 Cu(As. O 2) – used against Colorado potato beetle, 1865 – Scheele’s Green • Copper arsenite, Cu. HAs. O – Na. As. O 2 • still used in poison bait against grasshoppers – Pb. HAs. O 4 • used on apples against codling moths • Used against gypsy moth • 38, 000 kg in 1942 • LD 50 in rats, po: 800 mg/kg • Herbicide – Cacodylic acid: (CH 3)2 As. O(OH) • LD 50 in rats, po: 830 mg/kg • Fungicide – Chromated copper arsenate • wood treatment – introduced 1940 s – phased out 2003 • 12 x 2 x 6” board contains 27 g arsenic • 5 Tbs ash contains enough As to kill a 1, 100 lb cow • tastes salty

Human Toxicity of Arsenic • • • Acute toxicity – GI tract symptoms Cumulative toxicity (rats) – 1. 6 mg/kg/day is NOAEL of arsenites – 3. 2 mg/kg/day is NOAEL of arsenates Mechanism – Increases permeability of capillaries – Decreases blood pressure ---> shock, death • • • Skin lesions – Also used therapeutically to clear skin Polyneuropathy Cancer – Skin • Following ingestion, not topical application – Lung • Following inhalation only? • Evidence is epidemiological – People living downwind from copper smelters

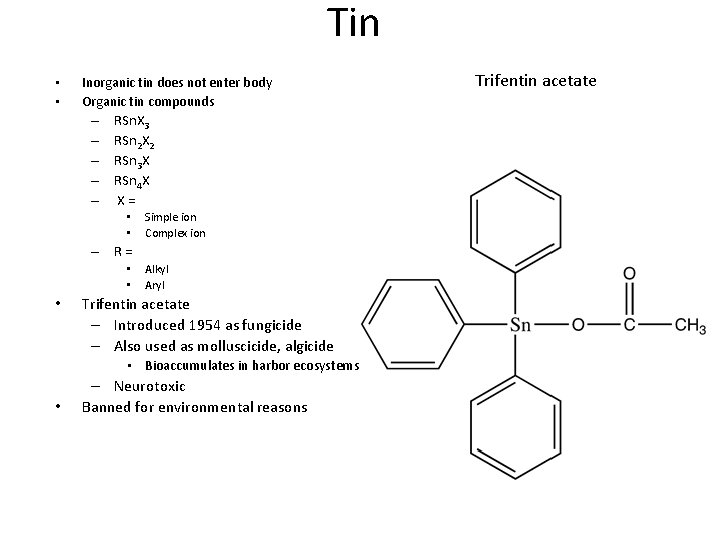
Tin • • Inorganic tin does not enter body Organic tin compounds – – – RSn. X 3 RSn 2 X 2 RSn 3 X RSn 4 X X= • • Simple ion Complex ion – R= • • • Alkyl Aryl Trifentin acetate – Introduced 1954 as fungicide – Also used as molluscicide, algicide • Bioaccumulates in harbor ecosystems • – Neurotoxic Banned for environmental reasons Trifentin acetate

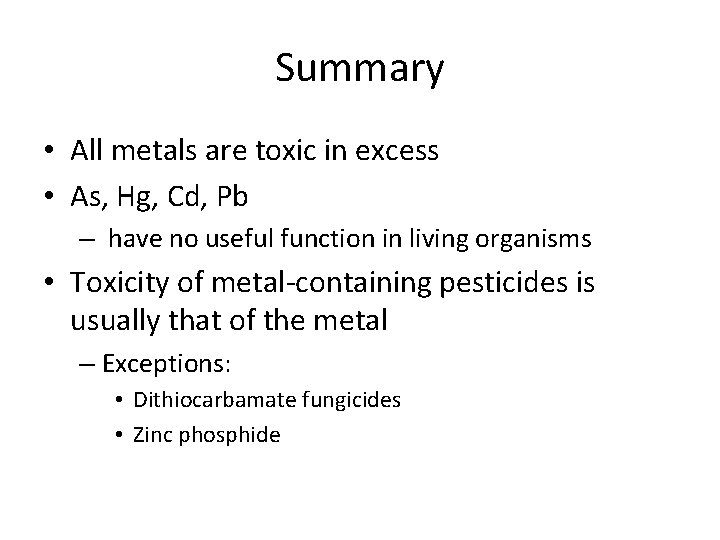
Summary • All metals are toxic in excess • As, Hg, Cd, Pb – have no useful function in living organisms • Toxicity of metal-containing pesticides is usually that of the metal – Exceptions: • Dithiocarbamate fungicides • Zinc phosphide

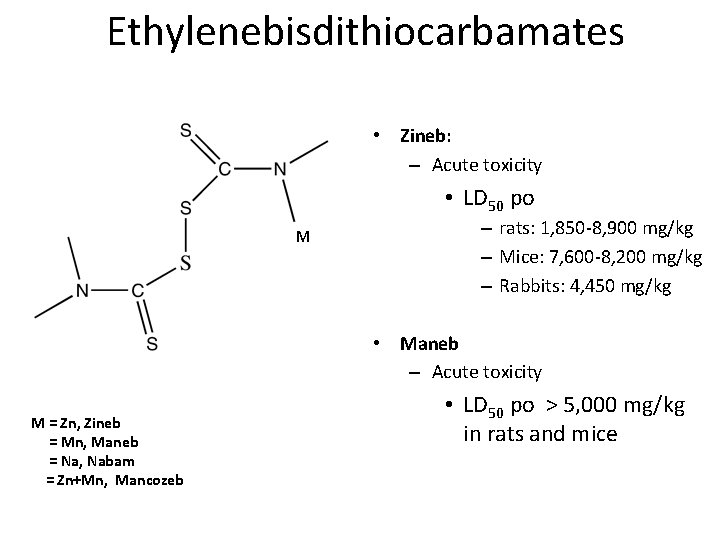
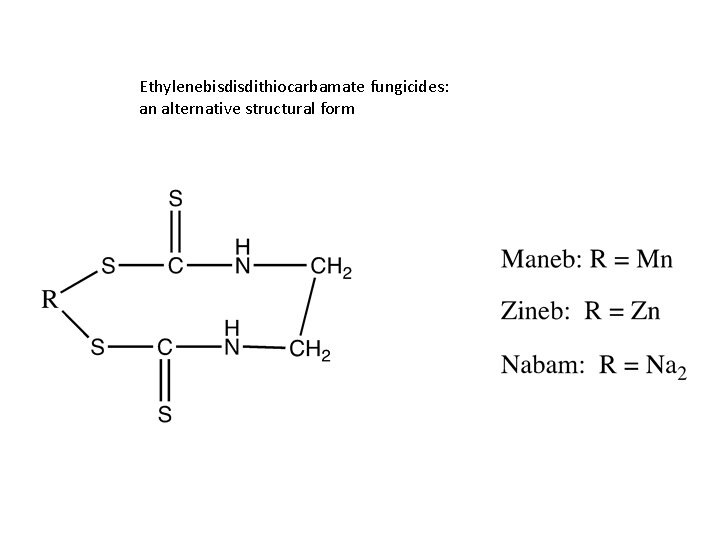
Dithiocarbamate Fungicides • Ethylenebisdithiocarbamates – Maneb – Nabam – Zineb • Dimethyldithiocarbamates – Ferbam – Ziram

Ethylenebisdithiocarbamates • Zineb: – Acute toxicity • LD 50 po M – rats: 1, 850 -8, 900 mg/kg – Mice: 7, 600 -8, 200 mg/kg – Rabbits: 4, 450 mg/kg • Maneb – Acute toxicity M = Zn, Zineb = Mn, Maneb = Na, Nabam = Zn+Mn, Mancozeb • LD 50 po > 5, 000 mg/kg in rats and mice

Ethylenebisdisdithiocarbamate fungicides: an alternative structural form

Dimethyldithiocarbamates – Ziram • LD 50 po – rats: 1400 mg/kg – Mice: 480 mg/kg – Guinea pigs: 100 -150 mg/kg

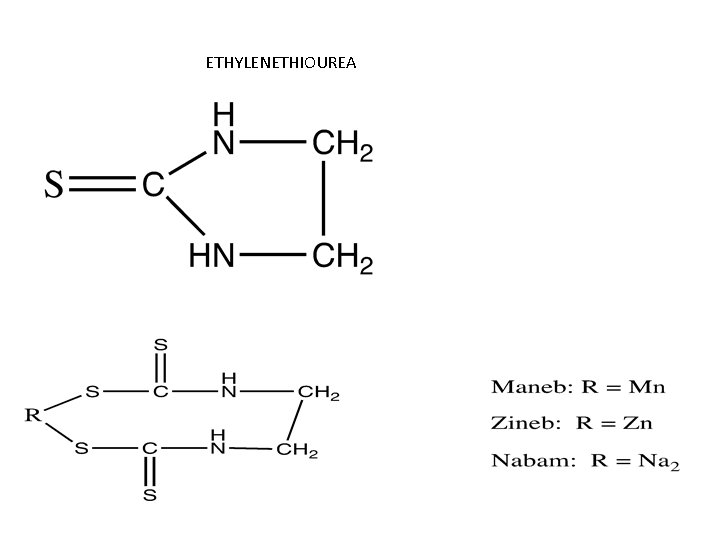
Common Features of Dithiocarbamates • Acute toxicity is low • Depress thyroid function • Common metabolite: – Ethylenethiourea • Interact with alcohol consumption – Blocks oxidation of Et. OH at acetaldehyde step – Acetaldehyde accumulates – Disulfiram (Antabuse) • Used to prevent Et. OH consumption by alcoholics Disulfiram

ETHYLENETHIOUREA

Developmental toxicity of ethylenethiourea • Severe developmental toxicity in rats – Craniofacial malformations • Little activity in mice • Similar pharmacokinetics in both species

Summary • Toxicity of most metal-containing pesticides depends on the metal • Toxicity of dithiocarbamates does not depend on the identity of the metal • Dithiocarbamates are – Thyrotoxic – Suspect carcinogens – Their metabolite, ethylenethiourea, • Is a developmental toxic in rats but not in mice • Many dithiocarbamates were voluntarily withdrawn by their manufacturers rather than carrying out currently required safety testing
 Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Example of a chemical change
Example of a chemical change D orbital shape
D orbital shape Magnesium and copper oxide
Magnesium and copper oxide Natural science grade 7 notes
Natural science grade 7 notes Nonmetals on the periodic table
Nonmetals on the periodic table Properties of materials grade 7
Properties of materials grade 7 Ferrous metals
Ferrous metals Example of non metal
Example of non metal Metal and non metal
Metal and non metal Iron sharpens iron friendship
Iron sharpens iron friendship Mass of iron in an iron tablet
Mass of iron in an iron tablet Essential non essential fatty acids
Essential non essential fatty acids Soybean aphid pesticides
Soybean aphid pesticides Classification of insecticides
Classification of insecticides Different types of pesticides
Different types of pesticides How pesticides work
How pesticides work Integrated pest management ____ than pesticides
Integrated pest management ____ than pesticides Example of pesticides
Example of pesticides Maine board of pesticides control
Maine board of pesticides control Agriscience unit 14 completion answers
Agriscience unit 14 completion answers Cannabis pesticides
Cannabis pesticides Bio pesticides example
Bio pesticides example Pesticide treadmill definition
Pesticide treadmill definition The pros and cons of pesticides
The pros and cons of pesticides