Ferrous Metals NonFerrous Metals Iron is a chemical



- Slides: 11

Ferrous Metals & Non-Ferrous Metals Iron is a chemical element with the symbol Fe (from Latin: ferrum) and atomic number 26 and atomic weight 56. It is a metal in the first transition metal series Ferrous metals are metals that contain iron E. g. Steel (iron and carbon) Non-ferrous metals are metals that do not contain iron E. g. Zinc (pure metal), Bronze (Copper and tin) (non-ferrous may contain slight traces of iron) Ferrous Metal = alloy metals that contains iron ( Primary base metal is iron) Non-ferrous Metal = alloy metals that do not contain iron Primary base metal does not contain iron)

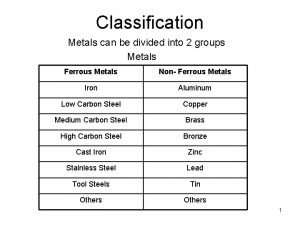
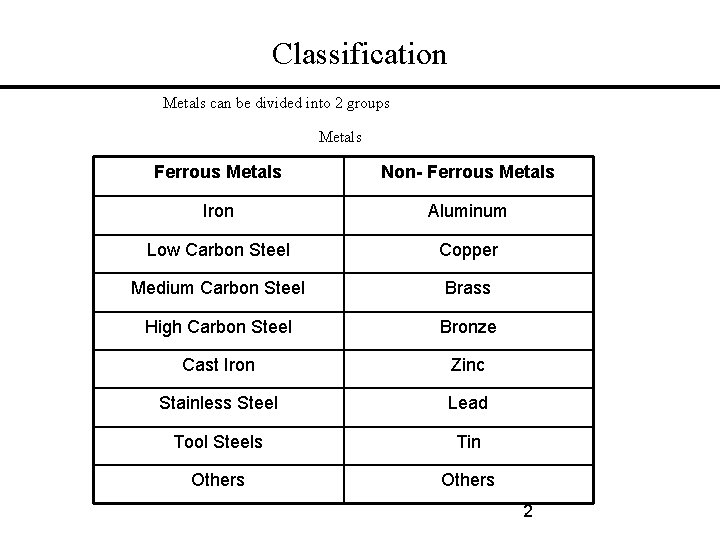
Classification Metals can be divided into 2 groups Metals Ferrous Metals Non- Ferrous Metals Iron Aluminum Low Carbon Steel Copper Medium Carbon Steel Brass High Carbon Steel Bronze Cast Iron Zinc Stainless Steel Lead Tool Steels Tin Others 2

Iron as inorganic metal carrier • Iron is the most abundant transition metal ion in biological systems--almost all organisms use it – Availability: » Most abundant transition metal on the Earth’s crust » Nuclear Binding Energy is maximized at 56 Fe – Versatility: » Fe 2+/Fe 3+ » High Spin/Low Spin » Hard/Soft » Labile/Inert » Coordination Number: 4, 5, 6

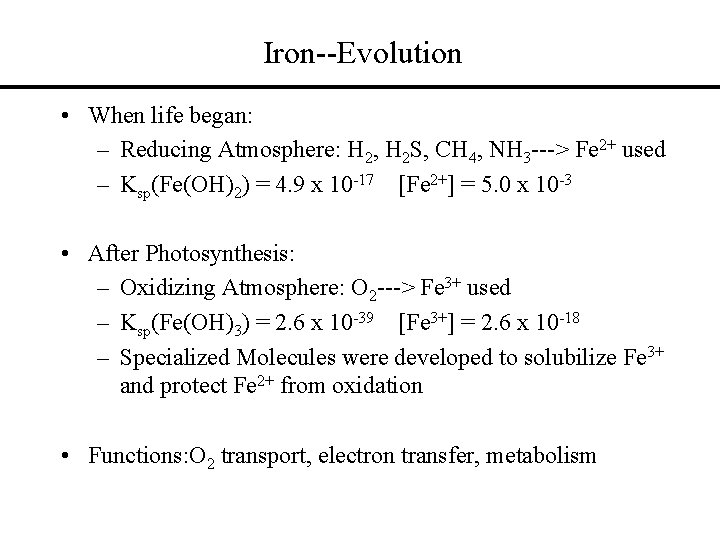
Iron--Evolution • When life began: – Reducing Atmosphere: H 2, H 2 S, CH 4, NH 3 ---> Fe 2+ used – Ksp(Fe(OH)2) = 4. 9 x 10 -17 [Fe 2+] = 5. 0 x 10 -3 • After Photosynthesis: – Oxidizing Atmosphere: O 2 ---> Fe 3+ used – Ksp(Fe(OH)3) = 2. 6 x 10 -39 [Fe 3+] = 2. 6 x 10 -18 – Specialized Molecules were developed to solubilize Fe 3+ and protect Fe 2+ from oxidation • Functions: O 2 transport, electron transfer, metabolism

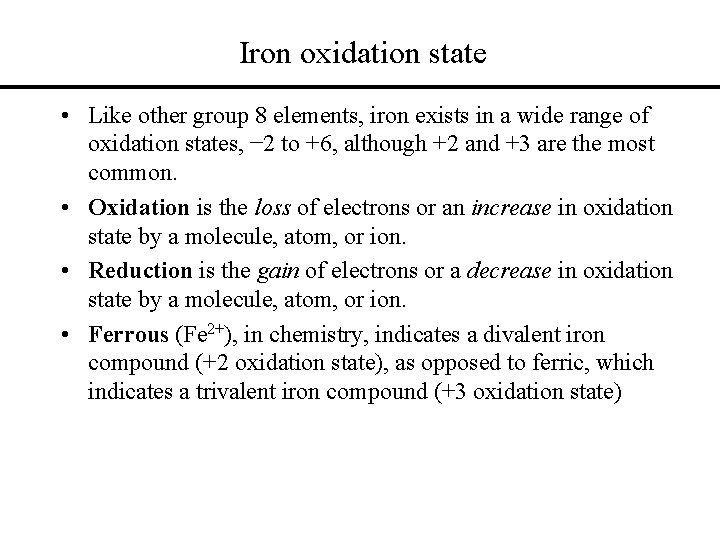
Iron oxidation state • Like other group 8 elements, iron exists in a wide range of oxidation states, − 2 to +6, although +2 and +3 are the most common. • Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. • Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion. • Ferrous (Fe 2+), in chemistry, indicates a divalent iron compound (+2 oxidation state), as opposed to ferric, which indicates a trivalent iron compound (+3 oxidation state)

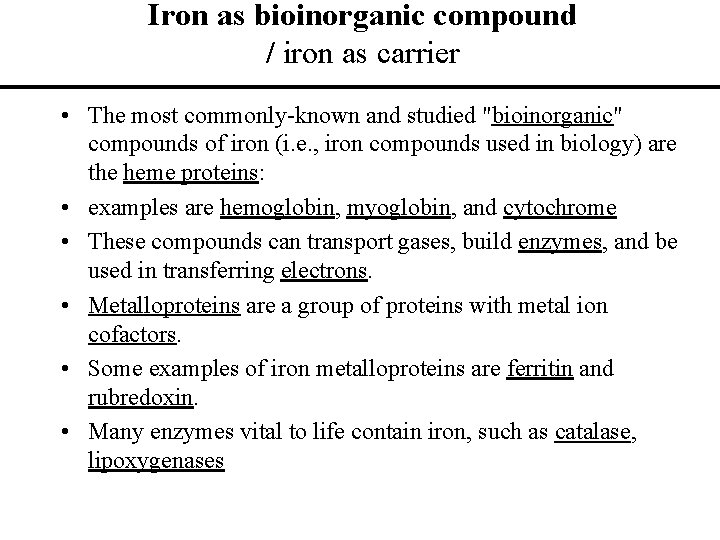
Iron as bioinorganic compound / iron as carrier • The most commonly-known and studied "bioinorganic" compounds of iron (i. e. , iron compounds used in biology) are the heme proteins: • examples are hemoglobin, myoglobin, and cytochrome • These compounds can transport gases, build enzymes, and be used in transferring electrons. • Metalloproteins are a group of proteins with metal ion cofactors. • Some examples of iron metalloproteins are ferritin and rubredoxin. • Many enzymes vital to life contain iron, such as catalase, lipoxygenases

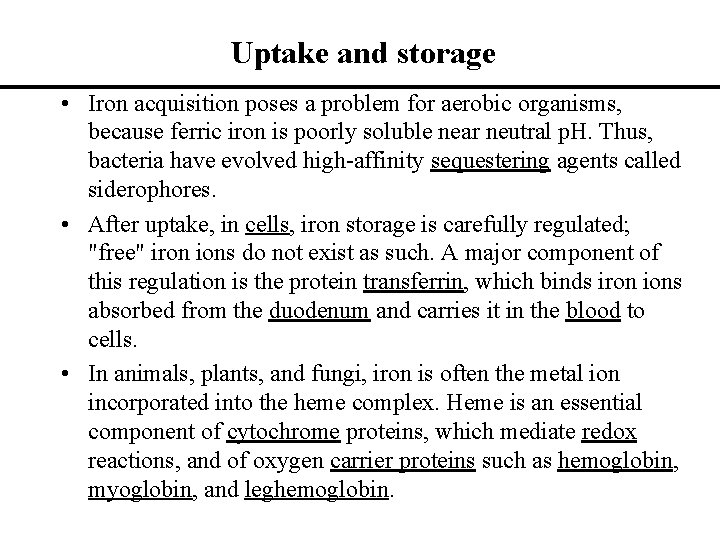
Uptake and storage • Iron acquisition poses a problem for aerobic organisms, because ferric iron is poorly soluble near neutral p. H. Thus, bacteria have evolved high-affinity sequestering agents called siderophores. • After uptake, in cells, iron storage is carefully regulated; "free" iron ions do not exist as such. A major component of this regulation is the protein transferrin, which binds iron ions absorbed from the duodenum and carries it in the blood to cells. • In animals, plants, and fungi, iron is often the metal ion incorporated into the heme complex. Heme is an essential component of cytochrome proteins, which mediate redox reactions, and of oxygen carrier proteins such as hemoglobin, myoglobin, and leghemoglobin.

Iron and Redox reactions • Inorganic iron contributes to redox reactions in the iron-sulfur clusters of many enzymes, such as nitrogenase (involved in the synthesis of ammonia from nitrogen and hydrogen) and hydrogenase. • Non-heme iron proteins include the enzymes • methane monooxygenase (oxidizes methane to methanol), ribonucleotide reductase (reduces ribose to deoxyribose; DNA biosynthesis), • Hemerythrins (oxygen transport and fixation in marine invertebrates) and • purple acid phosphatase (hydrolysis of phosphate esters). • Iron distribution is heavily regulated in mammals, partly because iron ions have a high potential for biological toxicity.

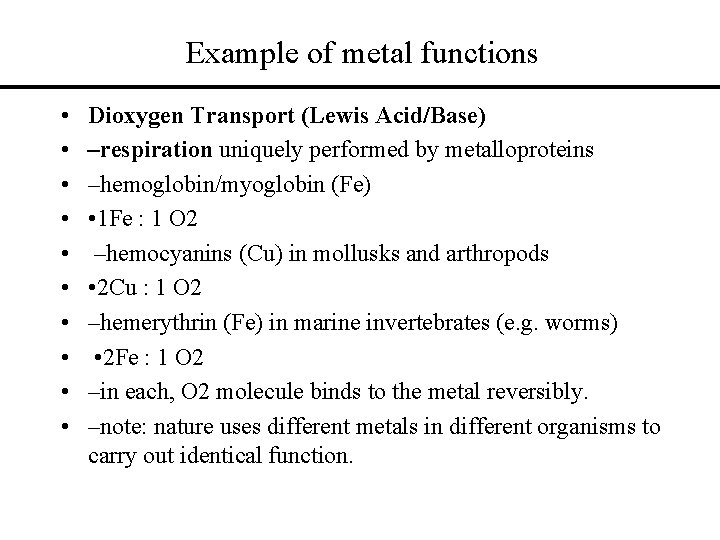
Example of metal functions • • • Dioxygen Transport (Lewis Acid/Base) –respiration uniquely performed by metalloproteins –hemoglobin/myoglobin (Fe) • 1 Fe : 1 O 2 –hemocyanins (Cu) in mollusks and arthropods • 2 Cu : 1 O 2 –hemerythrin (Fe) in marine invertebrates (e. g. worms) • 2 Fe : 1 O 2 –in each, O 2 molecule binds to the metal reversibly. –note: nature uses different metals in different organisms to carry out identical function.

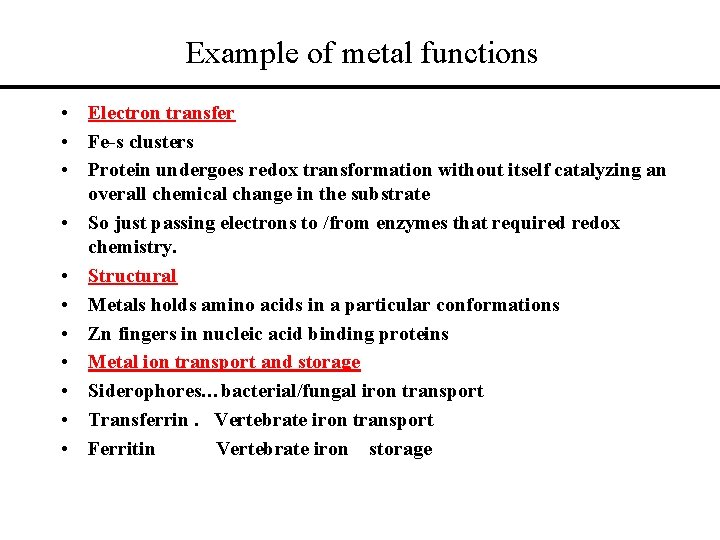
Example of metal functions • Electron transfer • Fe-s clusters • Protein undergoes redox transformation without itself catalyzing an overall chemical change in the substrate • So just passing electrons to /from enzymes that required redox chemistry. • Structural • Metals holds amino acids in a particular conformations • Zn fingers in nucleic acid binding proteins • Metal ion transport and storage • Siderophores…bacterial/fungal iron transport • Transferrin. Vertebrate iron transport • Ferritin Vertebrate iron storage

Examples of metal functions • • Metalloenzymes – hydrolytic enzymes • catalyze addition or removal of water in a substrate e. g. carbonic anhydrase catalyzes CO 2 + H 2 O → H 2 CO 3 –redox enzymes • catalyze oxidation/reduction of substrate • e. g. oxidation of hydrocarbons to alcohol by cyt-P 450 (Feporphyin) • • e. g. nitrogenase: reduces N 2 to NH 3 (6 electron transformation) • –rearrangements • • biological transformation without change in oxidation state of the substrate. • metal acts as template
 Ferrous vs non ferrous
Ferrous vs non ferrous Categories of metal
Categories of metal 5 non ferrous metals
5 non ferrous metals Classification of metal
Classification of metal 5 ferrous metals
5 ferrous metals Microstructure of ferrous metals
Microstructure of ferrous metals Mass of iron in an iron tablet
Mass of iron in an iron tablet Iron sharpens iron friendship
Iron sharpens iron friendship What is the percent yield of ferrous sulfide
What is the percent yield of ferrous sulfide Ferrous scrap
Ferrous scrap Physical properties of ferrous sulphate
Physical properties of ferrous sulphate Ferrous material
Ferrous material