Maryland Situation Update on Coronavirus Disease COVID19 for








- Slides: 29

Maryland Situation Update on Coronavirus Disease (COVID-19) for BHA Maryland Department of Health Infectious Disease Epidemiology and Outbreak Response Bureau July 24, 2020

Call Agenda Picture Courtesy of NIAID-RML • Review current situation of COVID-19 • Infection Control Updates • Droplet and Airborne disease • New Testing Technology • Questions and Answers 2

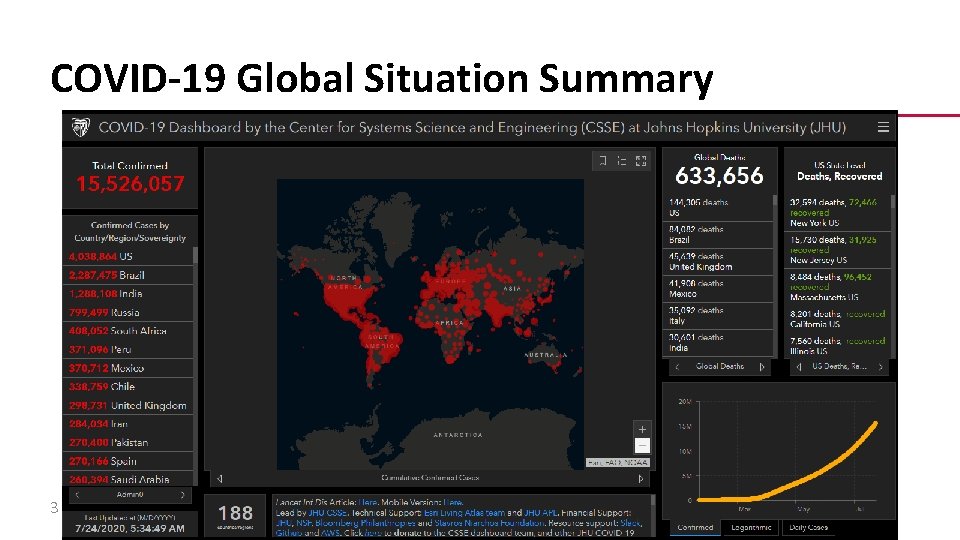
COVID-19 Global Situation Summary 3

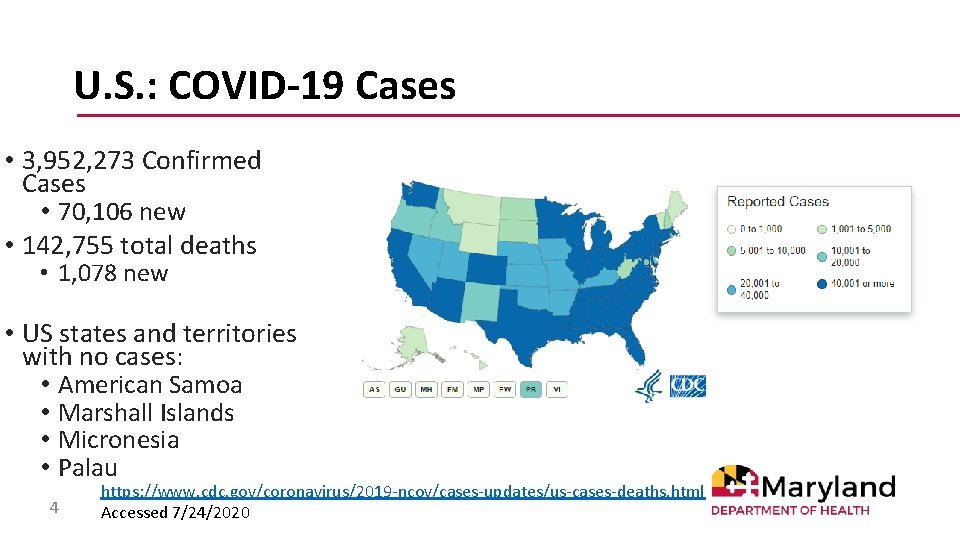
U. S. : COVID-19 Cases • 3, 952, 273 Confirmed Cases • 70, 106 new • 142, 755 total deaths • 1, 078 new • US states and territories with no cases: • American Samoa • Marshall Islands • Micronesia • Palau 4 https: //www. cdc. gov/coronavirus/2019 -ncov/cases-updates/us-cases-deaths. html Accessed 7/24/2020

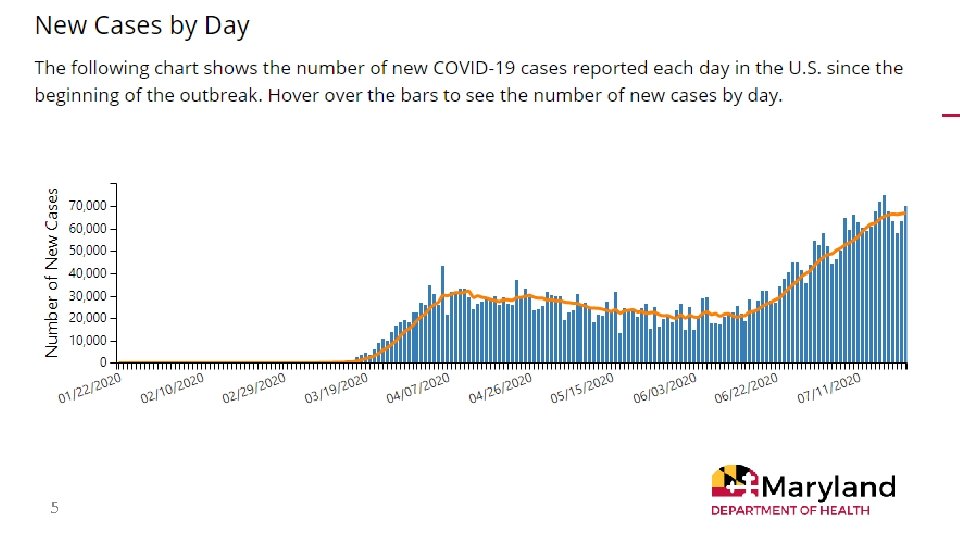
5

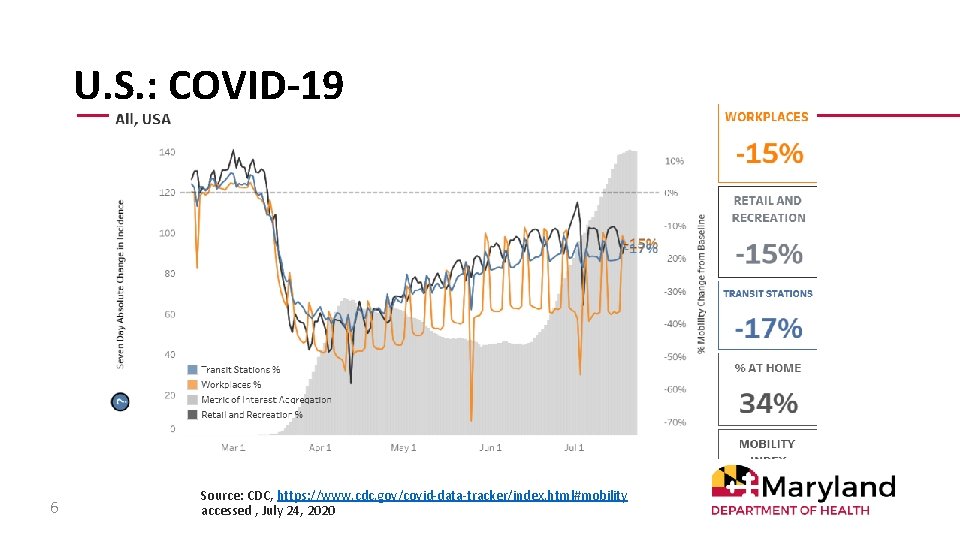
U. S. : COVID-19 Mobility 6 Source: CDC, https: //www. cdc. gov/covid-data-tracker/index. html#mobility accessed , July 24, 2020

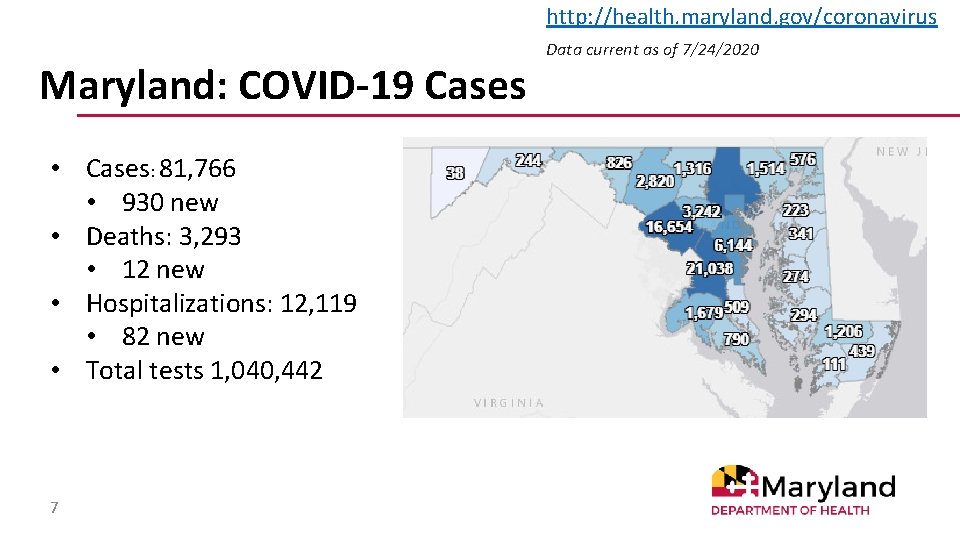
http: //health. maryland. gov/coronavirus Maryland: COVID-19 Cases • Cases: 81, 766 • 930 new • Deaths: 3, 293 • 12 new • Hospitalizations: 12, 119 • 82 new • Total tests 1, 040, 442 7 Data current as of 7/24/2020

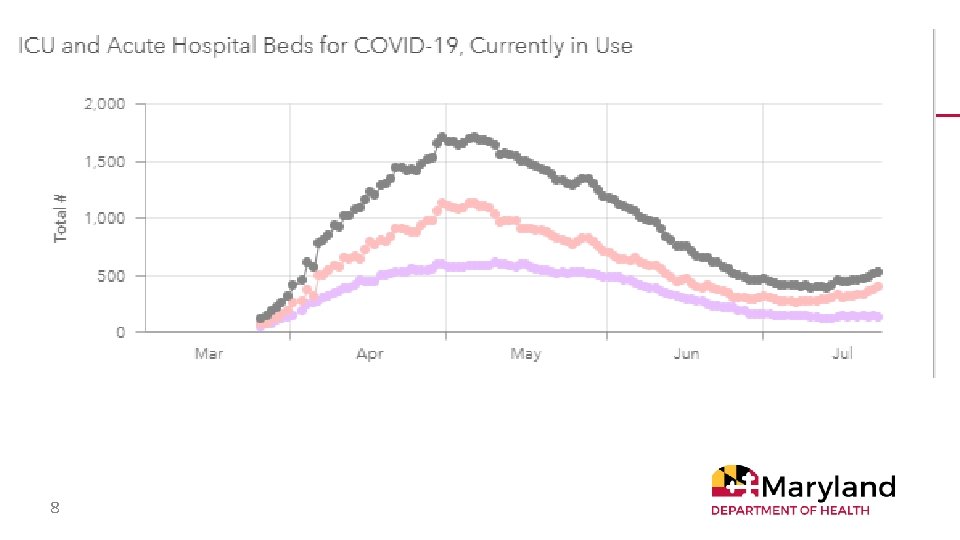
8

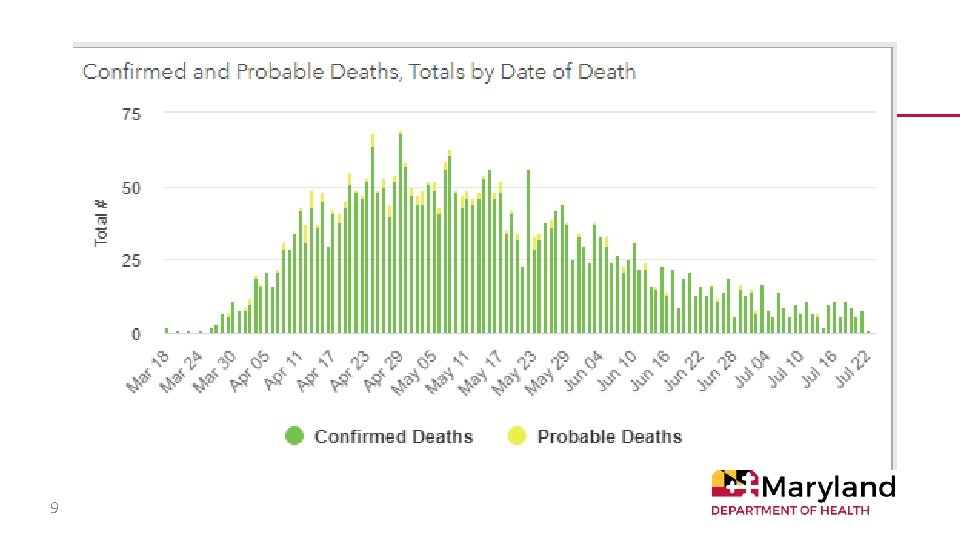
9

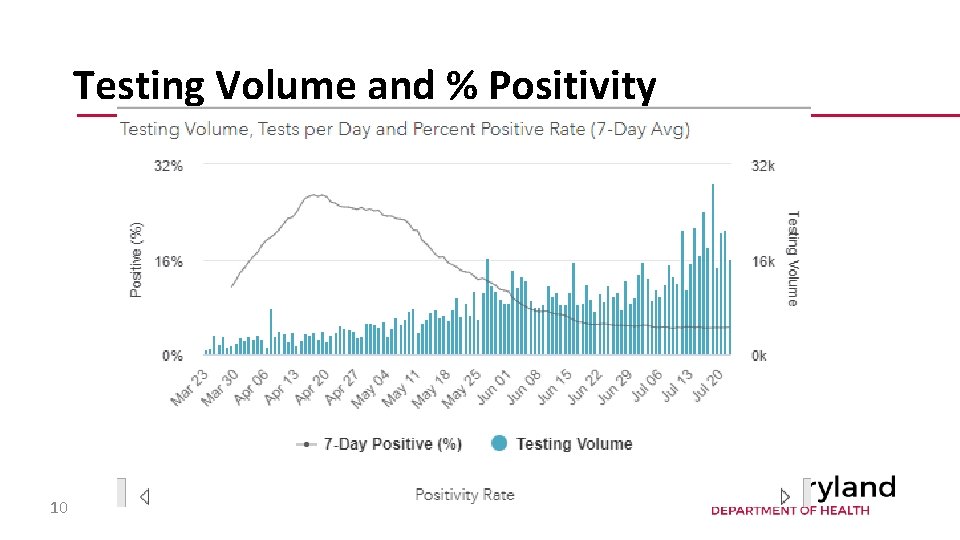
Testing Volume and % Positivity 10

Infection Prevention and Control Updates 11

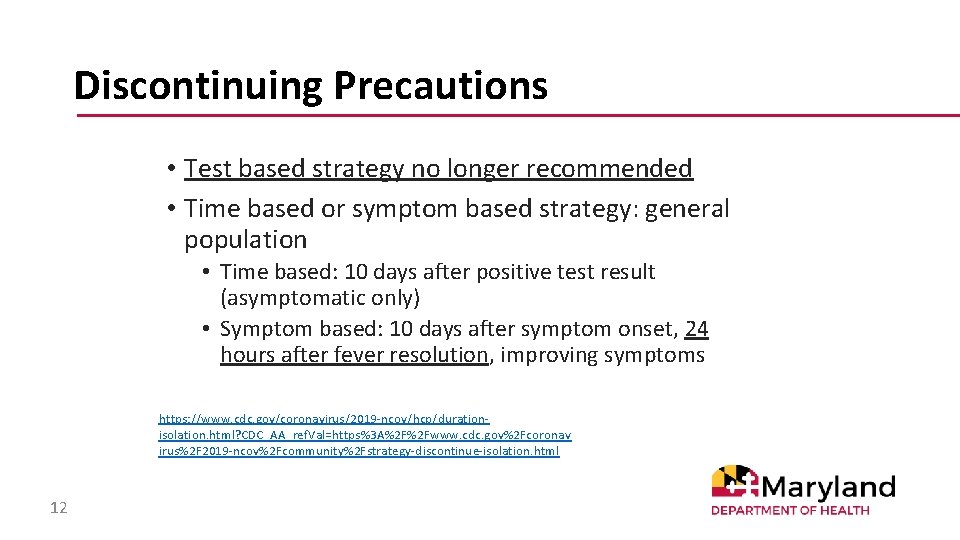
Discontinuing Precautions • Test based strategy no longer recommended • Time based or symptom based strategy: general population • Time based: 10 days after positive test result (asymptomatic only) • Symptom based: 10 days after symptom onset, 24 hours after fever resolution, improving symptoms https: //www. cdc. gov/coronavirus/2019 -ncov/hcp/durationisolation. html? CDC_AA_ref. Val=https%3 A%2 F%2 Fwww. cdc. gov%2 Fcoronav irus%2 F 2019 -ncov%2 Fcommunity%2 Fstrategy-discontinue-isolation. html 12

Where does this guidance apply? • Discontinue transmission based precautions in acute and long term care settings • Return to work criteria for HCP • End of isolation period for ill and asymptomatic positive individuals https: //www. cdc. gov/coronavirus/2019 -ncov/hcp/disposition-hospitalized-patients. html https: //www. cdc. gov/coronavirus/2019 -ncov/hcp/disposition-in-home-patients. html https: //www. cdc. gov/coronavirus/2019 -ncov/hcp/return-towork. html? CDC_AA_ref. Val=https%3 A%2 F%2 Fwww. cdc. gov%2 Fcoronavirus%2 F 2019 ncov%2 Fhealthcare-facilities%2 Fhcp-return-work. html 13

Special Circumstances • For HCW return to work and discontinuing transmission based precautions only • Individuals with severe disease or who are immunocompromised: • Time based: 20 days after positive test result (asymptomatic only) • Symptom based: 20 days after symptom onset, 24 hours after fever resolution, improving symptoms 14

Definitions • Severe illness • Individuals who have respiratory frequency >30 breaths per minute, Sp. O 2 <94% on room air at sea level (or, for patients with chronic hypoxemia, a decrease from baseline of >3%), ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (Pa. O 2/Fi. O 2) <300 mm. Hg, or lung infiltrates >50%. • Critical illness • Individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction. • Immunocompromised (not an exhaustive list) • Some conditions, such as being on chemotherapy for cancer, untreated HIV infection with CD 4 T lymphocyte count < 200, combined primary immunodeficiency disorder, and receipt of prednisone >20 mg/day for more than 14 days • Ultimately, the degree of immunocompromise for the patient is determined by the treating provider, and preventive actions are tailored to each individual and situation. 15

Re-testing and prolonged PCR positivity • For persons recovered from SARS-Co. V-2 infection, a positive PCR during the 90 days after illness onset more likely represents persistent shedding of viral RNA than reinfection • If such a person remains asymptomatic during this 90 -day period, then any re-testing is unlikely to yield useful information, even if the person had close contact with an infected person • If such a person becomes symptomatic during this 90 -day period an evaluation fails to identify a diagnosis other than SARS-Co. V -2 infection (e. g. , influenza), then the person may warrant evaluation for SARS-Co. V-2 reinfection 16

Implications • Individuals who have tested positive within the last 90 days and have met recovery criteria • Do not need to be quarantined after a close contact • Do not need to be excluded from work after a close contact • Still need to wear appropriate PPE • Do not need to be retested (if asymptomatic) • If people are retested and still come up positive, they do not need to be excluded from work or put on 10 days of isolation 17

Serologic Testing • Serologic testing should not be used to establish the presence or absence of SARS-Co. V-2 infection or reinfection • The utility of serologic testing to establish the absence or presence of infection or reinfection remains undefined 18

Modes of Transmission

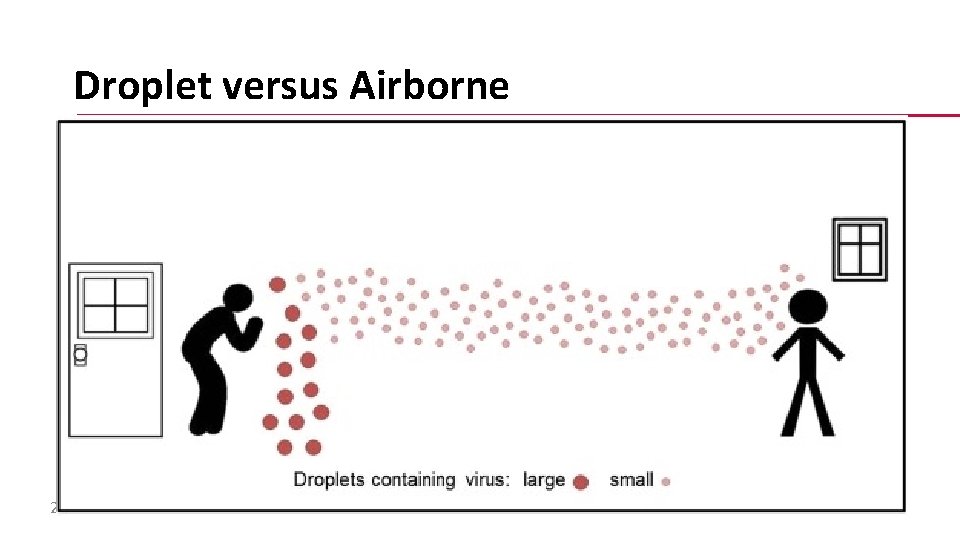
Droplet versus Airborne 20

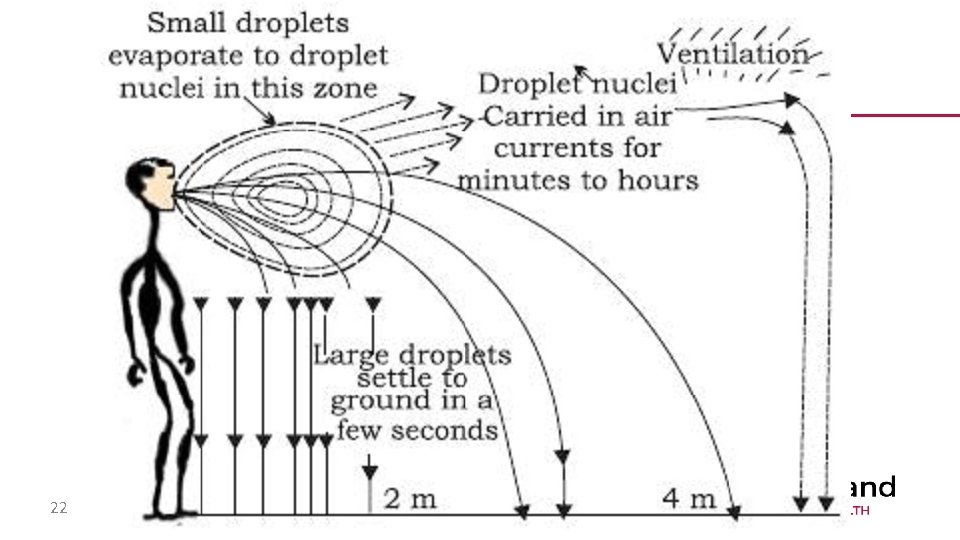
The life cycle of an airborne pathogen • Small droplets containing pathogen and liquid are expelled • The liquid evaporates before it hits the ground • Remaining bits of pathogen and protein called droplet nuclei • Droplet nuclei attach to microscopic bits of dust in the air • The dust particles can travel through HVAC systems, in the air, etc. • Dust particles get inhaled by a new host 21

22

Why do we wear masks? 23

Face Shield • Protects wearer’s eyes, nose and mouth from large droplets in the air • Stops large droplets from the wearer • Does not contain small or medium sized droplets • Not effective as source control 24

Cloth Face Mask • Loosely covers the nose and mouth of wearer • Will catch and contain small and medium droplets, and even some droplet nuclei produced by the wearer • May block large and some medium droplets in the air from getting into the nose and mouth of the wearer • Recommended for source control 25

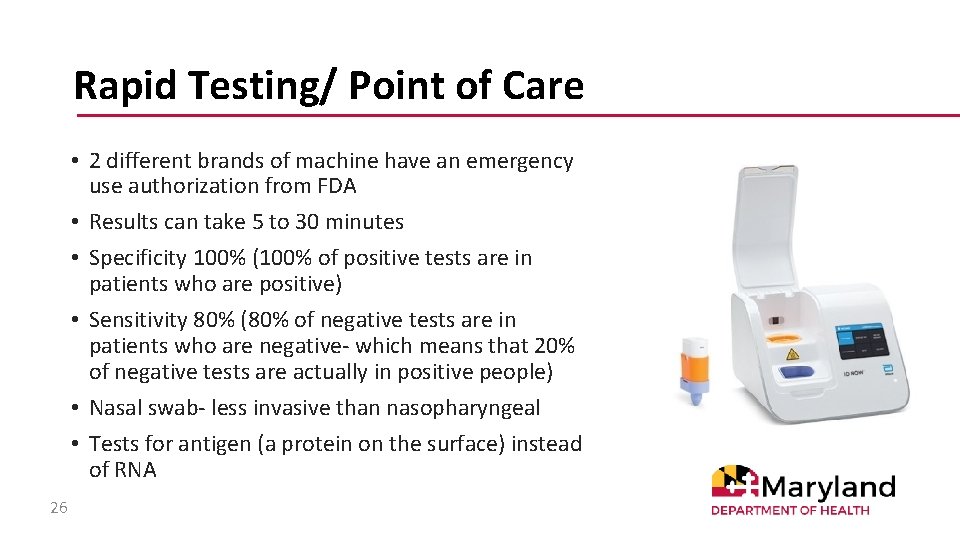
Rapid Testing/ Point of Care • 2 different brands of machine have an emergency use authorization from FDA • Results can take 5 to 30 minutes • Specificity 100% (100% of positive tests are in patients who are positive) • Sensitivity 80% (80% of negative tests are in patients who are negative- which means that 20% of negative tests are actually in positive people) • Nasal swab- less invasive than nasopharyngeal • Tests for antigen (a protein on the surface) instead of RNA 26

Where is Point of Care testing useful? • Rural areas with limited lab access • Rapid deployment to investigate newly identified clusters • Certain congregate living settings (nursing homes, corrections) for diagnostics or limited screening • Clinics and testing sites with highly mobile populations 27

Resources • MDH Novel Coronavirus: http: //health. maryland. gov/coronavirus • MDH Laboratory Coronavirus: https: //health. maryland. gov/laboratories/Pages/Novel. Coronavirus. aspx • COVID-19 People at risk https: //www. cdc. gov/coronavirus/2019 -ncov/specific-groups/highrisk-complications. html • CDC Guidance for Infection Control https: //www. cdc. gov/coronavirus/2019 -n. Co. V/infectioncontrol. html • Behavioral Health https: //www. cdc. gov/coronavirus/2019 ncov/hcp/infection-controlfaq. html? CDC_AA_ref. Val=https%3 A%2 F%2 Fwww. cdc. gov%2 Fcoron avirus%2 F 2019 -ncov%2 Finfection-control%2 Finfection-preventioncontrol-faq. html 28 • Memory Care https: //www. cdc. gov/coronavirus/2019 ncov/hcp/memory-care. html

Questions? Email : mdh. ipcovid@Maryland. gov 29
 Recovery techniques based on immediate update
Recovery techniques based on immediate update Bronquite coronavirus
Bronquite coronavirus 2 factores abioticos
2 factores abioticos Scissurite coronavirus
Scissurite coronavirus Allegato xlvi del d.lgs. 81/08 coronavirus
Allegato xlvi del d.lgs. 81/08 coronavirus Relazione finale classe quinta scuola primaria
Relazione finale classe quinta scuola primaria Contenitori per trasporto materiale biologico
Contenitori per trasporto materiale biologico Mehmet dorak
Mehmet dorak What do if test positive covid19
What do if test positive covid19 Http://apps.tujuhbukit.com/covid19/
Http://apps.tujuhbukit.com/covid19/ Vaksin covid19
Vaksin covid19 Do if you covid19
Do if you covid19 Covid19 athome rapid what know
Covid19 athome rapid what know Mdh situation update
Mdh situation update Communicable disease and non communicable disease
Communicable disease and non communicable disease Plats för toran ark
Plats för toran ark Kolposkopi px
Kolposkopi px Sju principer för tillitsbaserad styrning
Sju principer för tillitsbaserad styrning Romarriket tidslinje
Romarriket tidslinje Stig kerman
Stig kerman Modell för handledningsprocess
Modell för handledningsprocess Shingelfrisyren
Shingelfrisyren Ledningssystem för verksamhetsinformation
Ledningssystem för verksamhetsinformation Borstål, egenskaper
Borstål, egenskaper Shivaismen
Shivaismen Cks
Cks Jag har nigit för nymånens skära text
Jag har nigit för nymånens skära text Inköpsprocessen steg för steg
Inköpsprocessen steg för steg A gastrica
A gastrica Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning