Legal Aspects of Clinical Trials Shekhar Potkar Director



















- Slides: 20

Legal Aspects of Clinical Trials Shekhar Potkar Director Clinical Research Pfizer Limited

Why is law necessary in clinical research?

Jesse Gelsinger vs University of Penn n n n n OTC deficiency Gene therapy experiment with Adenovirus vector No direct benefit, minimal risks, benefit to others Conducted by Dr Wilson, IHGT at University of Penn sponsored by Genovo Died of jaundice, kidney failure, lung failure, brain death Alan Milstein sued the University, Dr Wilson and Genovo Risks not explained, negligence, conflicts of interests of Dr Wilson with Genovo ~ 10 million in settlement

Why Law in Clinical Research? n n Risk management perspective Stakeholders interests perspective

Agenda n n Legal agreements Legal implications

Agreements

Elements of a Contract n n n Parties Offer Consideration Acceptance Remedy

Major Agreements n n n Non Disclosure Agreement Investigator undertaking/agreement Clinical trial agreement Indemnity and insurance Services contract

Non Disclosure Agreement n n PI and Sponsor Protection of proprietary information Binding on site staff Site responsibility

Investigator Agreement n n n Undertaking by a PI to the regulatory agency Compliance to GCP and applicable laws Legal sanctions in case of violations

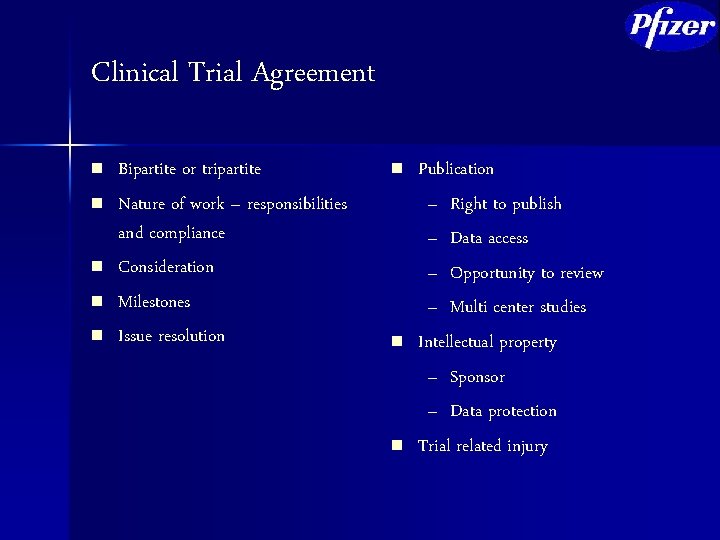
Clinical Trial Agreement n n n Bipartite or tripartite Nature of work – responsibilities and compliance Consideration Milestones Issue resolution n Publication – Right to publish – Data access – Opportunity to review – Multi center studies Intellectual property – Sponsor – Data protection Trial related injury

Indemnity/Insurance n Indemnity – Investigator, other staff and institute – Exceptions -Noncompliance/ Misconduct/ negligence – Role of a CRO n Clinical trial insurance

Services Agreement CROs, Labs, SMOs n Bipartite n Nature of services e. g. monitoring, lab analysis, site management n Terms n Consideration n Remedies

Legal Implications

Informed consent n n n Autonomy Valid consent – informed, competent (capacity), voluntary Competence - Understanding, reasoning, values Incompetence - Best interests, proxy, substituted judgement, advance directives Consent form as mechanism and evidence Battery versus Negligence - e. g. partial colectomy

Confidentiality n n n Fiduciary role – Patient autonomy – Implied promise – Virtue ethics – Consequentialism Balancing of public interests HIPAA – covered entities should use PHI for TPO only, consent necessary for any other use

Conflict of interests n n Therapeutic misconception Financial conflicts – Disclosure

Conclusions

Conclusions n n Ethics and law for human subject protection Legal framework for commercial aspects for research

Role of Ethics committee n Check risk management framework – Informed consent process, – Patient confidentiality – Conflict of interest – Financial aspects including trial related injury – Publication policy
 Importance of audience in theatre
Importance of audience in theatre Mpn clinical trials
Mpn clinical trials York clinical trials unit
York clinical trials unit Nida clinical trial network
Nida clinical trial network Clinical trials quality by design
Clinical trials quality by design Mrc clinical trials unit
Mrc clinical trials unit Protocol registration system
Protocol registration system Hawk irb
Hawk irb Site initiation visit
Site initiation visit Professor claire harrison
Professor claire harrison Randomization
Randomization Clinical trials.gov login
Clinical trials.gov login Clinical trials
Clinical trials Clinicaltrials.gov api
Clinicaltrials.gov api Clinical hysteria salem witch trials
Clinical hysteria salem witch trials Dhl atyrau
Dhl atyrau Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Ivr iwr for clinical trial
Ivr iwr for clinical trial Difference between inspection and audit
Difference between inspection and audit Clinical research statistician
Clinical research statistician Ohsu clinical trials office
Ohsu clinical trials office






































