Lecture 8 9 Multielectron atoms o Alkali atom

- Slides: 26

Lecture 8 -9: Multi-electron atoms o Alkali atom spectra. o Central field approximation. o Shell model. o Effective potentials and screening. o Experimental evidence for shell model. PY 3 P 05

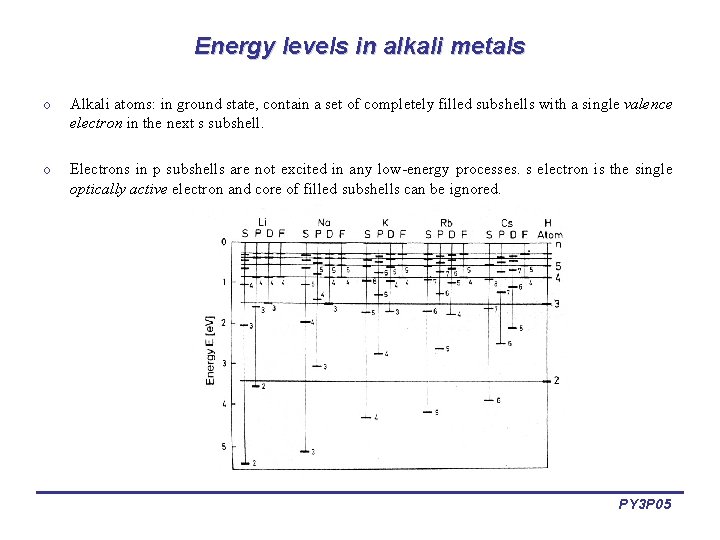
Energy levels in alkali metals o Alkali atoms: in ground state, contain a set of completely filled subshells with a single valence electron in the next s subshell. o Electrons in p subshells are not excited in any low-energy processes. s electron is the single optically active electron and core of filled subshells can be ignored. PY 3 P 05

Energy levels in alkali metals o In alkali atoms, the l degeneracy is lifted: states with the same principal quantum number n and different orbital quantum number l have different energies. o Relative to H-atom terms, alkali terms lie at lower energies. This shift increases the smaller l is. o For larger values of n, i. e. , greater orbital radii, the terms are only slightly different from hydrogen. o Also, electrons with small l are more strongly bound and their terms lie at lower energies. o These effects become stronger with increasing Z. o Non-Coulombic potential breaks degeneracy of levels with the same principal quantum number. PY 3 P 05

Hartree theory o For multi-electron atom, must consider Coulomb interactions between its Z electrons and its nucleus of charge +Ze. Largest effects due to large nuclear charge. o Must also consider Coulomb interactions between each electron and all other electrons in atom. Effect is weak. o Assume electrons are moving independently in a spherically symmetric net potential. o The net potential is the sum of the spherically symmetric attractive Coulomb potential due to the nucleus and a spherically symmetric repulsive Coulomb potential which represents the average effect of the electrons and its Z - 1 colleagues. o Hartree (1928) attempted to solve the time-independent Schrödinger equation for Z electrons in a net potential. o Total potential of the atom can be written as the sum of a set of Z identical net potentials V( r), each depending on r of the electron only. PY 3 P 05

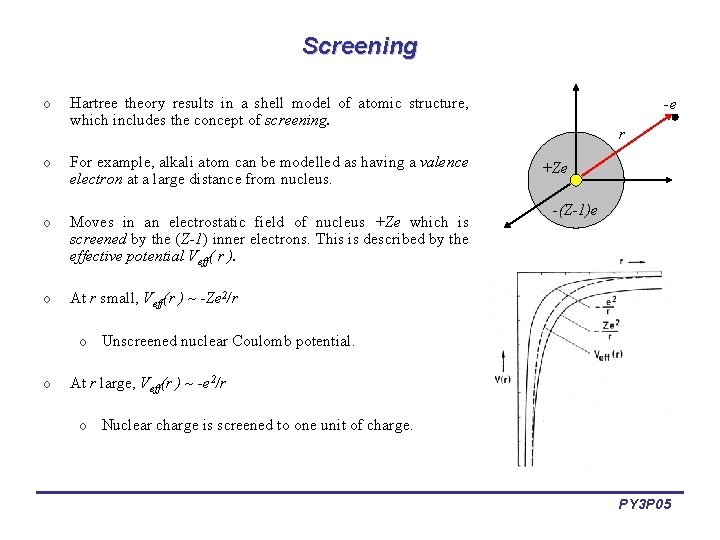
Screening o Hartree theory results in a shell model of atomic structure, which includes the concept of screening. o For example, alkali atom can be modelled as having a valence electron at a large distance from nucleus. o Moves in an electrostatic field of nucleus +Ze which is screened by the (Z-1) inner electrons. This is described by the effective potential Veff( r ). o At r small, Veff(r ) ~ -Ze 2/r -e r +Ze -(Z-1)e o Unscreened nuclear Coulomb potential. o At r large, Veff(r ) ~ -e 2/r o Nuclear charge is screened to one unit of charge. PY 3 P 05

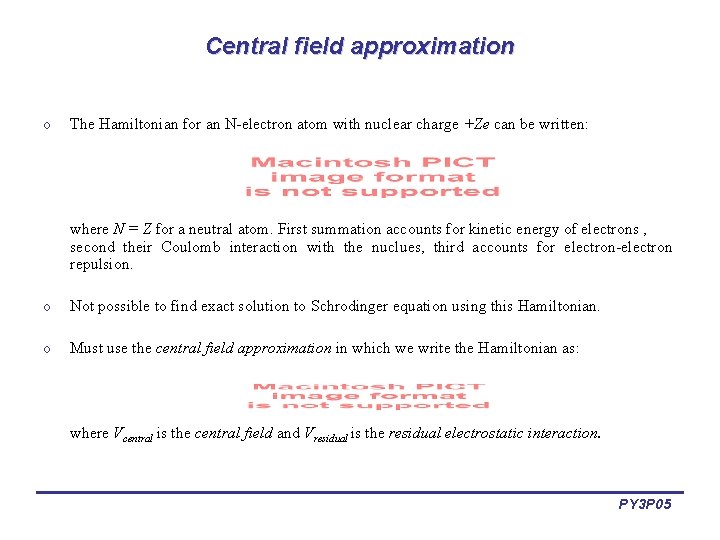
Central field approximation o The Hamiltonian for an N-electron atom with nuclear charge +Ze can be written: where N = Z for a neutral atom. First summation accounts for kinetic energy of electrons , second their Coulomb interaction with the nuclues, third accounts for electron-electron repulsion. o Not possible to find exact solution to Schrodinger equation using this Hamiltonian. o Must use the central field approximation in which we write the Hamiltonian as: where Vcentral is the central field and Vresidual is the residual electrostatic interaction. PY 3 P 05

Central field approximation o The central field approximation work in the limit where o In this case, Vresidual can be treated as a perturbation and solved later. o By writing we end up with N separate Schrödinger equations: with E = E 1 + E 2 + … + EN o Normally solved numerically, but analytic solutions can be found using the separation of variables technique. PY 3 P 05

Central field approximation o As potentials only depend on radial coordinate, can use separation of variables: where Ri(ri) are a set of radial wave functions and Yi( i, i ) are a set of spherical harmonic functions. o Following the same procedure as Lectures 3 -4, we end up with three equations, one for each polar coordinate. o Each electron will therefore have four quantum numbers: o l and ml: result from angular equations. o n: arises from solving radial equation. n and l determine the radial wave function Rnl(r ) and the energy of the electron. o ms: Electron can either have spin up (ms = +1/2) or down (ms = -1/2). o State of multi-electron atom is then found by working out the wave functions of the individual electrons and then finding the total energy of the atom (E = E 1 + E 2 + … + EN). PY 3 P 05

Shell model o Hartree theory predicts shell model structure, which only considers gross structure: 1. States are specified by four quantum numbers, n, l, ml, and ms. 2. Gross structure of spectrum is determined by n and l. 3. Each (n, l) term of the gross structure contains 2(2 l + 1) degenerate levels. 1. Shell model assumes that we can order energies of gross terms in a multielectron atom according to n and l. As electrons are added, electrons fill up the lowest available shell first. o Experimental evidence for shell model proves that central approximation is appropriate. PY 3 P 05

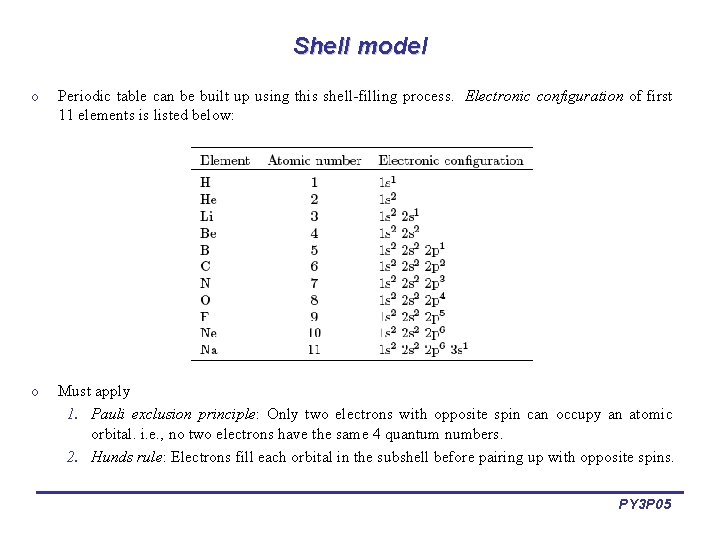
Shell model o Periodic table can be built up using this shell-filling process. Electronic configuration of first 11 elements is listed below: o Must apply 1. Pauli exclusion principle: Only two electrons with opposite spin can occupy an atomic orbital. i. e. , no two electrons have the same 4 quantum numbers. 2. Hunds rule: Electrons fill each orbital in the subshell before pairing up with opposite spins. PY 3 P 05

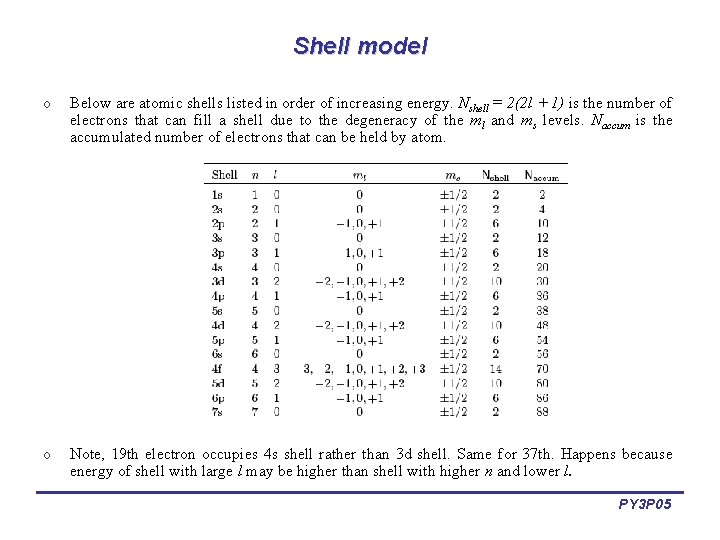
Shell model o Below are atomic shells listed in order of increasing energy. Nshell = 2(2 l + 1) is the number of electrons that can fill a shell due to the degeneracy of the ml and ms levels. Naccum is the accumulated number of electrons that can be held by atom. o Note, 19 th electron occupies 4 s shell rather than 3 d shell. Same for 37 th. Happens because energy of shell with large l may be higher than shell with higher n and lower l. PY 3 P 05

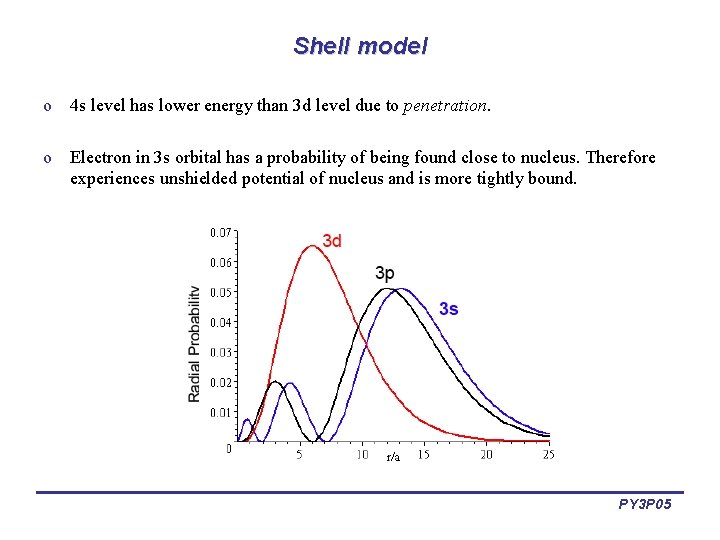
Shell model o 4 s level has lower energy than 3 d level due to penetration. o Electron in 3 s orbital has a probability of being found close to nucleus. Therefore experiences unshielded potential of nucleus and is more tightly bound. PY 3 P 05

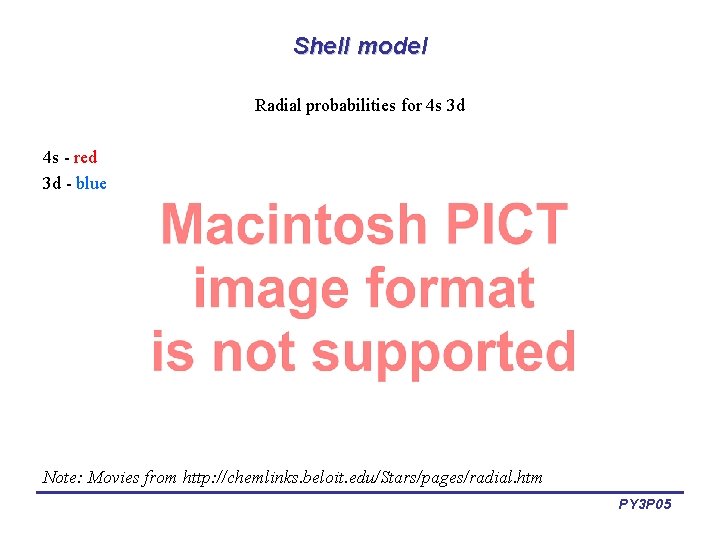
Shell model Radial probabilities for 4 s 3 d 4 s - red 3 d - blue Note: Movies from http: //chemlinks. beloit. edu/Stars/pages/radial. htm PY 3 P 05

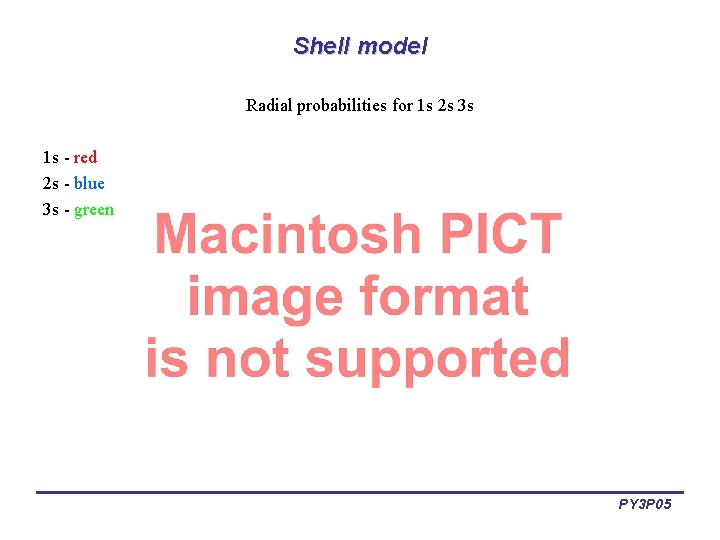
Shell model Radial probabilities for 1 s 2 s 3 s 1 s - red 2 s - blue 3 s - green PY 3 P 05

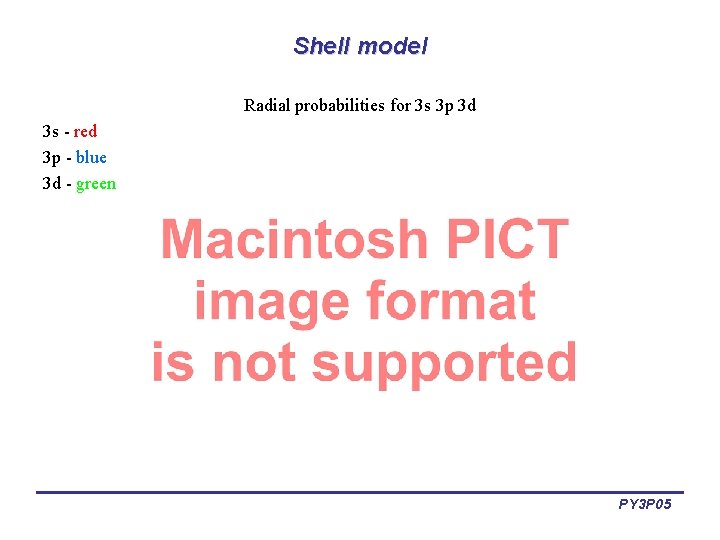
Shell model Radial probabilities for 3 s 3 p 3 d 3 s - red 3 p - blue 3 d - green PY 3 P 05

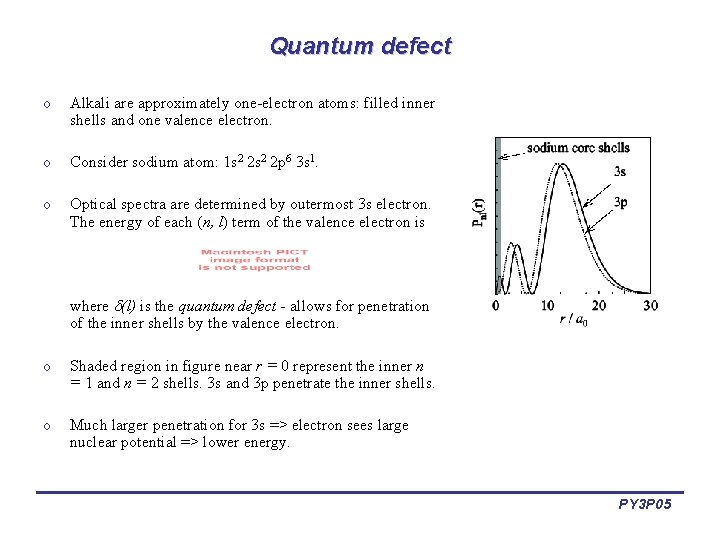
Quantum defect o Alkali are approximately one-electron atoms: filled inner shells and one valence electron. o Consider sodium atom: 1 s 2 2 p 6 3 s 1. o Optical spectra are determined by outermost 3 s electron. The energy of each (n, l) term of the valence electron is where (l) is the quantum defect - allows for penetration of the inner shells by the valence electron. o Shaded region in figure near r = 0 represent the inner n = 1 and n = 2 shells. 3 s and 3 p penetrate the inner shells. o Much larger penetration for 3 s => electron sees large nuclear potential => lower energy. PY 3 P 05

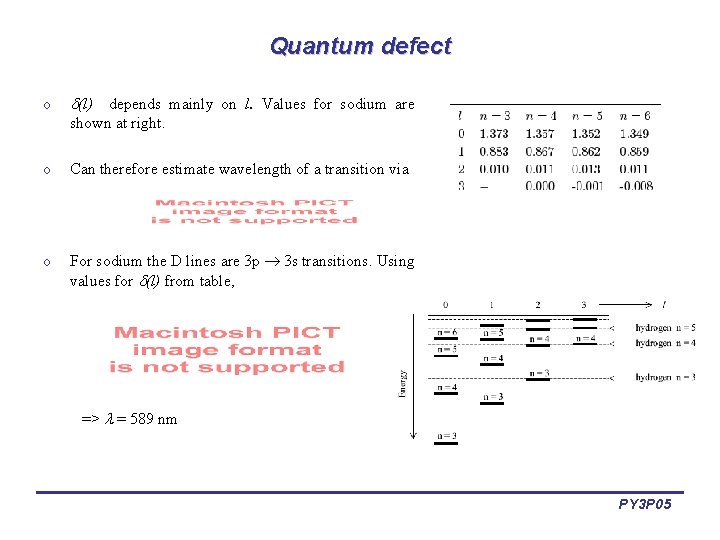
Quantum defect o (l) depends mainly on l. Values for sodium are shown at right. o Can therefore estimate wavelength of a transition via o For sodium the D lines are 3 p 3 s transitions. Using values for (l) from table, => = 589 nm PY 3 P 05

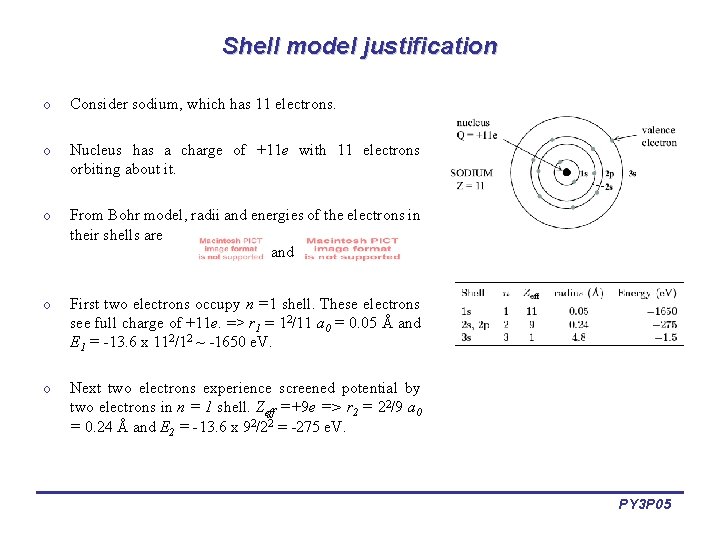
Shell model justification o Consider sodium, which has 11 electrons. o Nucleus has a charge of +11 e with 11 electrons orbiting about it. o From Bohr model, radii and energies of the electrons in their shells are and o First two electrons occupy n =1 shell. These electrons see full charge of +11 e. => r 1 = 12/11 a 0 = 0. 05 Å and E 1 = -13. 6 x 112/12 ~ -1650 e. V. o Next two electrons experience screened potential by two electrons in n = 1 shell. Zeff =+9 e => r 2 = 22/9 a 0 = 0. 24 Å and E 2 = -13. 6 x 92/22 = -275 e. V. PY 3 P 05

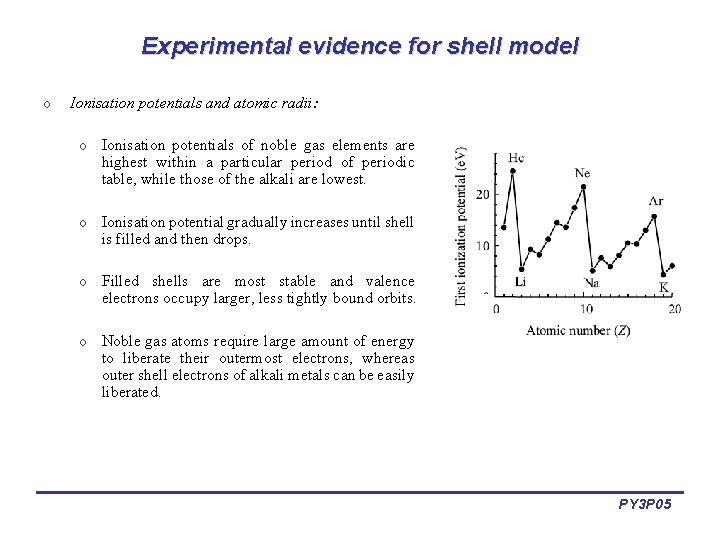
Experimental evidence for shell model o Ionisation potentials and atomic radii: o Ionisation potentials of noble gas elements are highest within a particular period of periodic table, while those of the alkali are lowest. o Ionisation potential gradually increases until shell is filled and then drops. o Filled shells are most stable and valence electrons occupy larger, less tightly bound orbits. o Noble gas atoms require large amount of energy to liberate their outermost electrons, whereas outer shell electrons of alkali metals can be easily liberated. PY 3 P 05

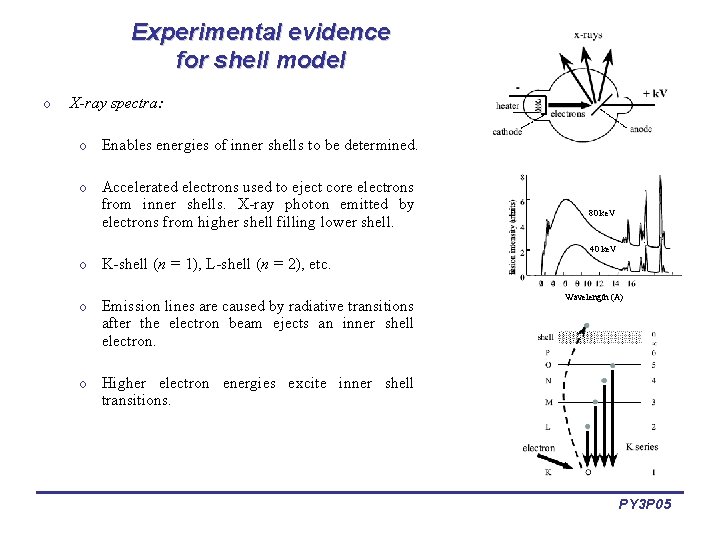
Experimental evidence for shell model o X-ray spectra: o Enables energies of inner shells to be determined. o Accelerated electrons used to eject core electrons from inner shells. X-ray photon emitted by electrons from higher shell filling lower shell. o K-shell (n = 1), L-shell (n = 2), etc. o Emission lines are caused by radiative transitions after the electron beam ejects an inner shell electron. 80 ke. V 40 ke. V Wavelength (A) o Higher electron energies excite inner shell transitions. PY 3 P 05

Experimental evidence for shell model o Wavelength of various series of emission lines are found to obey Moseley’s law. o For example, the K-shell lines are given by where accounts for the screening effect of other electrons. o Similarly, the L-shell spectra obey: o Same wavelength as predicted by Bohr, but now have and effective charge (Z - ) instead of Z. o L ~ 10 and K ~ 3. PY 3 P 05

Bohr model including screening o Assume net charge is ( Z - 1 )e. o Therefore, the potential energy is o Total energy of orbit is o Modified Bohr formula taking into account screening. o Can therefore easily show that PY 3 P 05

Shell model summary o Electrons in orbitals with large principal quantum numbers (n) will be shielded from the nucleus by innershell electrons. Zeff = Z - nl. o nl increases with n => Zeff decreases with n. o nl increases with l => Zeff decreases with l. PY 3 P 05

Shell model summary o o o In hydrogenic one-electron model, the energy levels of a given n are degenerate in l: 3 s 3 p 3 d Not the case in multi-electron atoms. Orbitals with the same n quantum number have different energies for differing values of l. As Zeff = Z - nl is a function of n and l, the l degeneracy is broken by modified potential. PY 3 P 05

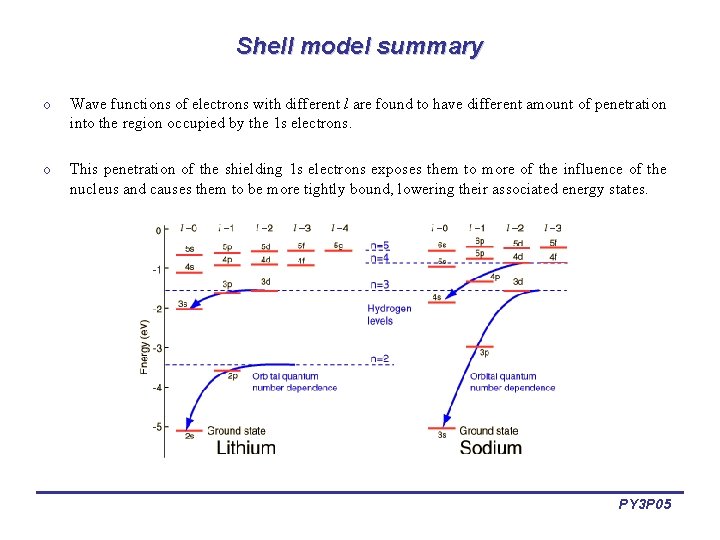
Shell model summary o Wave functions of electrons with different l are found to have different amount of penetration into the region occupied by the 1 s electrons. o This penetration of the shielding 1 s electrons exposes them to more of the influence of the nucleus and causes them to be more tightly bound, lowering their associated energy states. PY 3 P 05

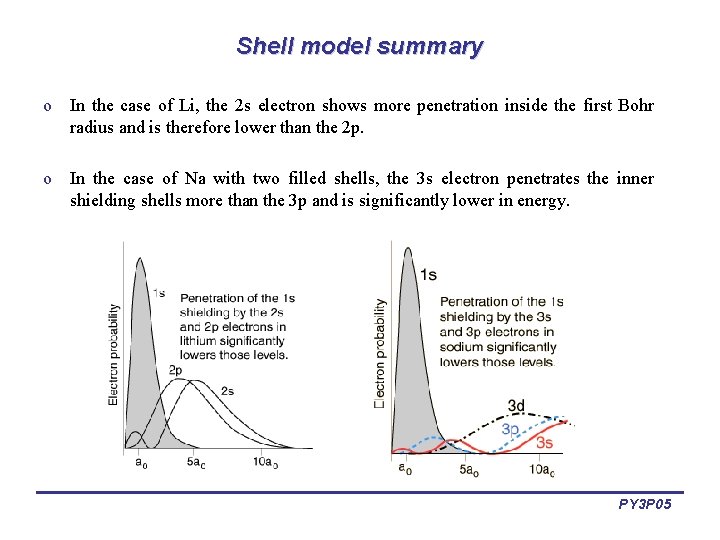
Shell model summary o In the case of Li, the 2 s electron shows more penetration inside the first Bohr radius and is therefore lower than the 2 p. o In the case of Na with two filled shells, the 3 s electron penetrates the inner shielding shells more than the 3 p and is significantly lower in energy. PY 3 P 05