Lecture 14 Liquid nitrogen VA group Nitrogen and









- Slides: 31

Lecture 14 Liquid nitrogen VA group. Nitrogen and Phosphorous and their compounds. Ph. D Halina Falfushynska

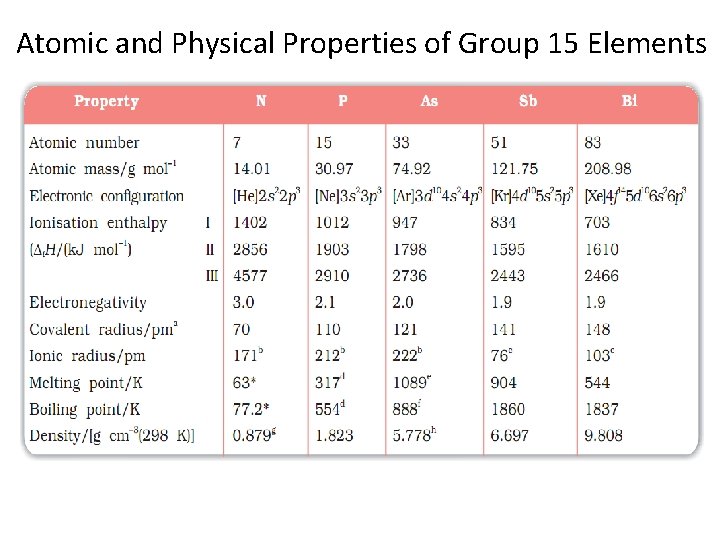
Atomic and Physical Properties of Group 15 Elements

Electronic Configuration. The valence shell electronic configuration of these elements is ns 2 np 3. The s orbital in these elements is completely filled and p orbitals are halffilled, making their electronic configuration extra stable. Atomic and Ionic Radii. Covalent and ionic (in a particular state) radii increase in size down the group. There is a considerable increase in covalent radius from N to P. However, from As to Bi only a small increase in covalent radius is observed. This is due to the presence of completely filled d and/or f orbitals in heavier members. Ionisation Enthalpy. Ionisation enthalpy decreases down the group due to gradual increase in atomic size. Because of the extra stable half-filled p orbitals electronic configuration and smaller size, the ionisation enthalpy of the group 15 elements is much greater than that of group 14 elements in the corresponding periods. The order of successive ionisation enthalpies, as expected is ΔH 1 < ΔH 2 < ΔH 3 Physical Properties. All the elements of this group are polyatomic. Dinitrogen is a diatomic gas while all others are solids. Metallic character increases down the group. Nitrogen and phosphorus are non-metals, arsenic and antimony metalloids and bismuth is a metal. This is due to decrease in ionisation enthalpy and increase in atomic size. The boiling points, in general, increase from top to bottom in the group but the melting point increases up to arsenic and then decreases up to bismuth. Except nitrogen, all the elements show allotropy.

Preparation of dinitrogen • In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite. NH 4 CI(aq) + Na. NO 2 (aq) → N 2 (g) + 2 H 2 O(l) + Na. Cl (aq) • It can be obtained by thermal decomposition of ammonium dichromate. (NH 4)2 Cr 2 O 7 (Heat) ��� → N 2 + 4 H 2 O + Cr 2 O 3 • Very pure nitrogen can be obtained by thermal decomposition of sodium or barium azide. Ba(N 3)2 → Ba + 3 N 2 • Air (4 N 2 + O 2) + C → 4 N 2 + CO 2 • NH 3 + 3 O 2 → 2 N 2 + 6 H 2 O • 2 NH 3 + 3 Cl 2 → N 2 + 6 HCl

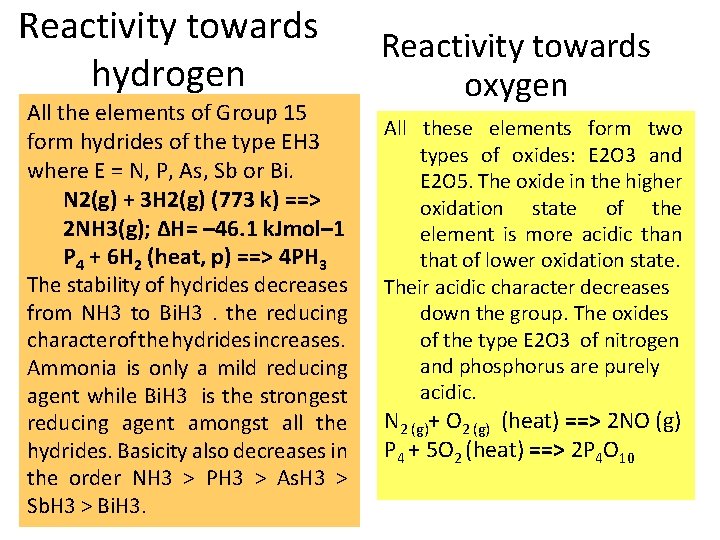
Reactivity towards hydrogen All the elements of Group 15 form hydrides of the type EH 3 where E = N, P, As, Sb or Bi. N 2(g) + 3 H 2(g) (773 k) ==> 2 NH 3(g); ΔH= – 46. 1 k. Jmol– 1 Р 4 + 6 Н 2 (heat, p) ==> 4 РН 3 The stability of hydrides decreases from NH 3 to Bi. H 3. the reducing character of the hydrides increases. Ammonia is only a mild reducing agent while Bi. H 3 is the strongest reducing agent amongst all the hydrides. Basicity also decreases in the order NH 3 > PH 3 > As. H 3 > Sb. H 3 > Bi. H 3. Reactivity towards oxygen All these elements form two types of oxides: E 2 O 3 and E 2 O 5. The oxide in the higher oxidation state of the element is more acidic than that of lower oxidation state. Their acidic character decreases down the group. The oxides of the type E 2 O 3 of nitrogen and phosphorus are purely acidic. N 2 (g)+ O 2 (g) (heat) ==> 2 NO (g) P 4 + 5 O 2 (heat) ==> 2 P 4 O 10

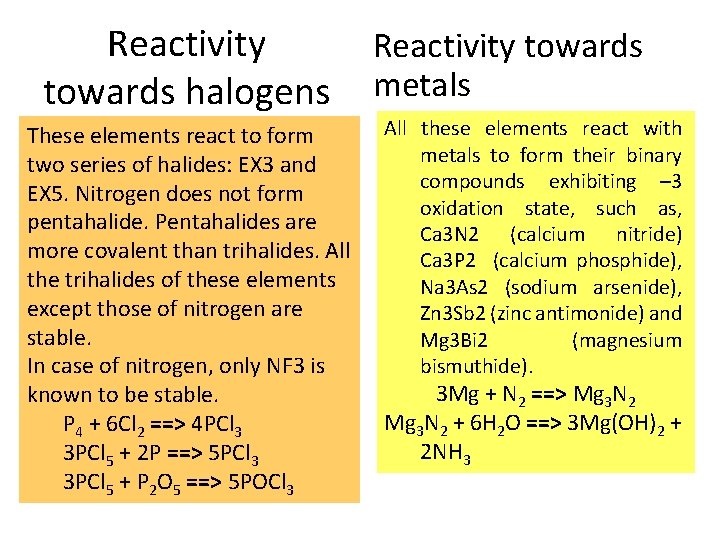
Reactivity towards halogens These elements react to form two series of halides: EX 3 and EX 5. Nitrogen does not form pentahalide. Pentahalides are more covalent than trihalides. All the trihalides of these elements except those of nitrogen are stable. In case of nitrogen, only NF 3 is known to be stable. P 4 + 6 Cl 2 ==> 4 PCl 3 3 PCl 5 + 2 P ==> 5 PCl 3 3 PCl 5 + P 2 O 5 ==> 5 POCl 3 Reactivity towards metals All these elements react with metals to form their binary compounds exhibiting – 3 oxidation state, such as, Ca 3 N 2 (calcium nitride) Ca 3 P 2 (calcium phosphide), Na 3 As 2 (sodium arsenide), Zn 3 Sb 2 (zinc antimonide) and Mg 3 Bi 2 (magnesium bismuthide). 3 Mg + N 2 ==> Mg 3 N 2 + 6 H 2 O ==> 3 Mg(OH)2 + 2 NH 3

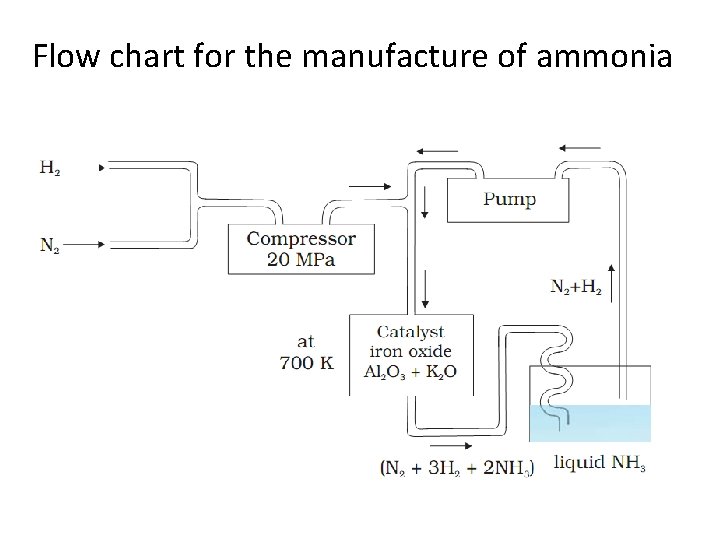
Ammonia • Ammonia is present in small quantities in air and soil where it is formed by the decay of nitrogenous organic matter e. g. , urea. • NH 2 CONH 2 +2 H 2 O → (NH 4)2 CO 3 → 2 NH 3 + H 2 O+ CO 2 • On a small scale ammonia is obtained from ammonium salts which decompose when treated with caustic soda or lime. 2 NH 4 Cl + Ca(OH)2 → 2 NH 3 + 2 H 2 O + Ca. Cl 2 (NH 4)2 SO 4 + 2 Na. OH → 2 NH 3 + 2 H 2 O + Na 2 SO 4 • On a large scale, ammonia is manufactured by Haber’s process. • N 2(g) + 3 H 2(g) → 2 NH 3(g); ΔH = – 46. 1 k. J mol-1

Flow chart for the manufacture of ammonia

Phosphine • Phosphine is prepared by the reaction of calcium phosphide with water or dilute HCl. • Ca 3 P 2 + 6 H 2 O → 3 Ca(OH)2 + 2 PH 3 • Ca 3 P 2 + 6 HCl → 3 Ca. Cl 2 + 2 PH 3 • In the laboratory, it is prepared by heating white phosphorus with concentrated Na. OH solution in an inert atmosphere of CO 2. • Р 4 + 3 КОН + 3 Н 2 О → РН 3 + 3 КН 2 РО 4 • PH 4 I (phosphonium iodide)+KOH→KI + H 2 O+PH 3

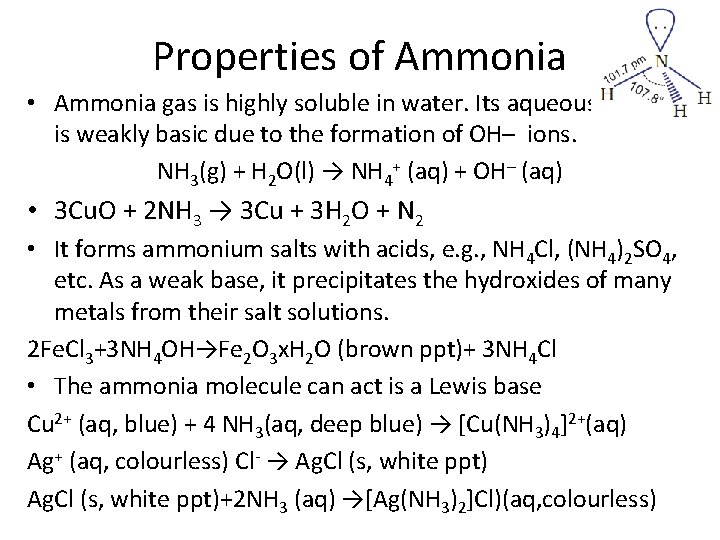
Properties of Ammonia • Ammonia gas is highly soluble in water. Its aqueous solution is weakly basic due to the formation of OH– ions. NH 3(g) + H 2 O(l) → NH 4+ (aq) + OH– (aq) • 3 Cu. O + 2 NH 3 → 3 Cu + 3 H 2 O + N 2 • It forms ammonium salts with acids, e. g. , NH 4 Cl, (NH 4)2 SO 4, etc. As a weak base, it precipitates the hydroxides of many metals from their salt solutions. 2 Fe. Cl 3+3 NH 4 OH→Fe 2 O 3 x. H 2 O (brown ppt)+ 3 NH 4 Cl • The ammonia molecule can act is a Lewis base Cu 2+ (aq, blue) + 4 NH 3(aq, deep blue) → [Cu(NH 3)4]2+(aq) Ag+ (aq, colourless) Cl- → Ag. Cl (s, white ppt)+2 NH 3 (aq) →[Ag(NH 3)2]Cl)(aq, colourless)

Properties of Ammonia Fountain. Demonstration Preparation of Potassium Amide. Potassium amide is prepared by of the high solubility of gaseous dissolution of potassium in liquid ammonia in water ammonia.

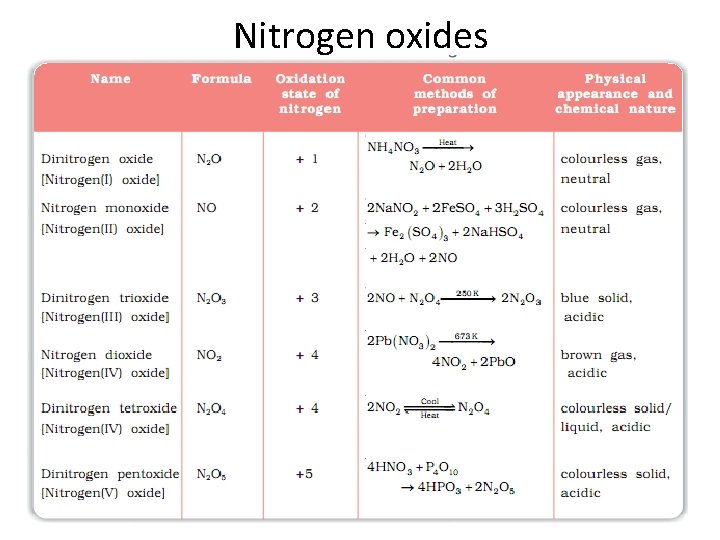
Nitrogen oxides


Nitric acid • In the laboratory, nitric acid is prepared by heating KNO 3 or Na. NO 3 and concentrated H 2 SO 4 in a glass retort: Na. NO 3 + H 2 SO 4 → Na. HSO 4+ HNO 3

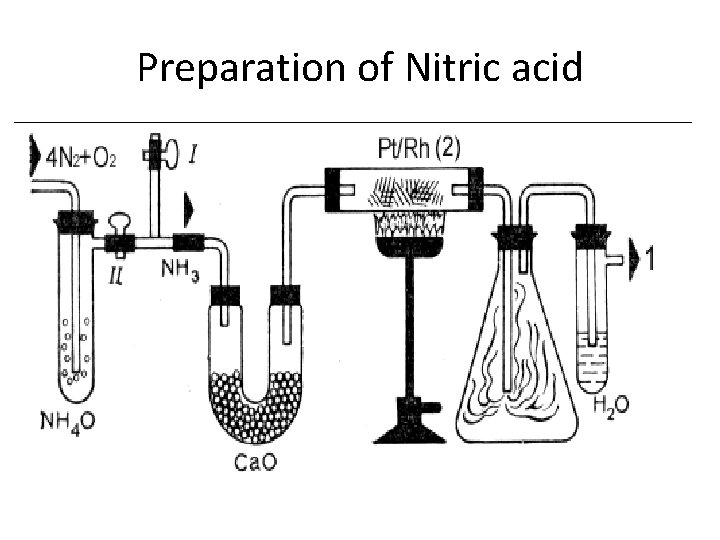
Preparation of Nitric acid

Nitric acid • In aqueous solution, nitric acid behaves as a strong acid giving hydronium and nitrate ions. • HNO 3(aq) + H 2 O(l) → H 3 O+ (aq) + NO 3– (aq) • Concentrated nitric acid is a strong oxidising agent and attacks most metals except noble metals such as gold and platinum. • 8 HNO 3 (dilute)+ 3 Cu → 3 Cu(NO 3)2 + 2 NO + 4 H 2 O • 4 Sn + 10 HNO 3 4 Sn(NO 3)2 + NH 4 NO 3 + 3 H 2 O

• Some metals (e. g. , Cr, Al) do not dissolve in concentrated nitric acid because of the formation of a passive film of oxide on the surface. • Concentrated HNO 3 also oxidises non–metals and their compounds. Iodine is oxidised to iodic acid, carbon to carbon dioxide, sulphur to H 2 SO 4, and phosphorus to phosphoric acid. • I 2 + 10 HNO 3 → 2 HIO 3 + 10 NO 2 + 4 H 2 O • C + 4 HNO 3 → CO 2 + 2 H 2 O + 4 NO 2 • S 8 + 48 HNO 3(conc. ) → 8 H 2 SO 4 + 48 NO 2 + 16 H 2 O • P 4 + 20 HNO 3(conc. ) → 4 H 3 PO 4 + 20 NO 2 + 4 H 2 O

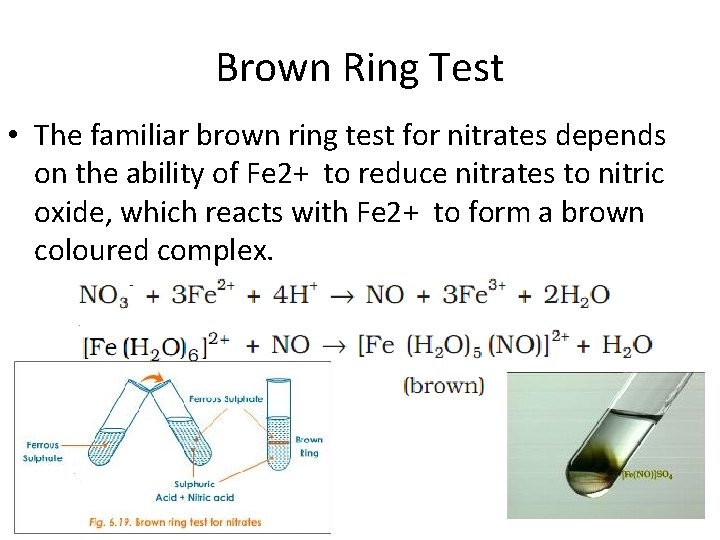
Brown Ring Test • The familiar brown ring test for nitrates depends on the ability of Fe 2+ to reduce nitrates to nitric oxide, which reacts with Fe 2+ to form a brown coloured complex.

Devarda's test • Devarda's alloy (Cu/Al/Zn) is a reducing agent. When reacted with nitrate in sodium hydroxide solution, ammonia is liberated. • 3 NO− 3 + 8 Al + 5 OH− + 18 H 2 O → 3 NH 3 + 8 [Al(OH)4]− • Aluminium is the reductant in this reaction.

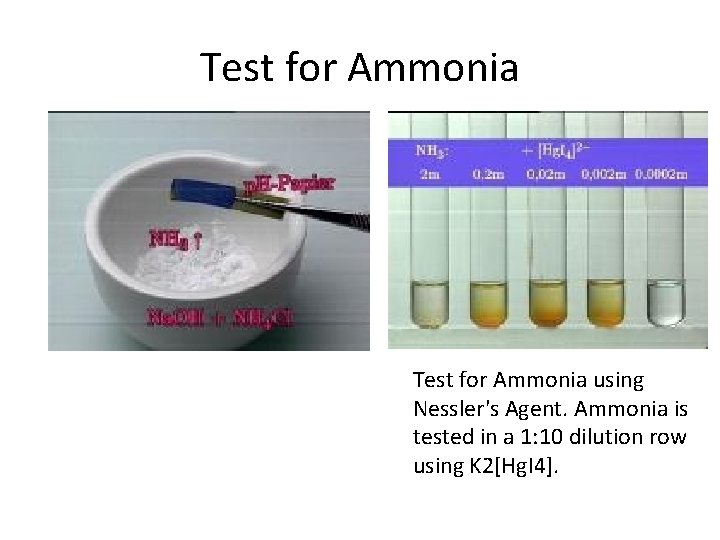
Test for Ammonia using Nessler's Agent. Ammonia is tested in a 1: 10 dilution row using K 2[Hg. I 4].

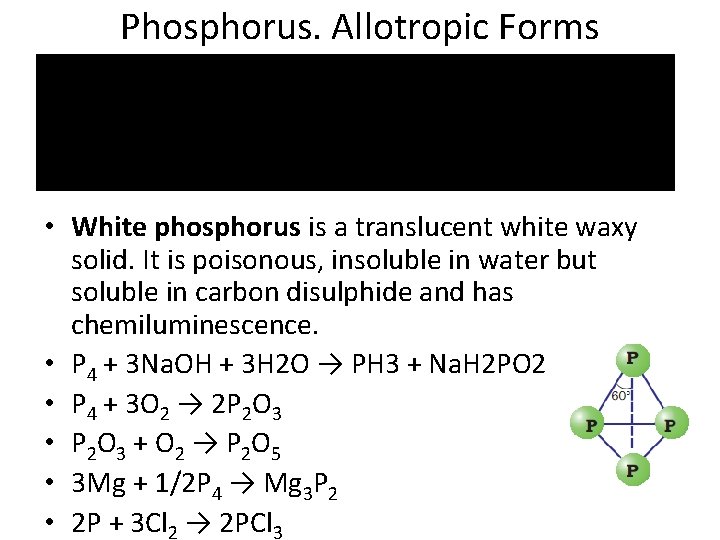
Phosphorus. Allotropic Forms • White phosphorus is a translucent white waxy solid. It is poisonous, insoluble in water but soluble in carbon disulphide and has chemiluminescence. • Р 4 + 3 Na. OH + 3 H 2 O → PH 3 + Na. H 2 PO 2 • Р 4 + 3 О 2 → 2 Р 2 О 3 • Р 2 О 3 + О 2 → Р 2 О 5 • 3 Mg + 1/2 P 4 → Mg 3 P 2 • 2 P + 3 Cl 2 → 2 PCl 3

White phosphorus exposed to air glows in the darkness

Red phosphorus • It is obtained by heating white phosphorus at 573 K in an inert atmosphere for several days. When red phosphorus is heated under high pressure, a series of phases of black phosphorus are formed. • Red phosphorus possesses iron grey lustre. It is odourless, non-poisonous and insoluble in water as well as in carbon disulphide. • Chemically, red phosphorus is much less reactive than white phosphorus. It does not glow in the dark.

Black phosphorus • It has two forms α-black phosphorus and β-black phosphorus. • α-Black phosphorus is formed when red phosphorus is heated in a sealed tube at 803 K. It can be sublimed in air and has opaque monoclinic or rhombohedral crystals. It does not oxidise in air. • β-Black phosphorus is prepared by heating white phosphorus at 473 K under high pressure. It does not burn in air up to 673 K.

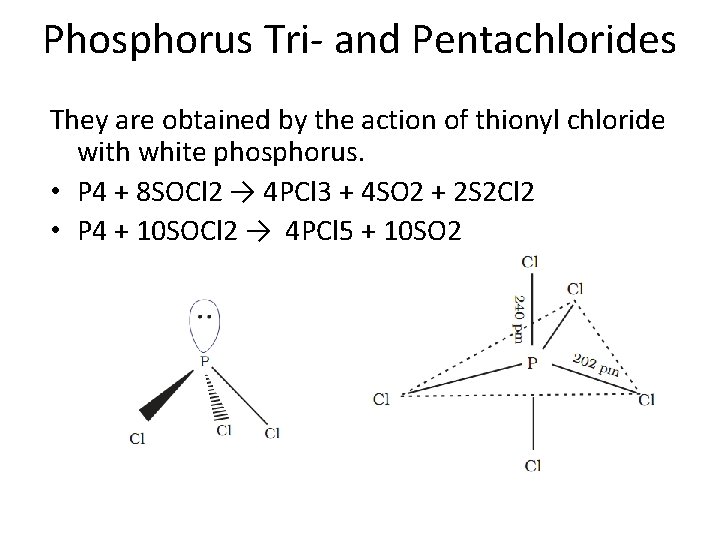
Phosphorus Tri- and Pentachlorides They are obtained by the action of thionyl chloride with white phosphorus. • P 4 + 8 SOCl 2 → 4 PCl 3 + 4 SO 2 + 2 S 2 Cl 2 • P 4 + 10 SOCl 2 → 4 PCl 5 + 10 SO 2

Reaction of Phosphorus Halides PCl 3 + 3 H 2 O → H 3 PO 3 + 3 HCl PCl 5 + H 2 O → POCl 3 + 2 HCl POCl 3 + 3 H 2 O → H 3 PO 4 + 3 HCl CH 3 COOH + PCl 5 → POCl 3 + HCl + CH 3 COCl H 2 SO 4 + PCl 5 → POCl 3 + HCl + SO 2(OH)Cl POCl 3 + 3 H 2 O → H 3 PO 4 + 3 HCl POCl 3 + 3 ROH → PO(OH)3 + 3 RCl 2 Ag + PCl 5 → 2 Ag. Cl + PCl 3 Sn + 2 PCl 5 → Sn. Cl 4 + 2 PCl 3

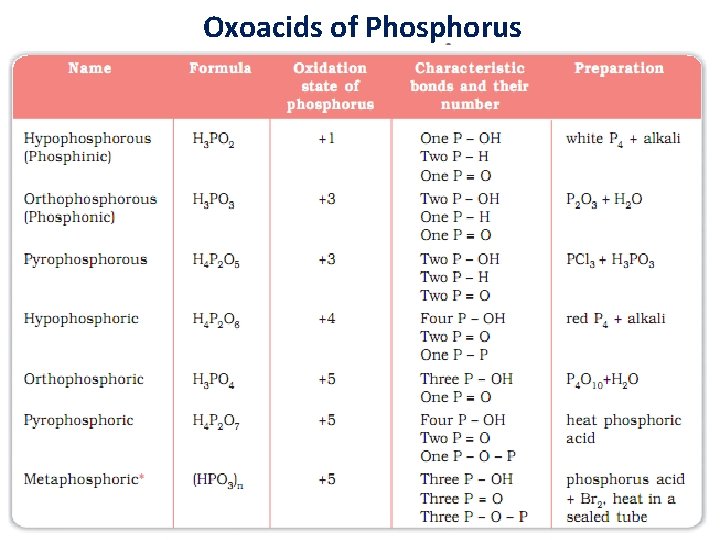
Oxoacids of Phosphorus

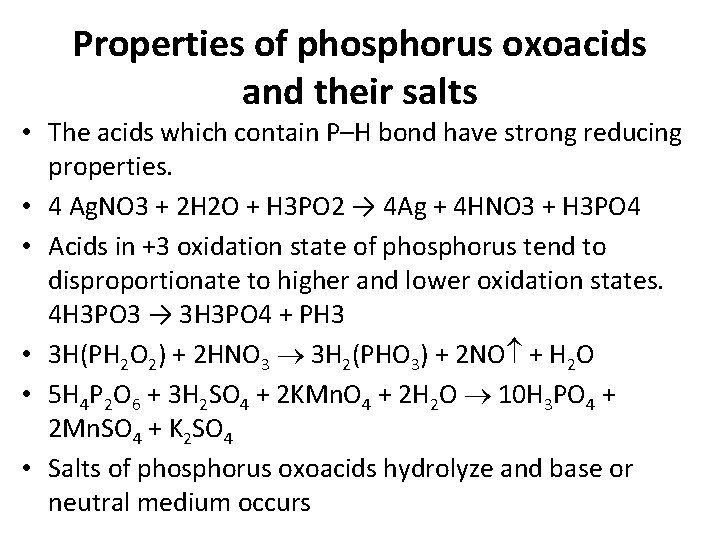
Properties of phosphorus oxoacids and their salts • The acids which contain P–H bond have strong reducing properties. • 4 Ag. NO 3 + 2 H 2 O + H 3 PO 2 → 4 Ag + 4 HNO 3 + H 3 PO 4 • Acids in +3 oxidation state of phosphorus tend to disproportionate to higher and lower oxidation states. 4 H 3 PO 3 → 3 H 3 PO 4 + PH 3 • 3 H(PH 2 O 2) + 2 HNO 3 3 H 2(PHO 3) + 2 NO + H 2 O • 5 H 4 P 2 O 6 + 3 H 2 SO 4 + 2 KMn. O 4 + 2 H 2 O 10 H 3 PO 4 + 2 Mn. SO 4 + K 2 SO 4 • Salts of phosphorus oxoacids hydrolyze and base or neutral medium occurs

Applications of nitrogen compounds • As a modified atmosphere, pure or mixed with carbon dioxide, to preserve the freshness of packaged or bulk foods • Nitrogen can be used instead of CO 2 to pressurize kegs of some beers, in particular, stouts and British ales, due to the smaller bubbles it produces, which make the dispensed beer smoother and headier • Liquid nitrogen is used in the cryopreservation of blood, reproductive cells (sperm and egg), and other biological samples. It is used in the clinical setting in cryotherapy to remove cysts and warts on the skin.

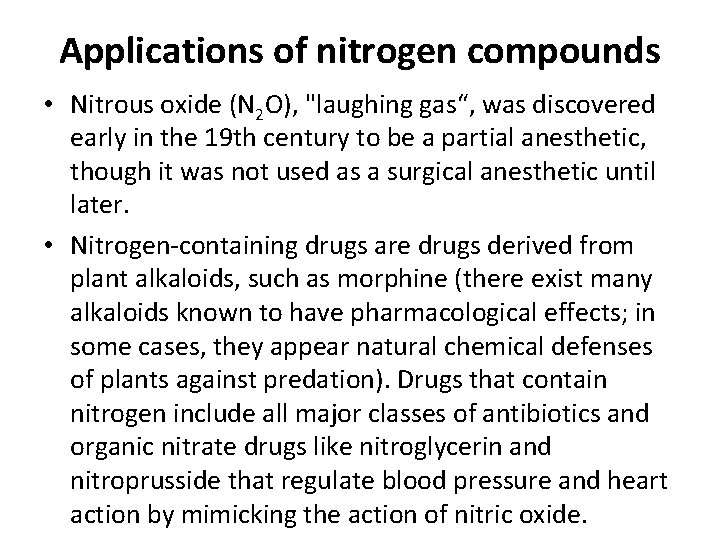
Applications of nitrogen compounds • Nitrous oxide (N 2 O), "laughing gas“, was discovered early in the 19 th century to be a partial anesthetic, though it was not used as a surgical anesthetic until later. • Nitrogen-containing drugs are drugs derived from plant alkaloids, such as morphine (there exist many alkaloids known to have pharmacological effects; in some cases, they appear natural chemical defenses of plants against predation). Drugs that contain nitrogen include all major classes of antibiotics and organic nitrate drugs like nitroglycerin and nitroprusside that regulate blood pressure and heart action by mimicking the action of nitric oxide.

Applications of phosphorous compounds • White phosphorus, called "WP" (slang term "Willie Peter") is used in military applications as incendiary bombs, for smoke-screening as smoke pots and smoke bombs, and in tracer ammunition. • The spontaneous combustion of phosphine is technically used in Holme’s signals. Containers containing calcium carbide and calcium phosphide are pierced and thrown in the sea when the gases evolved burn and serve as a signal. • Phosphine is also used in smoke screens.