Lecture 13 CATALYSIS OXIDATION OF ALKENES Copyright The

- Slides: 23

Lecture 13 CATALYSIS – OXIDATION OF ALKENES Copyright ©The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

Acetaldehyde – of commercial importance - oxidation to acetic acid - dehydration to acetic anhydride Previous synthetic route: Today, Wacker process is used producing 4 million tons of acetaldehyde from ethylene. ETHYLENE OXIDATION TO ACETALDEHYDE: WACKER PROCESS

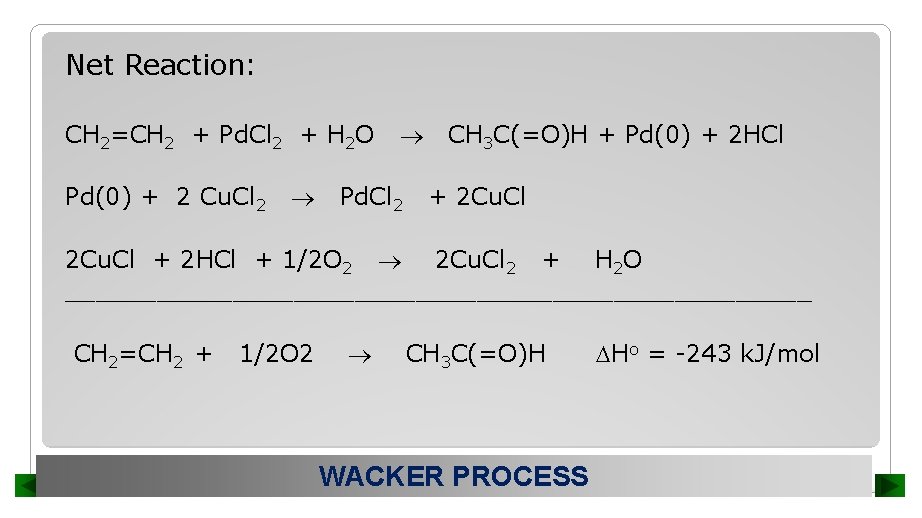
Net Reaction: CH 2=CH 2 + Pd. Cl 2 + H 2 O Pd(0) + 2 Cu. Cl 2 Pd. Cl 2 CH 3 C(=O)H + Pd(0) + 2 HCl + 2 Cu. Cl + 2 HCl + 1/2 O 2 2 Cu. Cl 2 + H 2 O _________________________ CH 2=CH 2 + 1/2 O 2 CH 3 C(=O)H WACKER PROCESS Ho = -243 k. J/mol

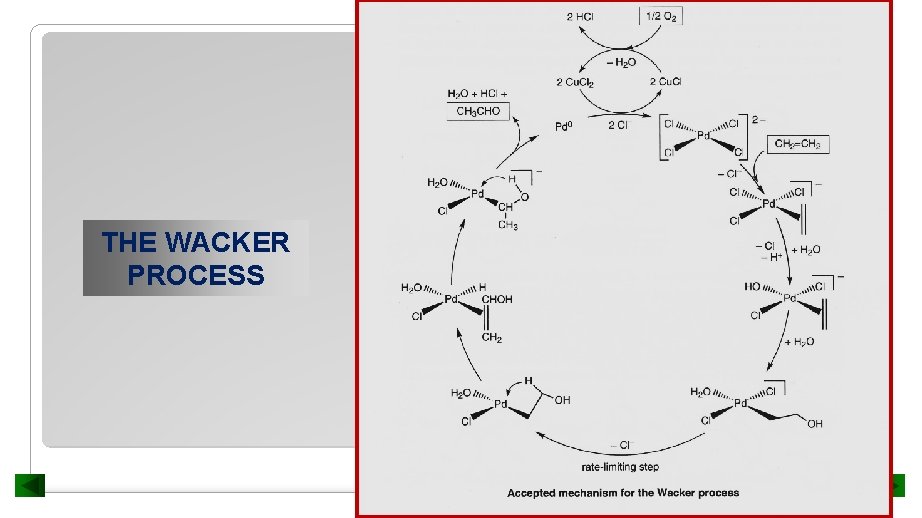
THE WACKER PROCESS

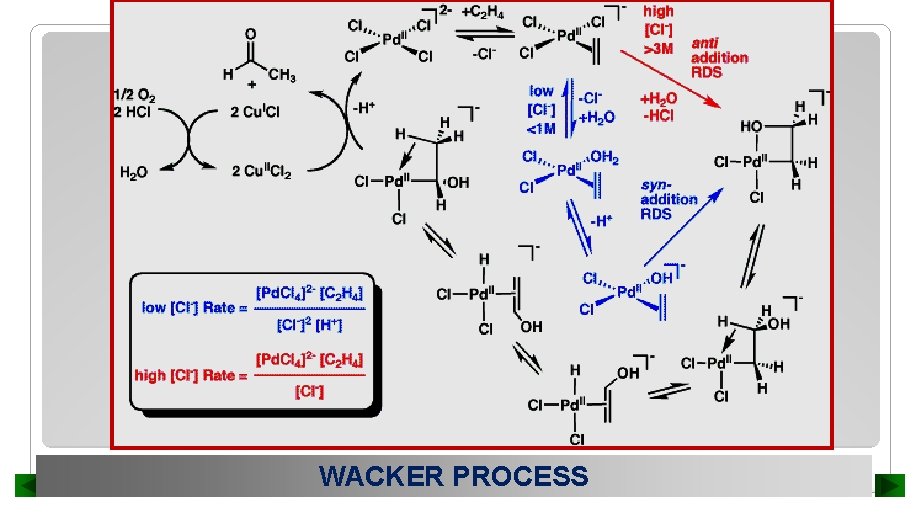
WACKER PROCESS

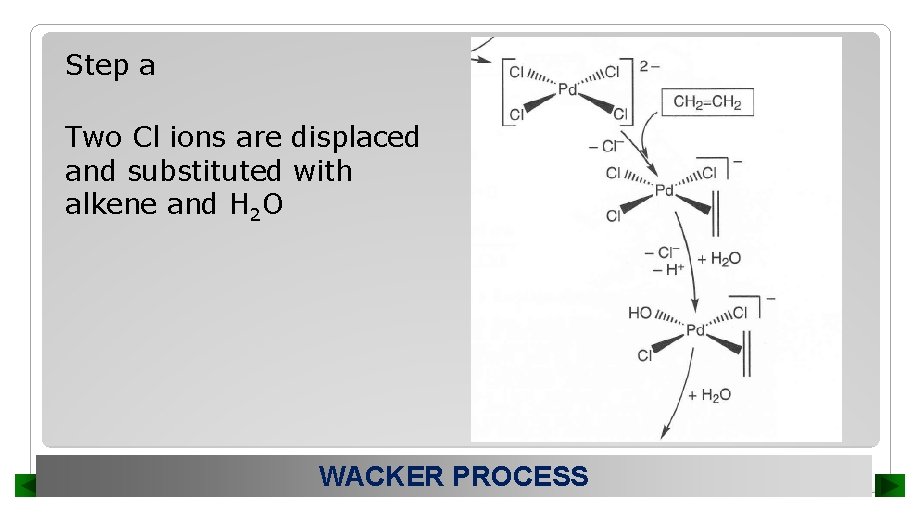
Step a Two Cl ions are displaced and substituted with alkene and H 2 O WACKER PROCESS

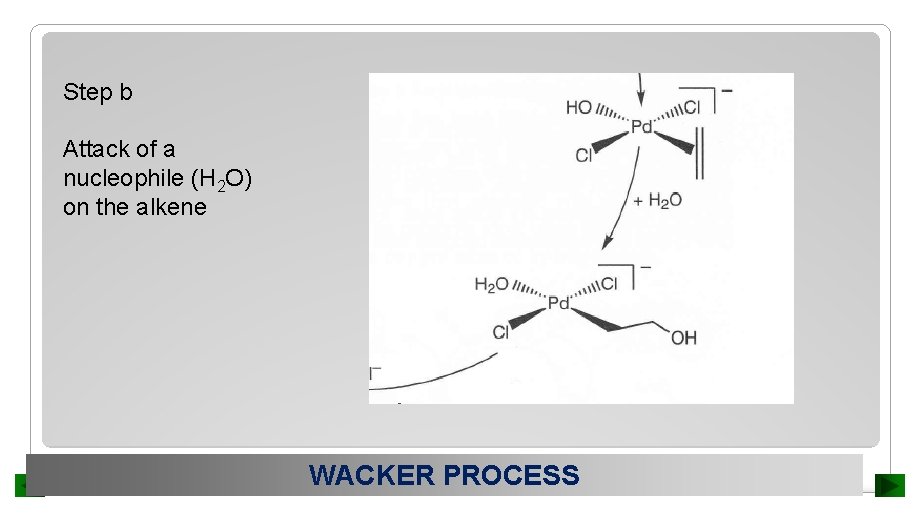
Step b Attack of a nucleophile (H 2 O) on the alkene WACKER PROCESS

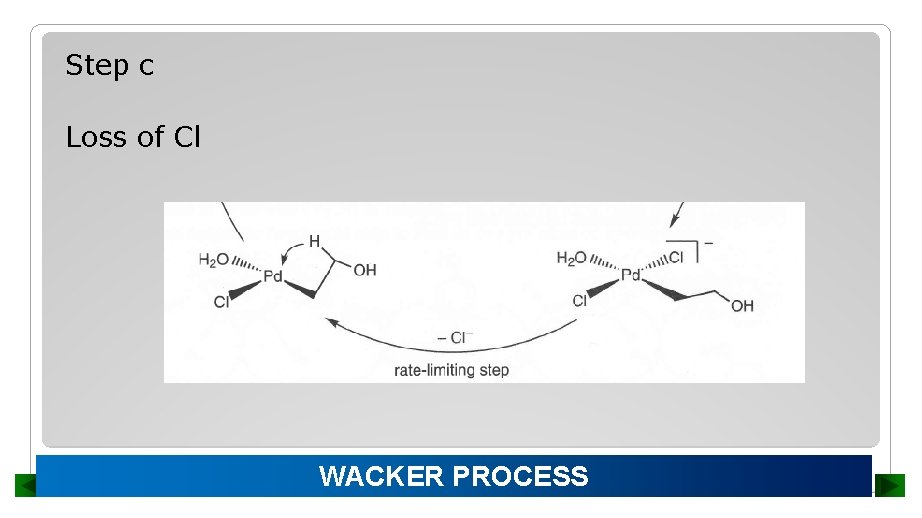
Step c Loss of Cl WACKER PROCESS

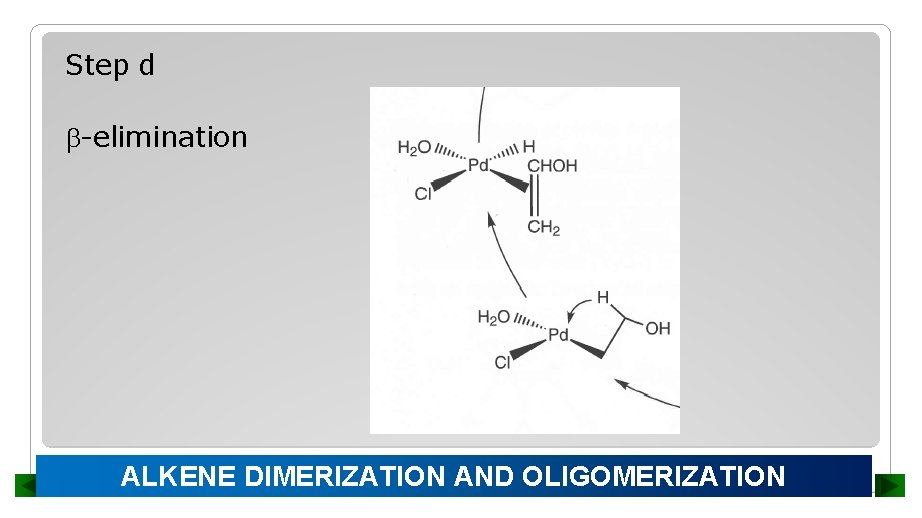
Step d -elimination ALKENE DIMERIZATION AND OLIGOMERIZATION

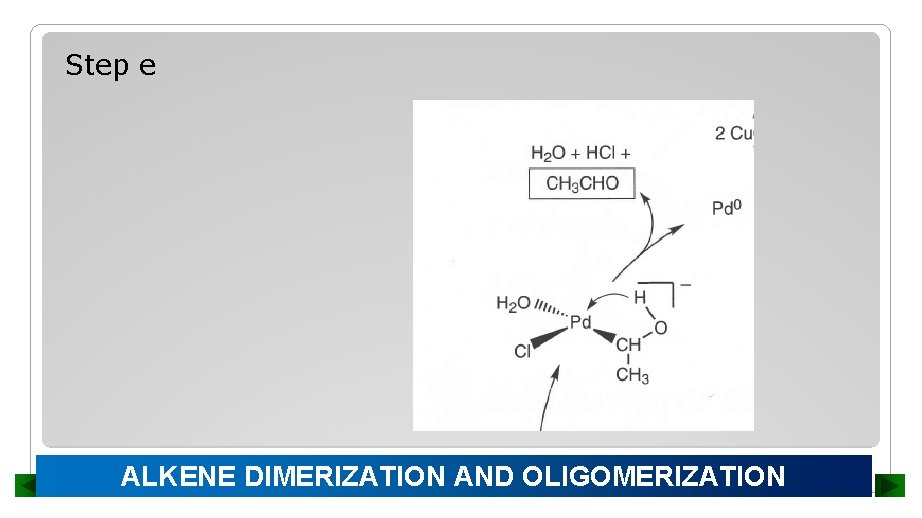
Step e ALKENE DIMERIZATION AND OLIGOMERIZATION

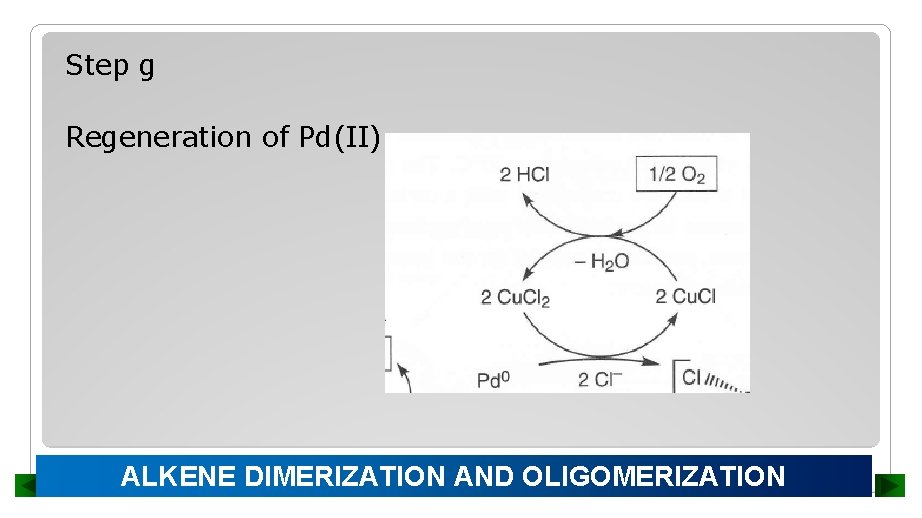
Step g Regeneration of Pd(II) ALKENE DIMERIZATION AND OLIGOMERIZATION

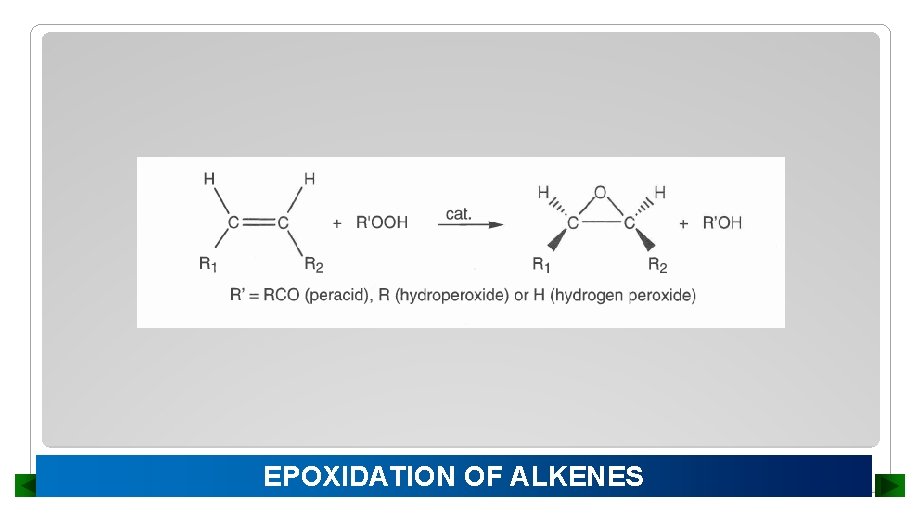
EPOXIDATION OF ALKENES

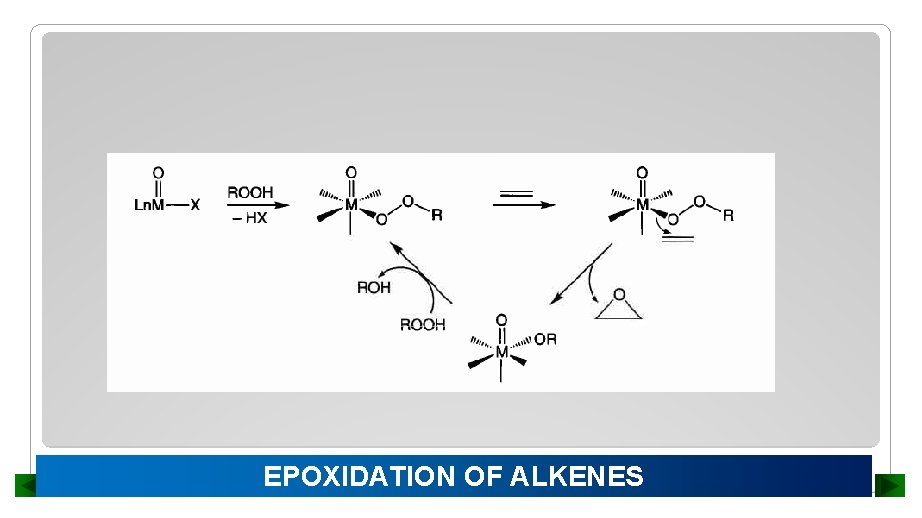
EPOXIDATION OF ALKENES

Lecture 13 CATALYSIS – CARBONYLATION AND CARBOXYLATION REACTIONS Copyright ©The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

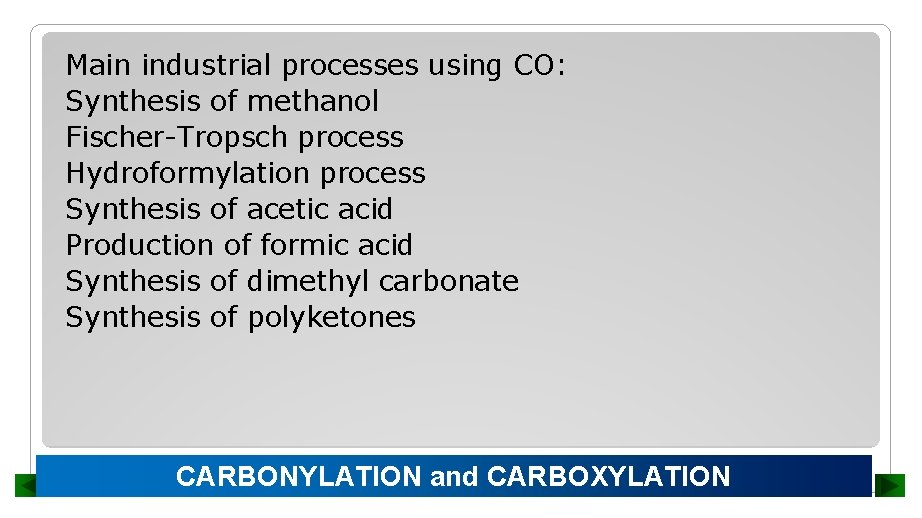
Main industrial processes using CO: Synthesis of methanol Fischer-Tropsch process Hydroformylation process Synthesis of acetic acid Production of formic acid Synthesis of dimethyl carbonate Synthesis of polyketones CARBONYLATION and CARBOXYLATION

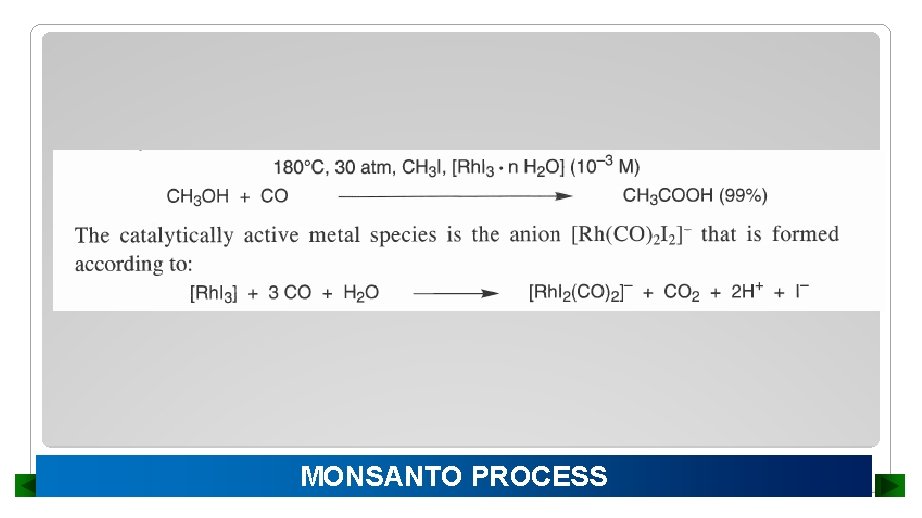
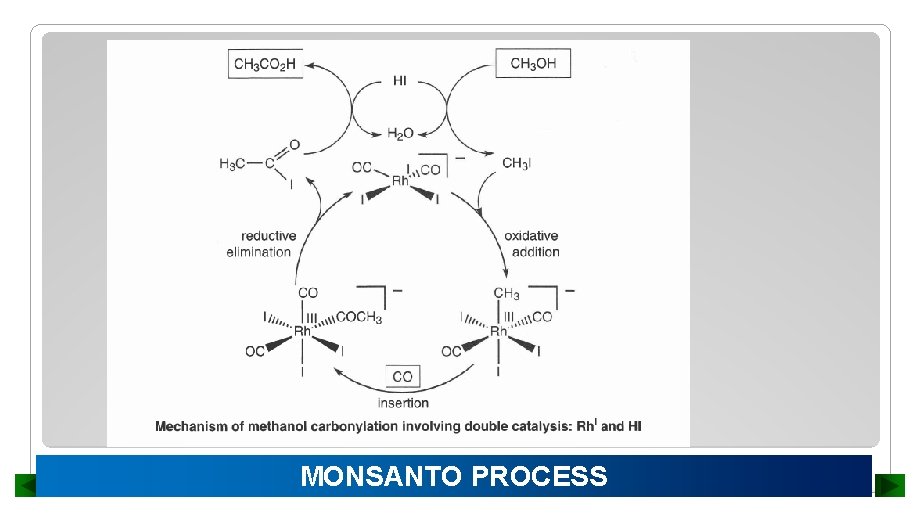
MONSANTO PROCESS

MONSANTO PROCESS

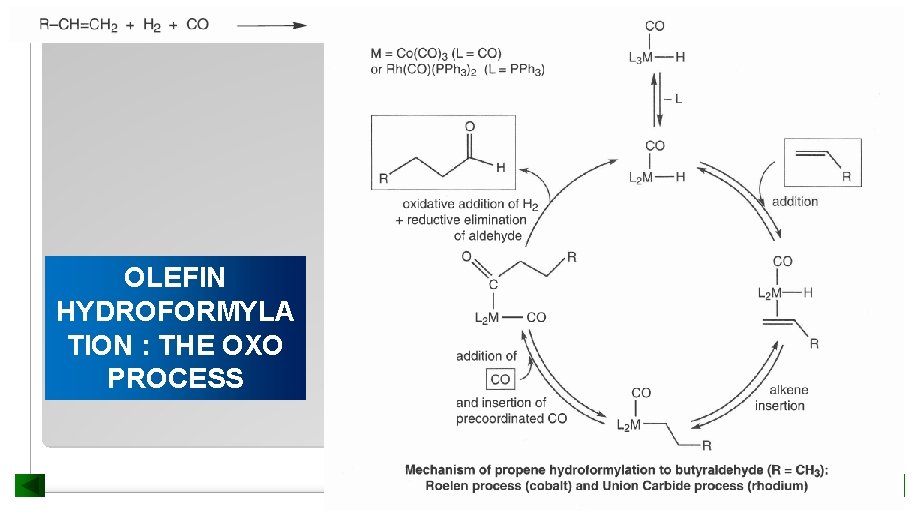
OLEFIN HYDROFORMYLA TION : THE OXO PROCESS

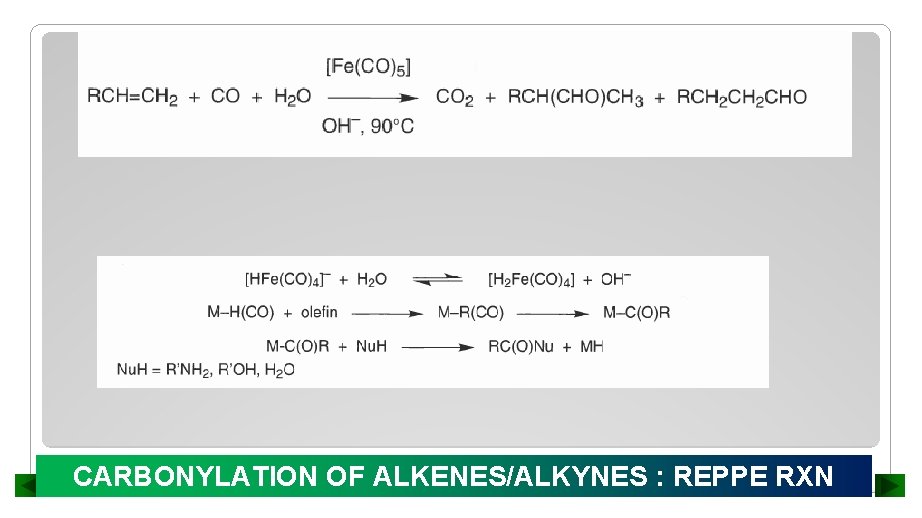
CARBONYLATION OF ALKENES/ALKYNES : REPPE RXN

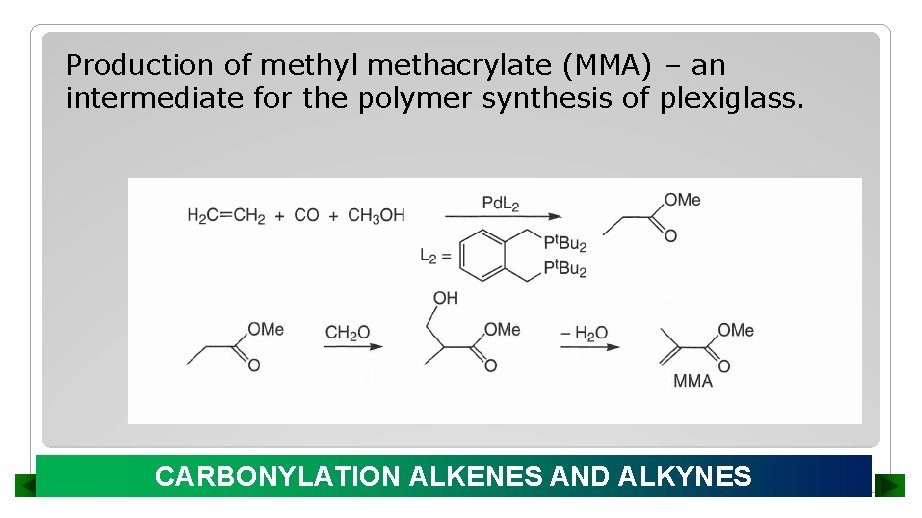
Production of methyl methacrylate (MMA) – an intermediate for the polymer synthesis of plexiglass. CARBONYLATION ALKENES AND ALKYNES

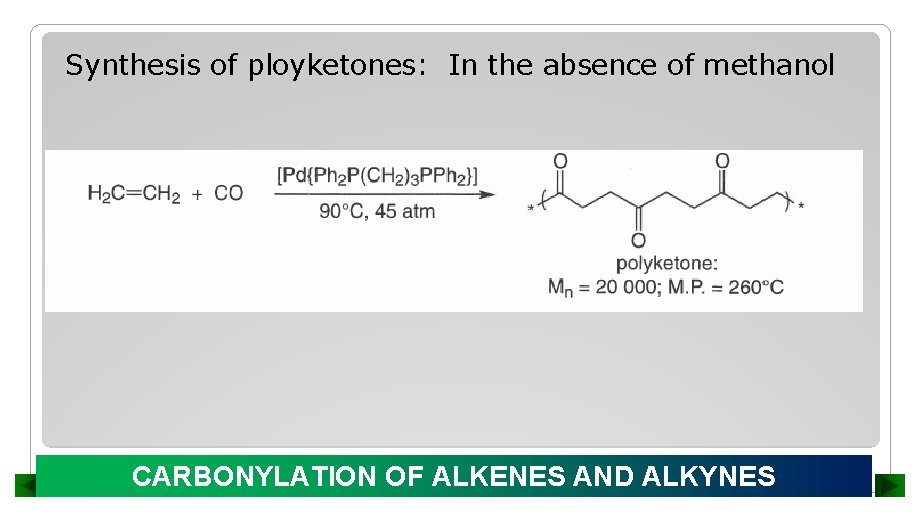
Synthesis of ployketones: In the absence of methanol CARBONYLATION OF ALKENES AND ALKYNES

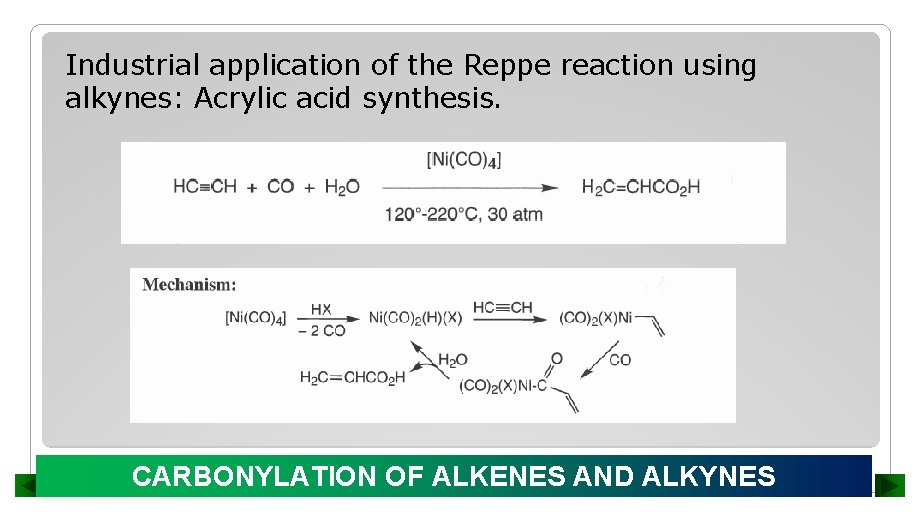
Industrial application of the Reppe reaction using alkynes: Acrylic acid synthesis. CARBONYLATION OF ALKENES AND ALKYNES

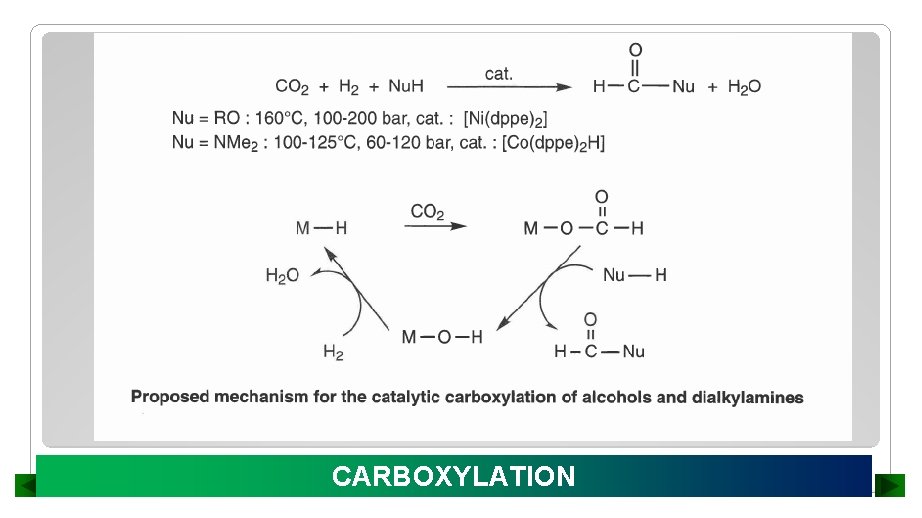
CARBOXYLATION
 Erzeng xue
Erzeng xue Oxide thickness color chart
Oxide thickness color chart Km in enzyme kinetics
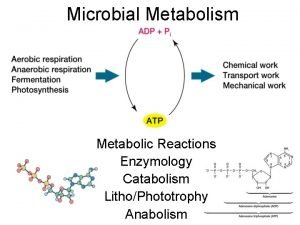
Km in enzyme kinetics Catabolism
Catabolism Honh2 dissociation equation
Honh2 dissociation equation Specific acid base catalysis
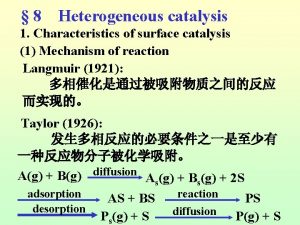
Specific acid base catalysis Langmuir-hinshelwood mechanism heterogeneous catalysis
Langmuir-hinshelwood mechanism heterogeneous catalysis Catalysis by approximation
Catalysis by approximation Specific acid base catalysis
Specific acid base catalysis Energy catalysis and biosynthesis
Energy catalysis and biosynthesis What is covalent catalysis
What is covalent catalysis Specific acid base catalysis
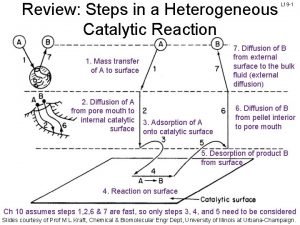
Specific acid base catalysis 7 steps of heterogeneous catalysis
7 steps of heterogeneous catalysis Site:slidetodoc.com
Site:slidetodoc.com What is covalent catalysis
What is covalent catalysis 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Diol formation from alkene
Diol formation from alkene Ozonolysis reaction
Ozonolysis reaction Rco3h reaction
Rco3h reaction Hybridization of alkenes
Hybridization of alkenes Chemsheets as 1139 answers
Chemsheets as 1139 answers Alkenes introduction
Alkenes introduction Physical properties of alkenes
Physical properties of alkenes Bromine test for unsaturation
Bromine test for unsaturation