LayerStructured Transition Metal Oxides as Cathodes for KIon



- Slides: 16

Layer-Structured Transition Metal Oxides as Cathodes for K-Ion Batteries Dec. 01 2017 04: 45 PM Abstract #: ES 04. 23. 057 Haegyeom Kim, Jae Chul Kim, Dong-Hwa Seo, Shou-Hang Bo, Deok-Hwang Kwon, Tan Shi, and Gerbrand Ceder* Post-doc Fellow in Ceder group Materials Sciences Division Lawrence Berkeley National Laboratory Download these slides at http: //ceder. berkeley. edu H. Kim et al. Adv. Energy Mater. 1700098 (2017) H. Kim et al. Adv. Mater. 1702480 (2017) 1

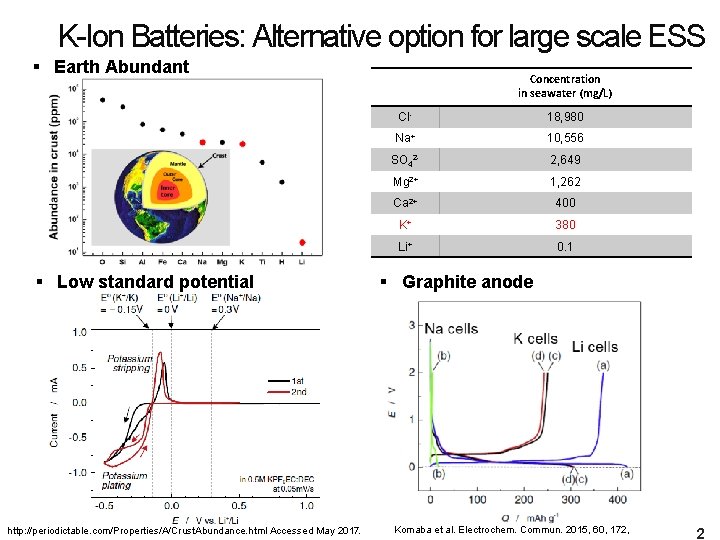
K-Ion Batteries: Alternative option for large scale ESS § Earth Abundant § Low standard potential http: //periodictable. com/Properties/A/Crust. Abundance. html Accessed May 2017. Concentration in seawater (mg/L) Cl- 18, 980 Na+ 10, 556 SO 42 - 2, 649 Mg 2+ 1, 262 Ca 2+ 400 K+ 380 Li+ 0. 1 § Graphite anode Komaba et al. Electrochem. Commun. 2015, 60, 172, 2

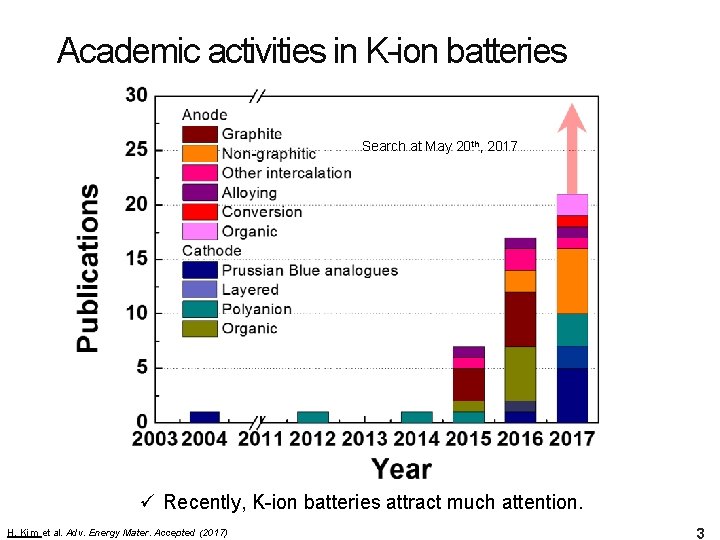
Academic activities in K-ion batteries Search at May 20 th, 2017 ü Recently, K-ion batteries attract much attention. H. Kim et al. Adv. Energy Mater. Accepted (2017) 3

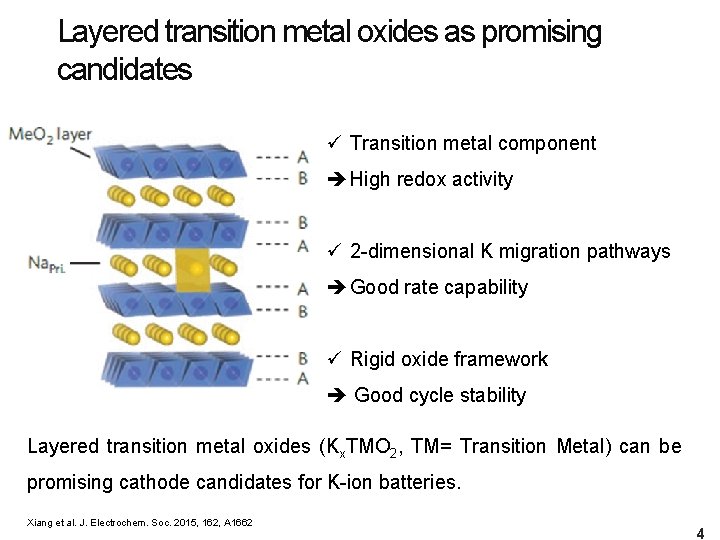
Layered transition metal oxides as promising candidates ü Transition metal component High redox activity ü 2 -dimensional K migration pathways Good rate capability ü Rigid oxide framework Good cycle stability Layered transition metal oxides (Kx. TMO 2, TM= Transition Metal) can be promising cathode candidates for K-ion batteries. Xiang et al. J. Electrochem. Soc. 2015, 162, A 1662 4

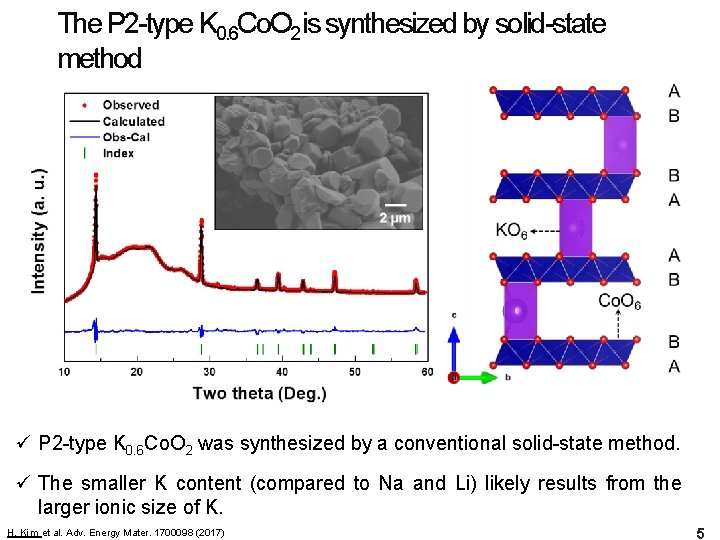
The P 2 -type K 0. 6 Co. O 2 is synthesized by solid-state method ü P 2 -type K 0. 6 Co. O 2 was synthesized by a conventional solid-state method. ü The smaller K content (compared to Na and Li) likely results from the larger ionic size of K. H. Kim et al. Adv. Energy Mater. 1700098 (2017) 5

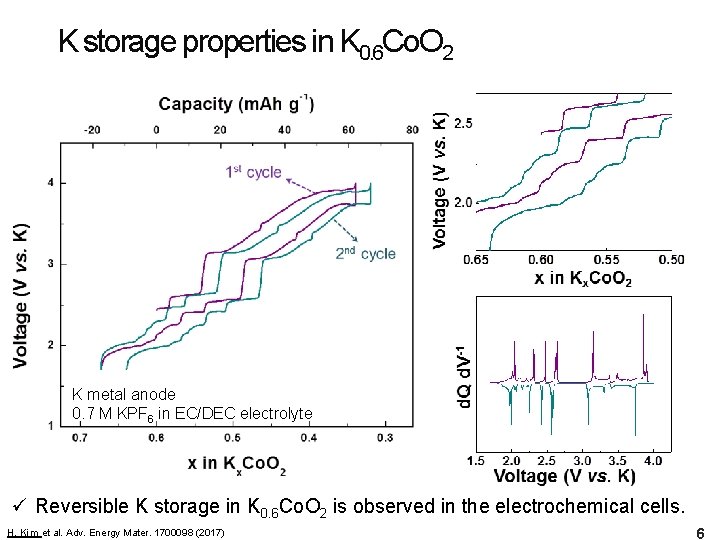
K storage properties in K 0. 6 Co. O 2 K metal anode 0. 7 M KPF 6 in EC/DEC electrolyte ü Reversible K storage in K 0. 6 Co. O 2 is observed in the electrochemical cells. H. Kim et al. Adv. Energy Mater. 1700098 (2017) 6

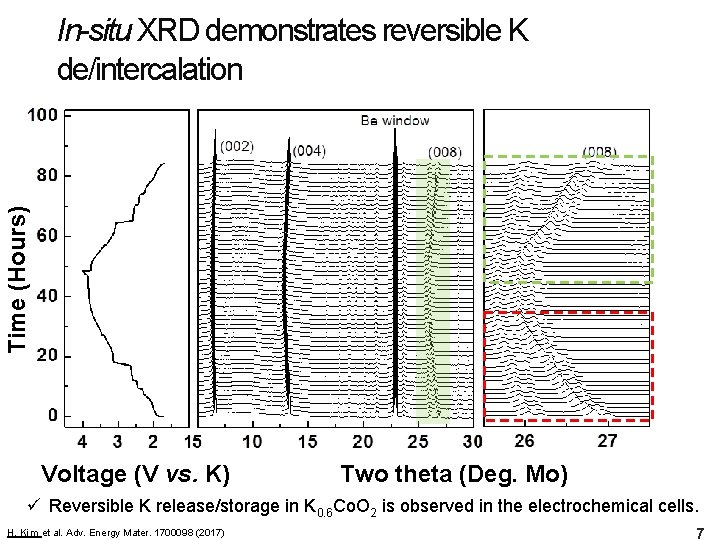
Time (Hours) In-situ XRD demonstrates reversible K de/intercalation Voltage (V vs. K) Two theta (Deg. Mo) ü Reversible K release/storage in K 0. 6 Co. O 2 is observed in the electrochemical cells. H. Kim et al. Adv. Energy Mater. 1700098 (2017) 7

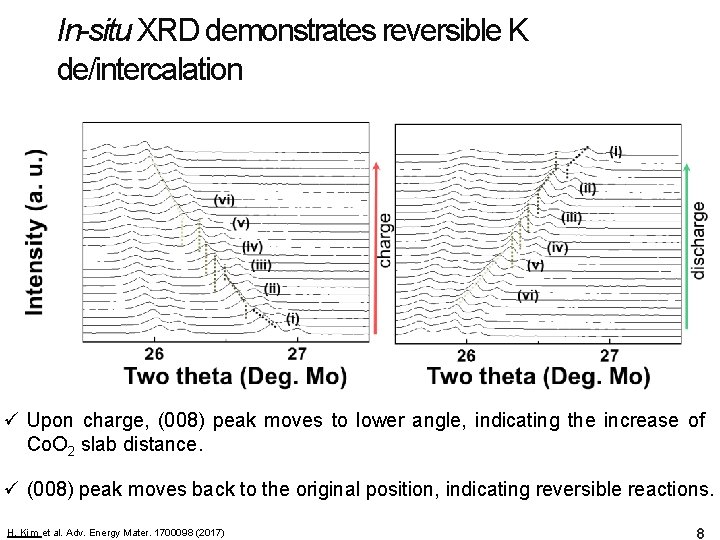
In-situ XRD demonstrates reversible K de/intercalation ü Upon charge, (008) peak moves to lower angle, indicating the increase of Co. O 2 slab distance. ü (008) peak moves back to the original position, indicating reversible reactions. H. Kim et al. Adv. Energy Mater. 1700098 (2017) 8

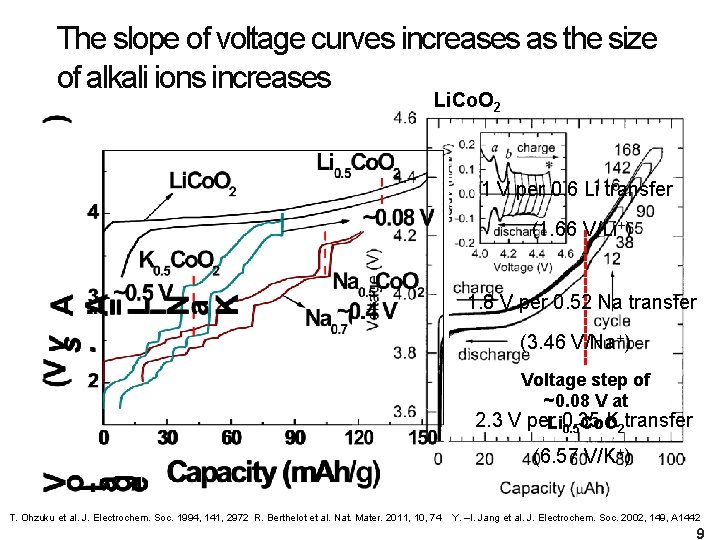
The slope of voltage curves increases as the size of alkali ions increases Li. Co. O 2 1 V per 0. 6 Li transfer (1. 66 V/Li+) 1. 8 V per 0. 52 Na transfer (3. 46 V/Na+) Voltage step of ~0. 08 V at 2. 3 V per. Li 0. 35 K transfer 0. 5 Co. O 2 (6. 57 V/K+) T. Ohzuku et al. J. Electrochem. Soc. 1994, 141, 2972 R. Berthelot et al. Nat. Mater. 2011, 10, 74 Y. –I. Jang et al. J. Electrochem. Soc. 2002, 149, A 1442 9

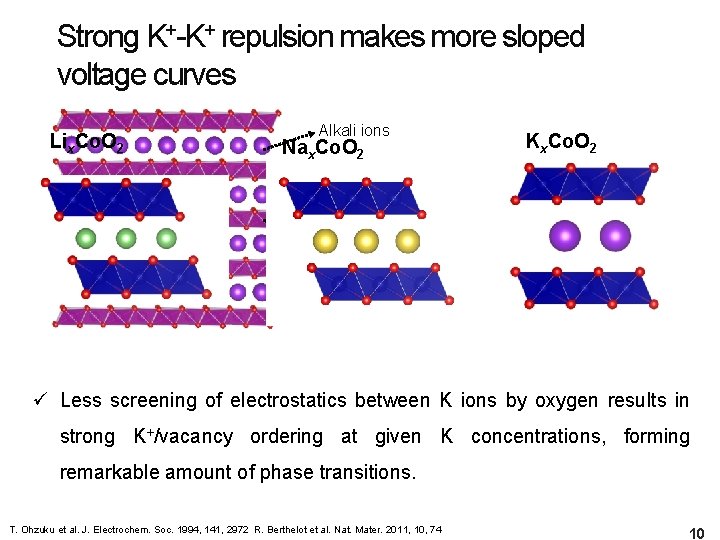
Strong K+-K+ repulsion makes more sloped voltage curves Lix. Co. O 2 Alkali ions Nax. Co. O 2 Kx. Co. O 2 Transition metals Oxygen Ionic size of alkali ions + (0. 76 Å) +(1. 38 Å) Na+ (1. 02 Å) K ions by K ü Li Less screening of electrostatics between oxygen results in strong K+/vacancy ordering at given K concentrations, forming Slab distance for alkali ions in remarkable amount of phase transitions. Lix. Co. O 2 (2. 64 Å) Nax. Co. O 2 (3. 43 Å) Kx. Co. O 2(4. 25 Å) T. Ohzuku et al. J. Electrochem. Soc. 1994, 141, 2972 R. Berthelot et al. Nat. Mater. 2011, 10, 74 10

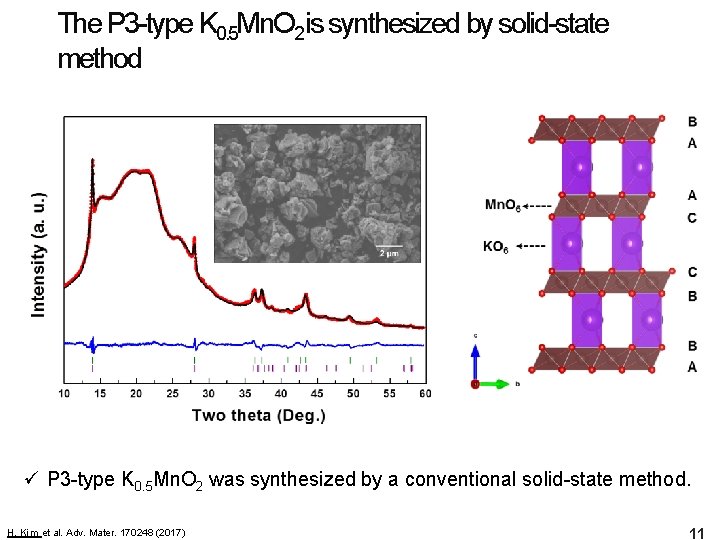
The P 3 -type K 0. 5 Mn. O 2 is synthesized by solid-state method ü P 3 -type K 0. 5 Mn. O 2 was synthesized by a conventional solid-state method. H. Kim et al. Adv. Mater. 170248 (2017) 11

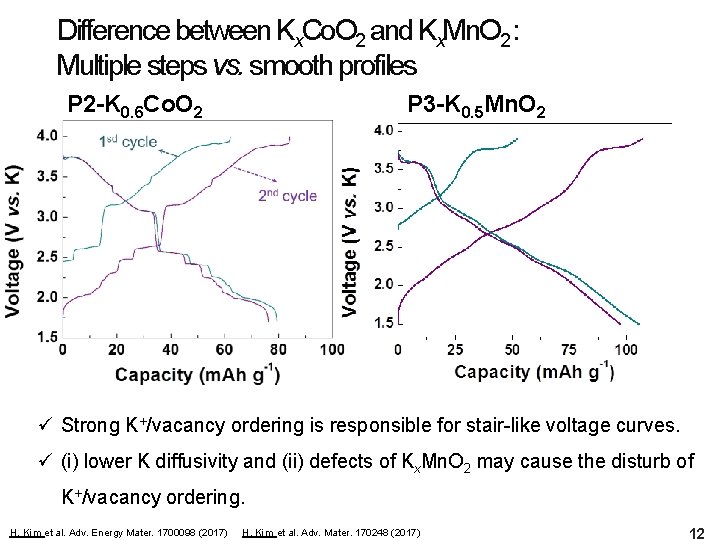
Difference between Kx. Co. O 2 and Kx. Mn. O 2 : Multiple steps vs. smooth profiles P 2 -K 0. 6 Co. O 2 P 3 -K 0. 5 Mn. O 2 ü Strong K+/vacancy ordering is responsible for stair-like voltage curves. ü (i) lower K diffusivity and (ii) defects of Kx. Mn. O 2 may cause the disturb of K+/vacancy ordering. H. Kim et al. Adv. Energy Mater. 1700098 (2017) H. Kim et al. Adv. Mater. 170248 (2017) 12

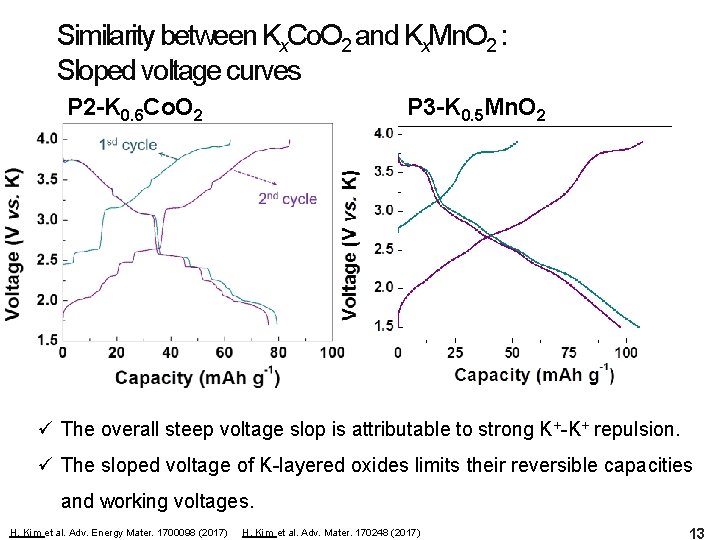
Similarity between Kx. Co. O 2 and Kx. Mn. O 2 : Sloped voltage curves P 2 -K 0. 6 Co. O 2 P 3 -K 0. 5 Mn. O 2 ü The overall steep voltage slop is attributable to strong K+-K+ repulsion. ü The sloped voltage of K-layered oxides limits their reversible capacities and working voltages. H. Kim et al. Adv. Energy Mater. 1700098 (2017) H. Kim et al. Adv. Mater. 170248 (2017) 13

Summary ü New P 2 -type K 0. 6 Co. O 2 and P 3 -type K 0. 5 Mn. O 2 cathodes are proposed for KIBs. ü P 2 -type K 0. 6 Co. O 2 shows stair-like voltage curves, but P 3 type K 0. 5 Mn. O 2 has smooth voltage profiles. ü However, K-layered oxide frameworks result in low specific energy (low capacity and low voltage). ü Our preliminary study demonstrates the polyanionic frameworks can be promising candidates for high-energy cathode for KIBs. 14

Acknowledgement Gerbrand Ceder, Chancellor’s Professor Department of Materials Science and Engineering Dr. Jae Chul Kim Dr. Dong-Hwa Seo The Laboratory Directed Research Prof. Shouhang Bo and Development Program of Lawrence Berkeley National Laboratory under U. S. Department of Energy (DE-AC 02 -05 CH 11231) Dr. Deok-Hwang Kwon Mr. Tan Shi 15

Thank you Download these slides at http: //ceder. berkeley. edu H. Kim et al. “K‐Ion Batteries Based on a P 2‐Type K 0. 6 Co. O 2 Cathode. ” Adv. Energy Mater. 1700098 (2017) H. Kim et al. “Investigation of Potassium Storage in Layered P 3‐Type K 0. 5 Mn. O 2 Cathode. ” Adv. Mater. 1702480 (2017) 16