Law of Conservation of Matter The Law of





- Slides: 26


Law of Conservation of Matter • The Law of Conservation of Matter states that matter can neither be created nor destroyed. • Because the same atoms are present in a reaction at the beginning and at the end, the amount of matter in a system does not change Antoine Lavoisier, (1788)

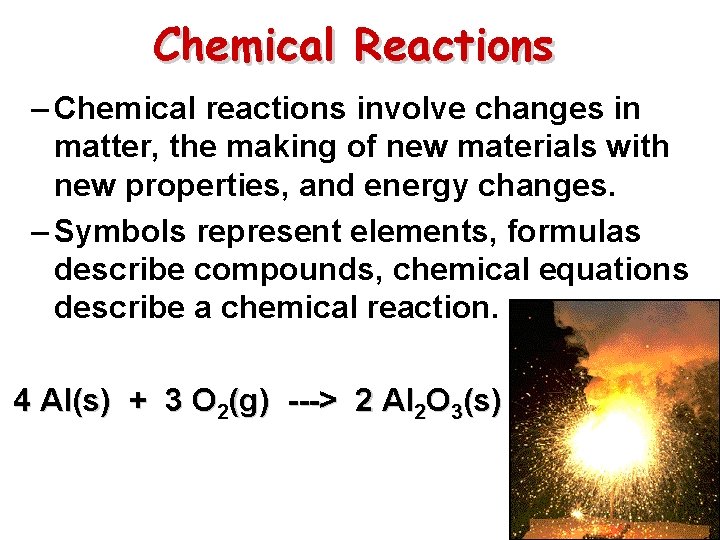
Chemical Reactions – Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. – Symbols represent elements, formulas describe compounds, chemical equations describe a chemical reaction. 4 Al(s) + 3 O 2(g) ---> 2 Al 2 O 3(s)

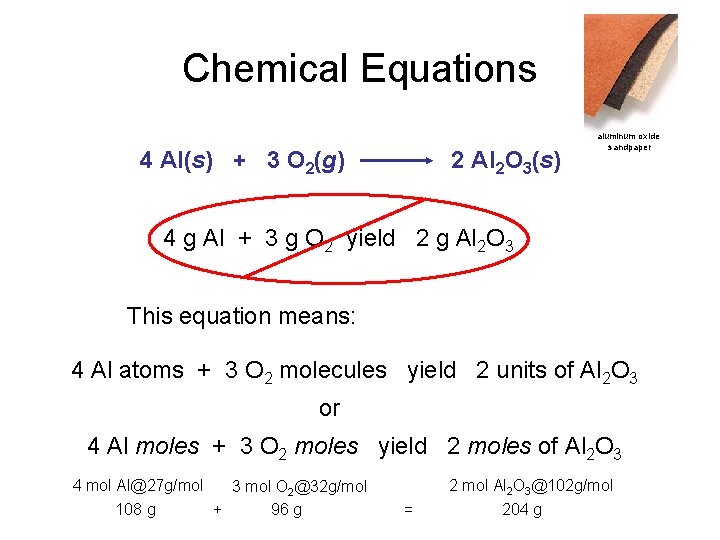
Chemical Equations 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) aluminum oxide sandpaper 4 g Al + 3 g O 2 yield 2 g Al 2 O 3 This equation means: 4 Al atoms + 3 O 2 molecules yield 2 units of Al 2 O 3 or 4 Al moles + 3 O 2 moles yield 2 moles of Al 2 O 3 4 mol Al@27 g/mol 3 mol O 2@32 g/mol 108 g + 96 g 2 mol Al 2 O 3@102 g/mol = 204 g

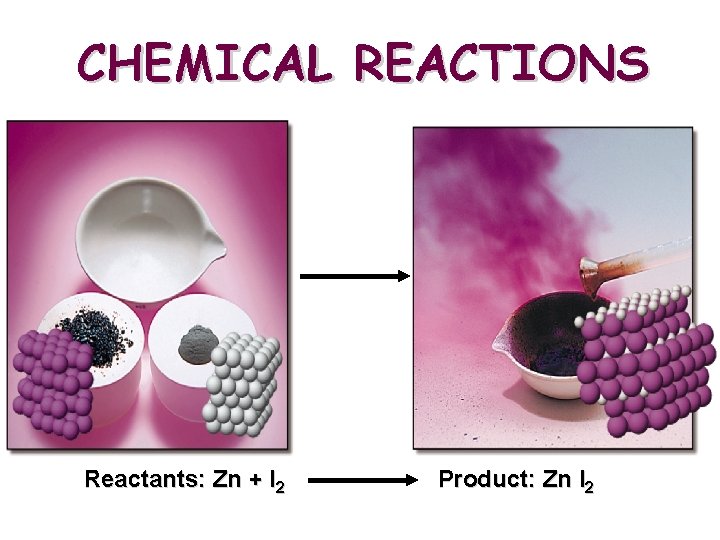
CHEMICAL REACTIONS Reactants: Zn + I 2 Product: Zn I 2

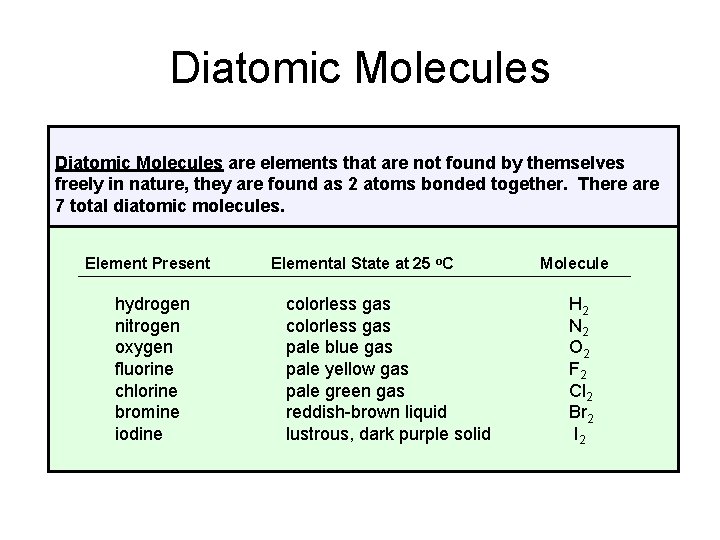
Diatomic Molecules are elements that are not found by themselves freely in nature, they are found as 2 atoms bonded together. There are 7 total diatomic molecules. Element Present hydrogen nitrogen oxygen fluorine chlorine bromine iodine Elemental State at 25 o. C colorless gas pale blue gas pale yellow gas pale green gas reddish-brown liquid lustrous, dark purple solid Molecule H 2 N 2 O 2 F 2 Cl 2 Br 2 I 2


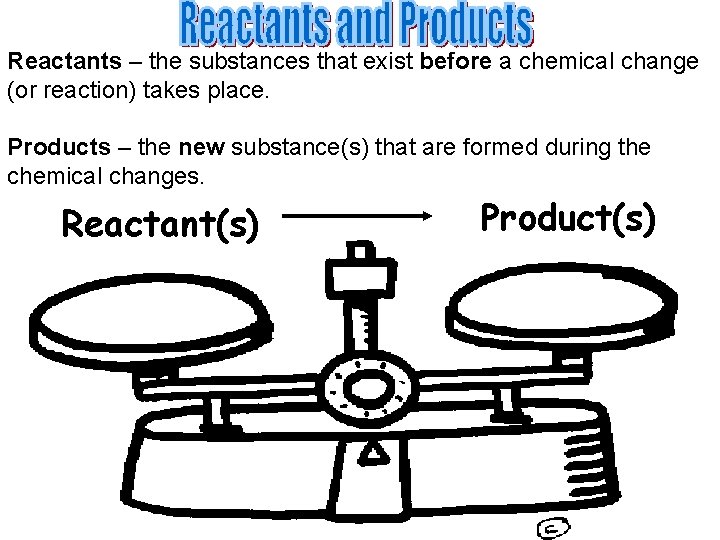
Reactants – the substances that exist before a chemical change (or reaction) takes place. Products – the new substance(s) that are formed during the chemical changes. Reactant(s) Product(s)

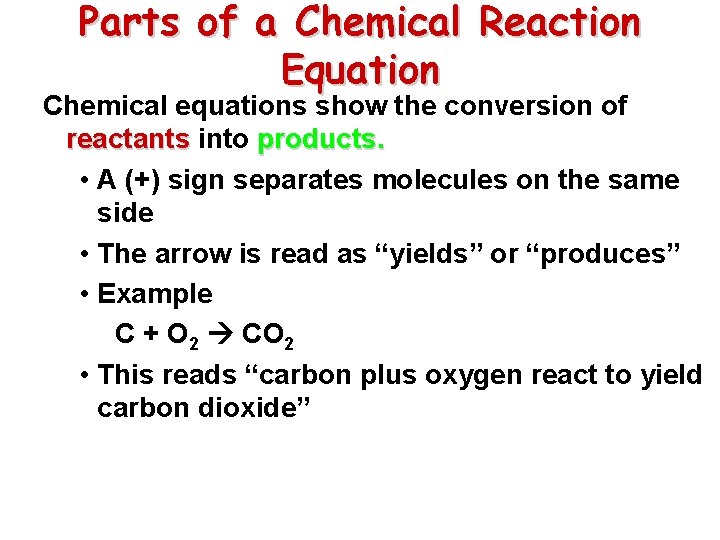
Parts of a Chemical Reaction Equation Chemical equations show the conversion of reactants into products. • A (+) sign separates molecules on the same side • The arrow is read as “yields” or “produces” • Example C + O 2 CO 2 • This reads “carbon plus oxygen react to yield carbon dioxide”

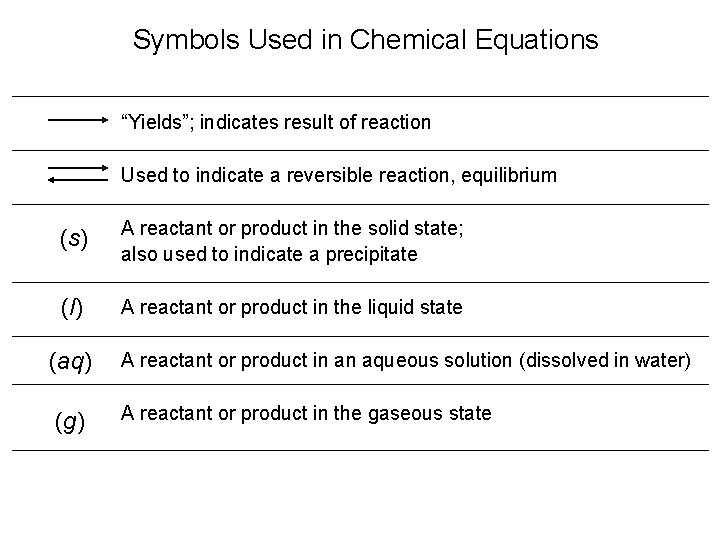
Symbols Used in Chemical Equations “Yields”; indicates result of reaction Used to indicate a reversible reaction, equilibrium (s) A reactant or product in the solid state; also used to indicate a precipitate (l) A reactant or product in the liquid state (aq) A reactant or product in an aqueous solution (dissolved in water) (g) A reactant or product in the gaseous state

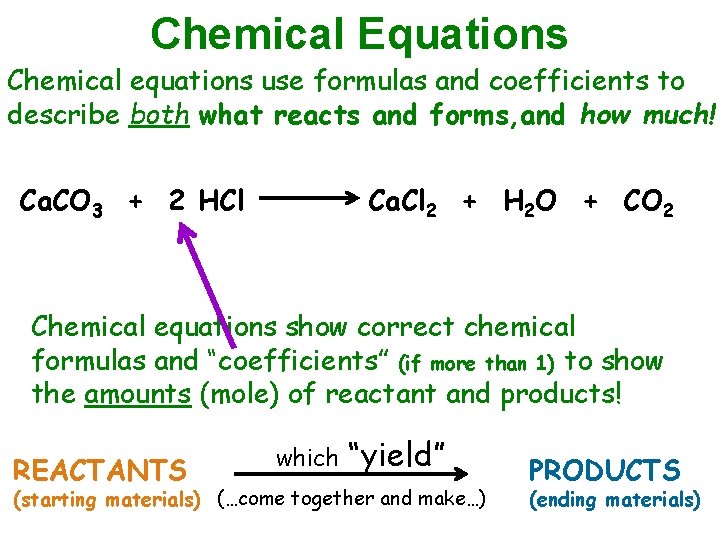
Chemical Equations Chemical equations use formulas and coefficients to describe both what reacts and forms, and how much! Ca. CO 3 + 2 HCl Ca. Cl 2 + H 2 O + CO 2 Chemical equations show correct chemical formulas and “coefficients” (if more than 1) to show the amounts (mole) of reactant and products! REACTANTS which “yield” (starting materials) (…come together and make…) PRODUCTS (ending materials)

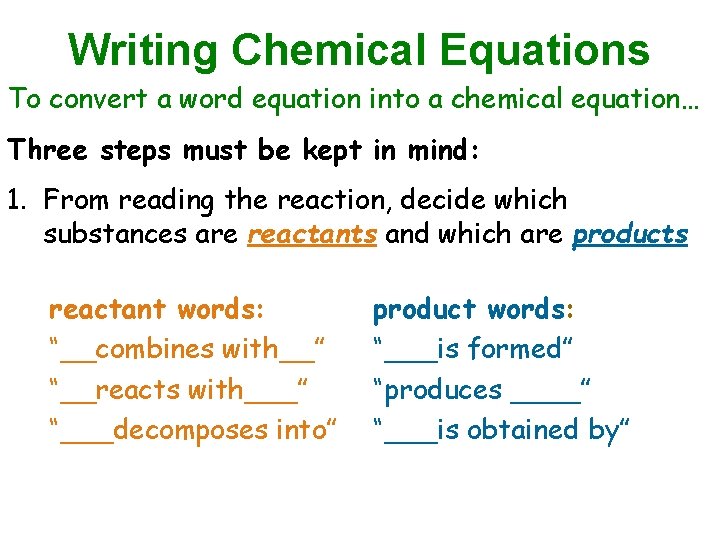
Writing Chemical Equations To convert a word equation into a chemical equation… Three steps must be kept in mind: 1. From reading the reaction, decide which substances are reactants and which are products reactant words: “__combines with__” “__reacts with___” “___decomposes into” product words: “___is formed” “produces ____” “___is obtained by”

Writing Chemical Equations 2. Be sure the FORMULAS of all substances are written CORRECTLY! Be sure you know how to use your oxidation table of ions and their charges! (If the wrong formula is used for ANY reactant or product, the chemical equation will never be correct!)

Writing Chemical Equations 3. Write the reactant formulas to the left of the “yields” sign, and… 4. write the product formulas to the right of the “yields” sign. 5. (It doesn’t matter what order the reactants are written, so long as they are all on the left side of the “yields” sign…) 6. (The same is true for products, but on the right side of the “yields” sign. ) 7. (Note: the reaction written with just the correct formulas is called a “skeleton equation”).

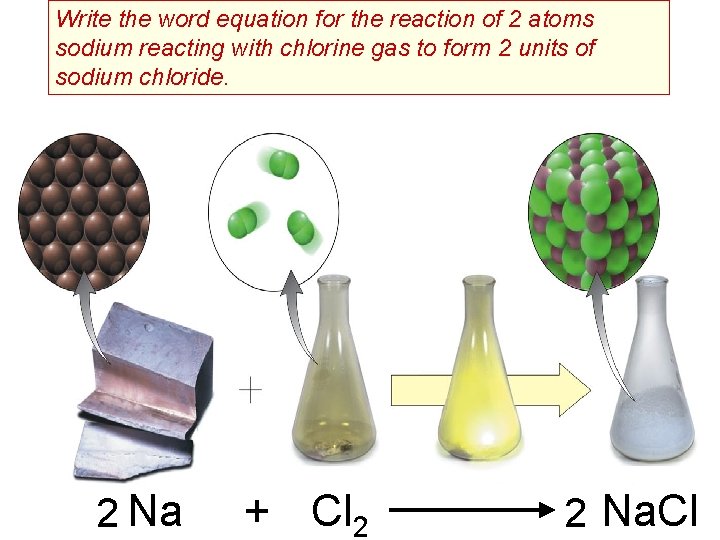
Write the word equation for the reaction of 2 atoms sodium reacting with chlorine gas to form 2 units of sodium chloride. 2 Na + Cl 2 2 Na. Cl

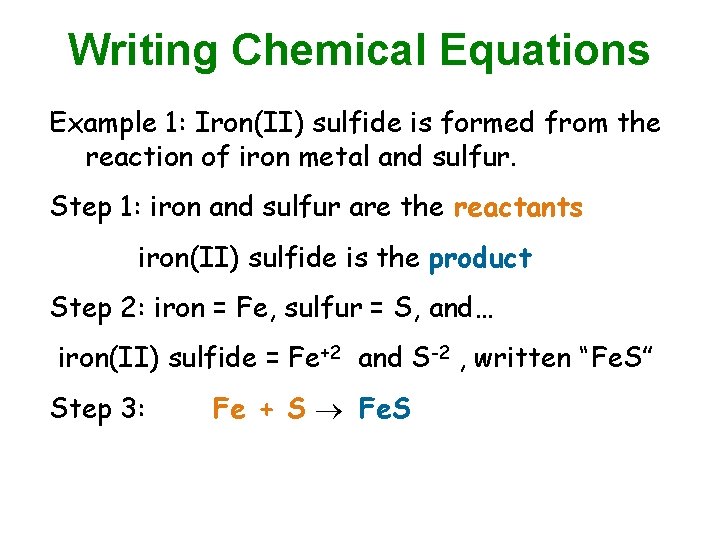
Writing Chemical Equations Example 1: Iron(II) sulfide is formed from the reaction of iron metal and sulfur. Step 1: iron and sulfur are the reactants iron(II) sulfide is the product Step 2: iron = Fe, sulfur = S, and… iron(II) sulfide = Fe+2 and S-2 , written “Fe. S” Step 3: Fe + S Fe. S

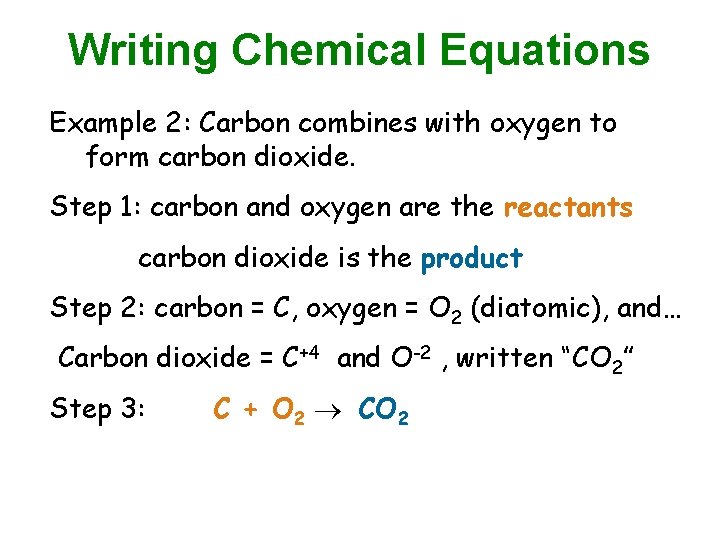
Writing Chemical Equations Example 2: Carbon combines with oxygen to form carbon dioxide. Step 1: carbon and oxygen are the reactants carbon dioxide is the product Step 2: carbon = C, oxygen = O 2 (diatomic), and… Carbon dioxide = C+4 and O-2 , written “CO 2” Step 3: C + O 2 CO 2

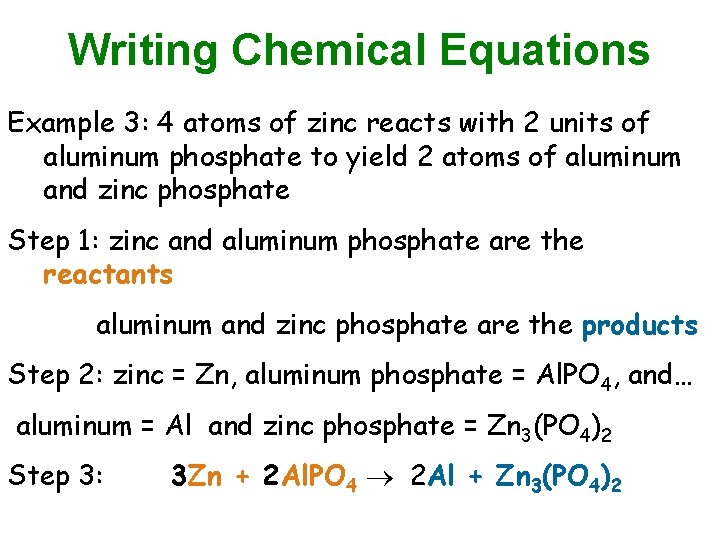
Writing Chemical Equations Example 3: 4 atoms of zinc reacts with 2 units of aluminum phosphate to yield 2 atoms of aluminum and zinc phosphate Step 1: zinc and aluminum phosphate are the reactants aluminum and zinc phosphate are the products Step 2: zinc = Zn, aluminum phosphate = Al. PO 4, and… aluminum = Al and zinc phosphate = Zn 3(PO 4)2 Step 3: 3 Zn + 2 Al. PO 4 2 Al + Zn 3(PO 4)2

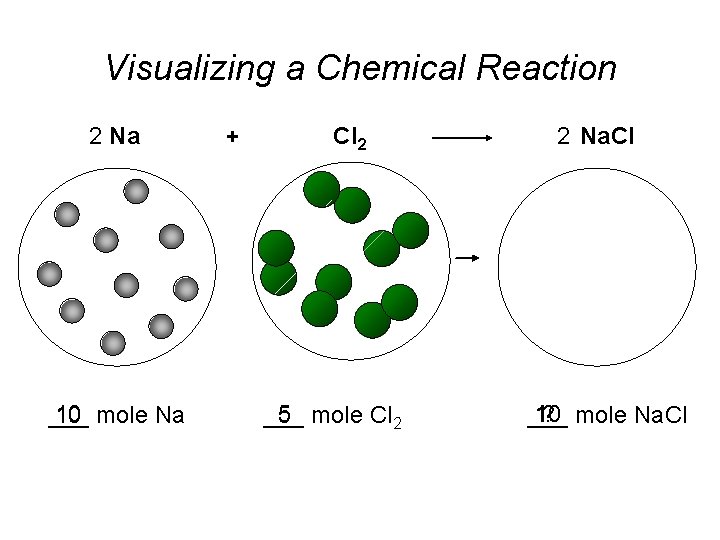
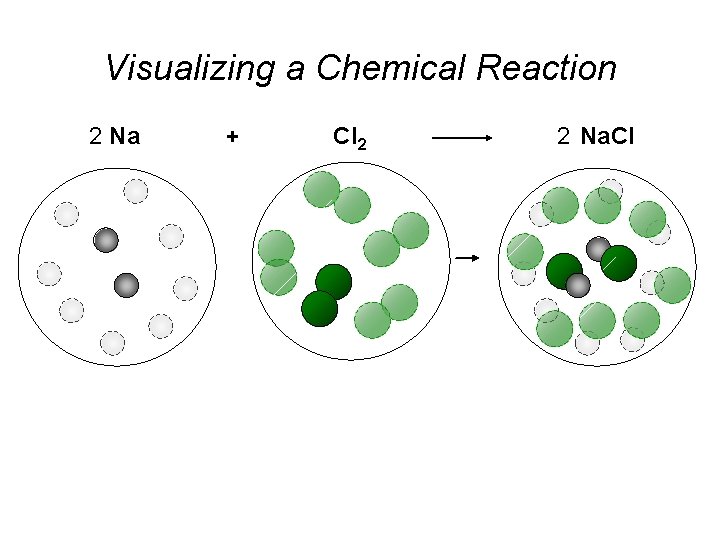
Visualizing a Chemical Reaction 2 Na 10 mole Na ___ + Cl 2 5 mole Cl 2 ___ 2 Na. Cl 10 ? mole Na. Cl ___

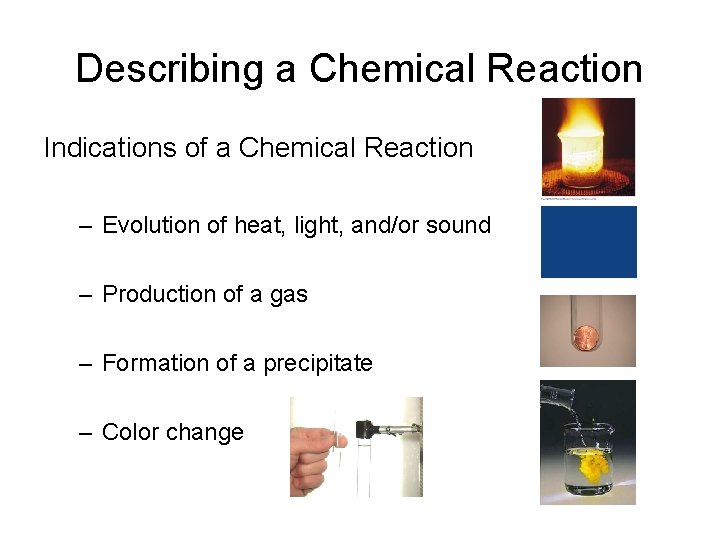
Describing a Chemical Reaction Indications of a Chemical Reaction – Evolution of heat, light, and/or sound – Production of a gas – Formation of a precipitate – Color change

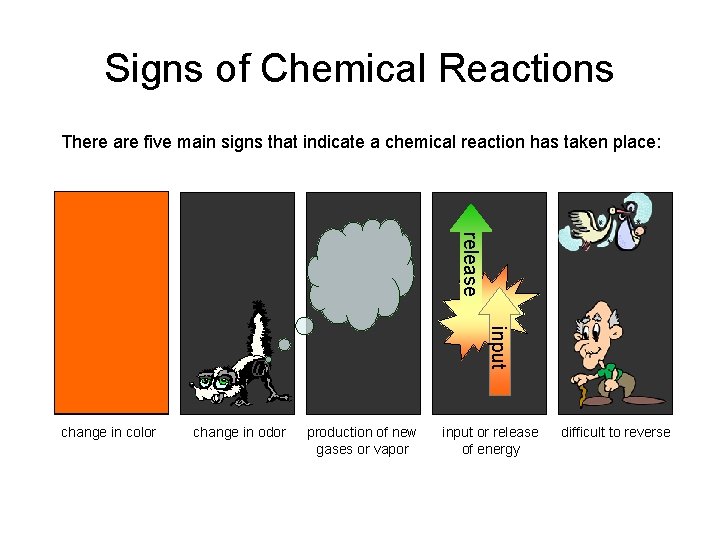
Signs of Chemical Reactions There are five main signs that indicate a chemical reaction has taken place: release input change in color change in odor production of new gases or vapor input or release of energy difficult to reverse

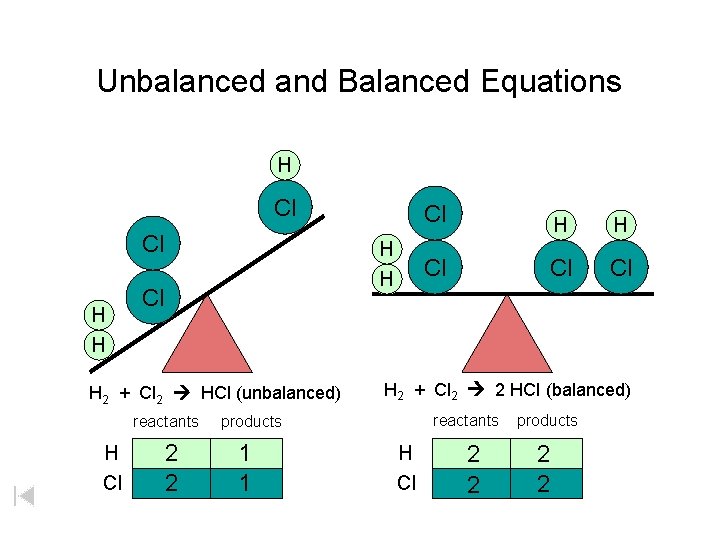
Unbalanced and Balanced Equations H Cl Cl H H Cl H 2 + Cl 2 HCl (unbalanced) reactants H Cl 2 2 H H Cl Cl Cl H 2 + Cl 2 2 HCl (balanced) reactants products 1 1 Cl H Cl 2 2 products 2 2

Visualizing a Chemical Reaction 2 Na + Cl 2 2 Na. Cl

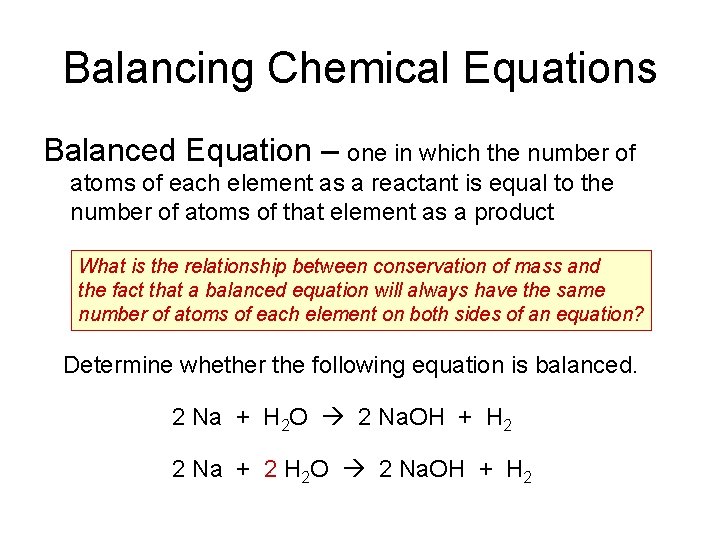
Balancing Chemical Equations Balanced Equation – one in which the number of atoms of each element as a reactant is equal to the number of atoms of that element as a product What is the relationship between conservation of mass and the fact that a balanced equation will always have the same number of atoms of each element on both sides of an equation? Determine whether the following equation is balanced. 2 Na + H 2 O 2 Na. OH + H 2 2 Na + 2 H 2 O 2 Na. OH + H 2

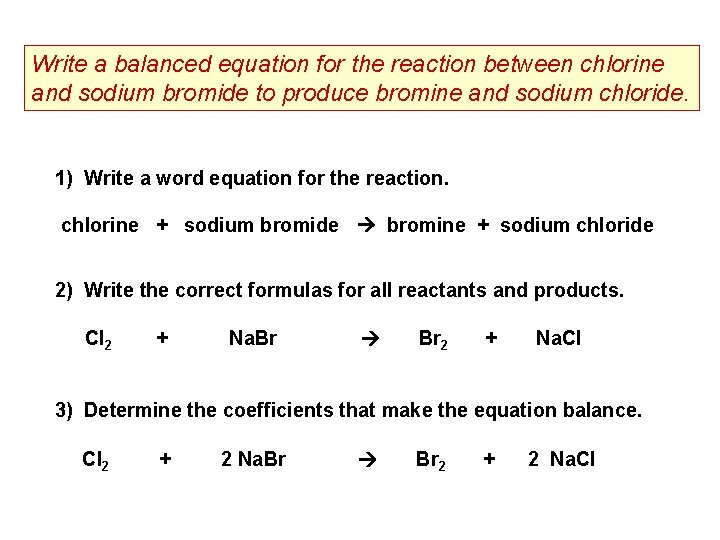
Write a balanced equation for the reaction between chlorine and sodium bromide to produce bromine and sodium chloride. 1) Write a word equation for the reaction. chlorine + sodium bromide bromine + sodium chloride 2) Write the correct formulas for all reactants and products. Cl 2 + Na. Br Br 2 + Na. Cl 3) Determine the coefficients that make the equation balance. Cl 2 + 2 Na. Br Br 2 + 2 Na. Cl

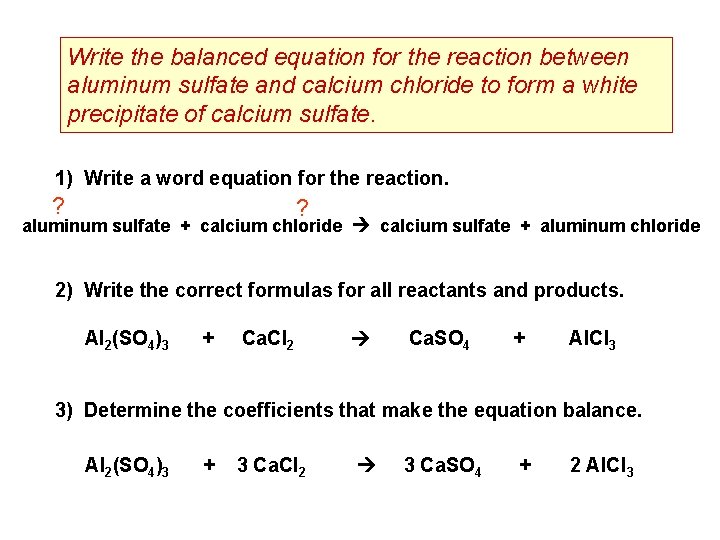
Write the balanced equation for the reaction between aluminum sulfate and calcium chloride to form a white precipitate of calcium sulfate. 1) Write a word equation for the reaction. ? ? aluminum sulfate + calcium chloride calcium sulfate + aluminum chloride 2) Write the correct formulas for all reactants and products. Al 2(SO 4)3 + Ca. Cl 2 Ca. SO 4 + Al. Cl 3 3) Determine the coefficients that make the equation balance. Al 2(SO 4)3 + 3 Ca. Cl 2 3 Ca. SO 4 + 2 Al. Cl 3
 Law of conservation of matter definition
Law of conservation of matter definition Law of conservation of mass
Law of conservation of mass Section 1 composition of matter
Section 1 composition of matter Gray and white matter
Gray and white matter Composition of matter section 1
Composition of matter section 1 Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Primary taste cortex
Primary taste cortex Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter Telencephalon
Telencephalon Ecological succession
Ecological succession Formula of kinetic energy
Formula of kinetic energy Conservation starters
Conservation starters How momentum conservation is applied in vehicular accidents
How momentum conservation is applied in vehicular accidents Law of conservation of linear momentum
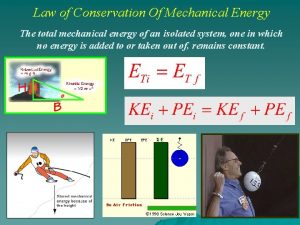
Law of conservation of linear momentum Conservation of mechanical energy
Conservation of mechanical energy Antoine lavoisier law
Antoine lavoisier law Law of conservation of energy worksheets
Law of conservation of energy worksheets Energy conservation law
Energy conservation law The law of conservation of energy states that
The law of conservation of energy states that Law of conservation of energy examples
Law of conservation of energy examples Law of conservation of angular momentum
Law of conservation of angular momentum Conservation mechanical energy
Conservation mechanical energy Whats angular momentum
Whats angular momentum Law of conservation od mass
Law of conservation od mass Lab conservation of mass worksheet answers
Lab conservation of mass worksheet answers Grade 7 term 1 natural science
Grade 7 term 1 natural science