Latest Developments in the Treatment of Invasive Aspergillosis


















- Slides: 57


Latest Developments in the Treatment of Invasive Aspergillosis William J. Steinbach, MD Assistant Professor of Pediatrics, Molecular Genetics, and Microbiology Pediatric Infectious Diseases Duke University Medical Center Durham, NC USA

Possible Areas for Improving Outcome in IA l l l l l Understanding IA epidemiology Host factors: Underlying & concomitant diseases Immunosuppression / Corticosteroids Antifungal prophylaxis Early diagnosis Early therapy Antifungal resistance Antifungal therapies Immune reconstitution, Immunotherapy

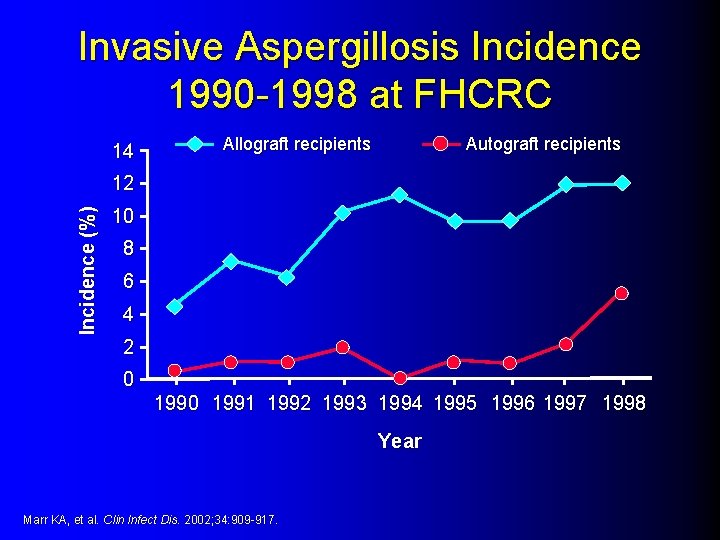
Invasive Aspergillosis Incidence 1990 -1998 at FHCRC 14 Allograft recipients Autograft recipients Incidence (%) 12 10 8 6 4 2 0 1991 1992 1993 1994 1995 1996 1997 1998 Year Marr KA, et al. Clin Infect Dis. 2002; 34: 909 -917.

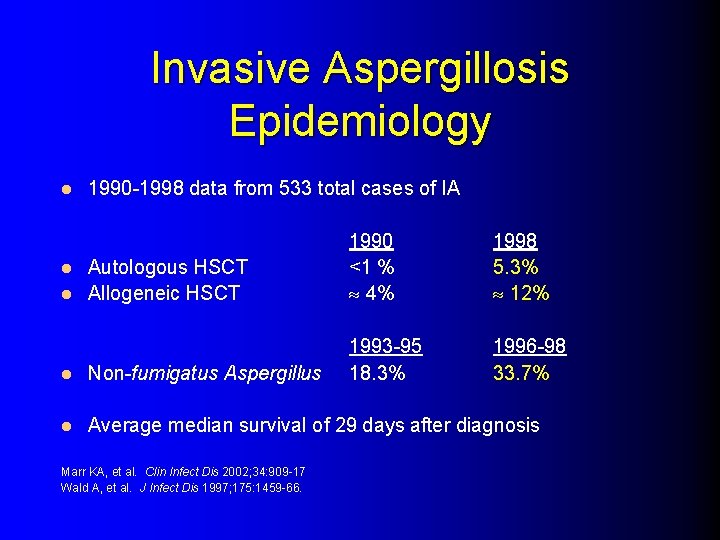
Invasive Aspergillosis Epidemiology l 1990 -1998 data from 533 total cases of IA Autologous HSCT l Allogeneic HSCT l 1990 <1 % 4% 1998 5. 3% 12% 1993 -95 18. 3% 1996 -98 33. 7% l Non-fumigatus Aspergillus l Average median survival of 29 days after diagnosis Marr KA, et al. Clin Infect Dis 2002; 34: 909 -17 Wald A, et al. J Infect Dis 1997; 175: 1459 -66.

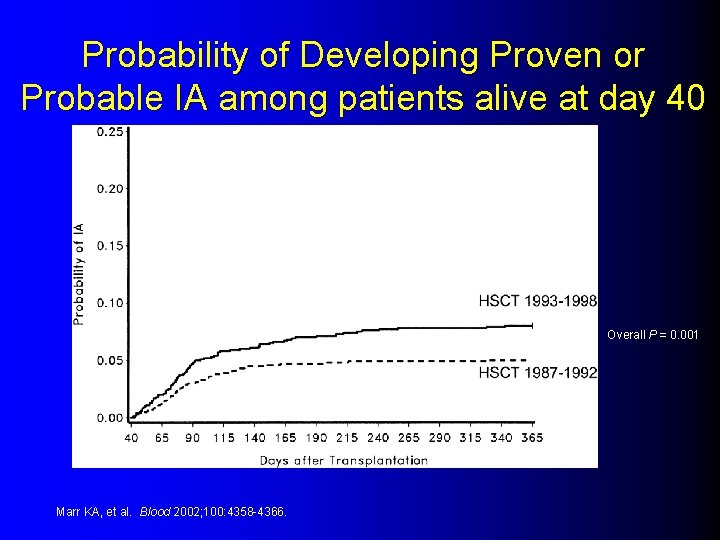
Probability of Developing Proven or Probable IA among patients alive at day 40 Overall P = 0. 001 Marr KA, et al. Blood 2002; 100: 4358 -4366.

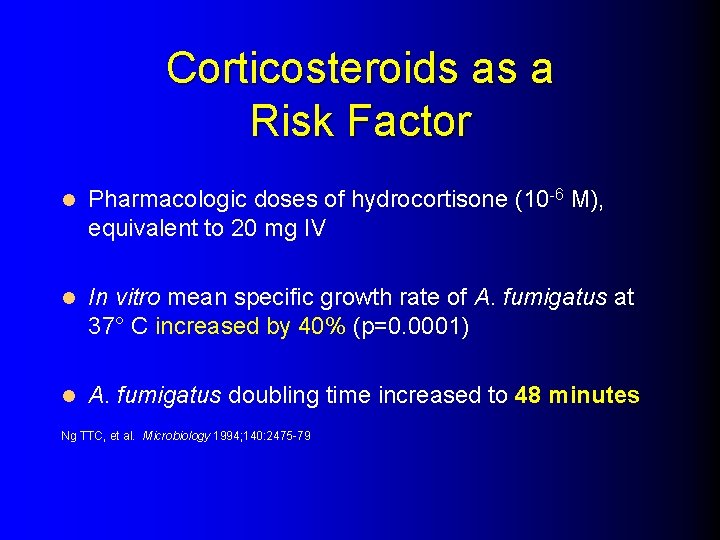
Corticosteroids as a Risk Factor l Pharmacologic doses of hydrocortisone (10 -6 M), equivalent to 20 mg IV l In vitro mean specific growth rate of A. fumigatus at 37° C increased by 40% (p=0. 0001) l A. fumigatus doubling time increased to 48 minutes Ng TTC, et al. Microbiology 1994; 140: 2475 -79

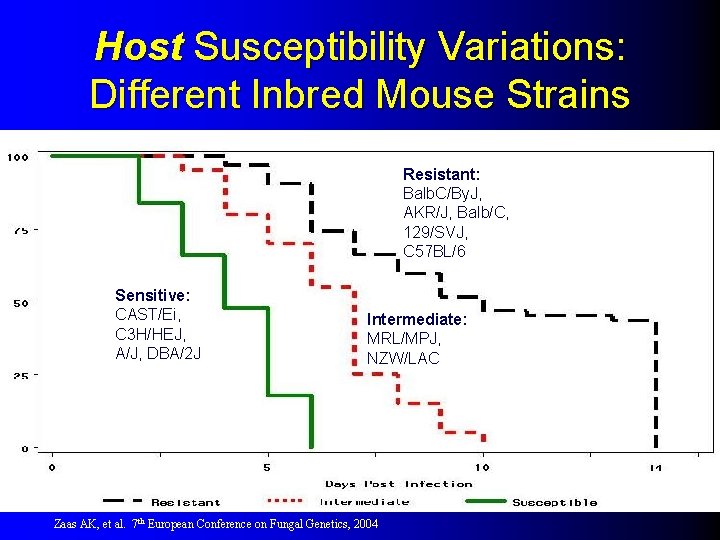
Host Susceptibility Variations: Different Inbred Mouse Strains Resistant: Balb. C/By. J, AKR/J, Balb/C, 129/SVJ, C 57 BL/6 Sensitive: CAST/Ei, C 3 H/HEJ, A/J, DBA/2 J Intermediate: MRL/MPJ, NZW/LAC Zaas AK, et al. 7 th European Conference on Fungal Genetics, 2004

Antifungal Therapy for Invasive Aspergillosis

A. terreus Infection l Murine model – Amphotericin B resistance confirmed Graybill JR, et al. Antimicrob Agents Chemother 2004; 48: 3715 -19. l Review of 28 in vitro analyses, 9 animal models, and 60 previously reported clinical cases – Am. B resistance shown in vitro and in vivo Steinbach WJ, et al. Antimicrob Agents Chemother 2004; 48: 3217 -25. l Multicenter retrospective analysis of 83 cases (1997 -2002) – Mortality at 12 weeks decreased in those who received voriconazole (HR 0. 29; 95% CI, 0. 15 -0. 56) vs. Am. B Steinbach WJ, et al. Clin Infect Dis 2004; 39: 192 -8.

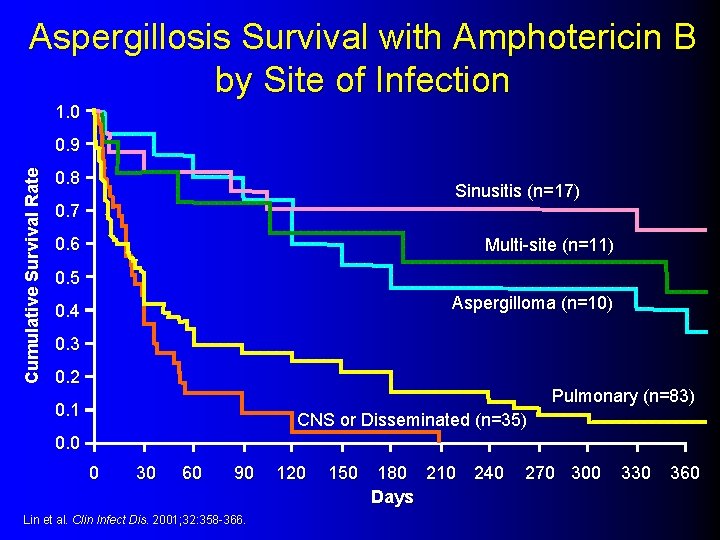
Aspergillosis Survival with Amphotericin B by Site of Infection 1. 0 Cumulative Survival Rate 0. 9 0. 8 Sinusitis (n=17) 0. 7 0. 6 Multi-site (n=11) 0. 5 Aspergilloma (n=10) 0. 4 0. 3 0. 2 Pulmonary (n=83) 0. 1 CNS or Disseminated (n=35) 0. 0 0 30 60 90 Lin et al. Clin Infect Dis. 2001; 32: 358 -366. 120 150 180 210 240 Days 270 300 330 360

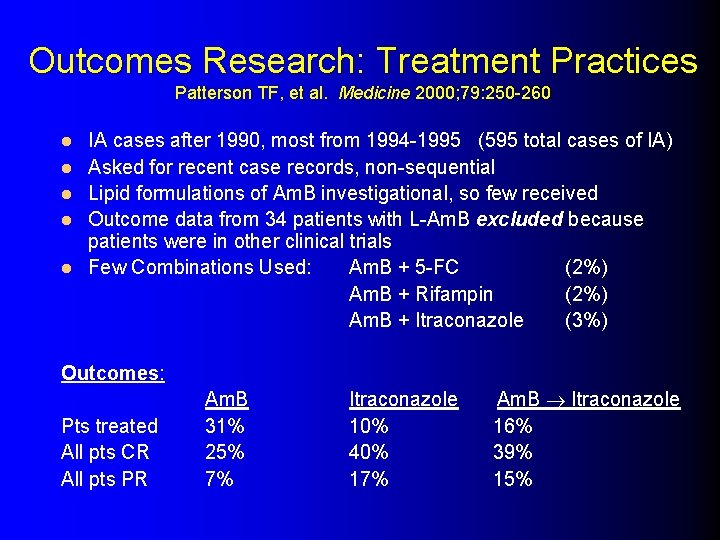
Outcomes Research: Treatment Practices Patterson TF, et al. Medicine 2000; 79: 250 -260 l l l IA cases after 1990, most from 1994 -1995 (595 total cases of IA) Asked for recent case records, non-sequential Lipid formulations of Am. B investigational, so few received Outcome data from 34 patients with L-Am. B excluded because patients were in other clinical trials Few Combinations Used: Am. B + 5 -FC (2%) Am. B + Rifampin (2%) Am. B + Itraconazole (3%) Outcomes: Pts treated All pts CR All pts PR Am. B 31% 25% 7% Itraconazole 10% 40% 17% Am. B Itraconazole 16% 39% 15%

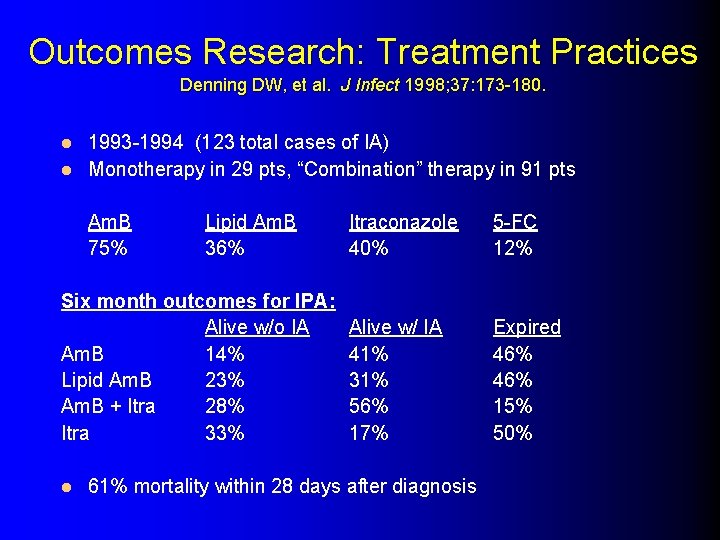
Outcomes Research: Treatment Practices Denning DW, et al. J Infect 1998; 37: 173 -180. 1993 -1994 (123 total cases of IA) l Monotherapy in 29 pts, “Combination” therapy in 91 pts l Am. B 75% Lipid Am. B 36% Six month outcomes for IPA: Alive w/o IA Am. B 14% Lipid Am. B 23% Am. B + Itra 28% Itra 33% l Itraconazole 40% 5 -FC 12% Alive w/ IA 41% 31% 56% 17% Expired 46% 15% 50% 61% mortality within 28 days after diagnosis

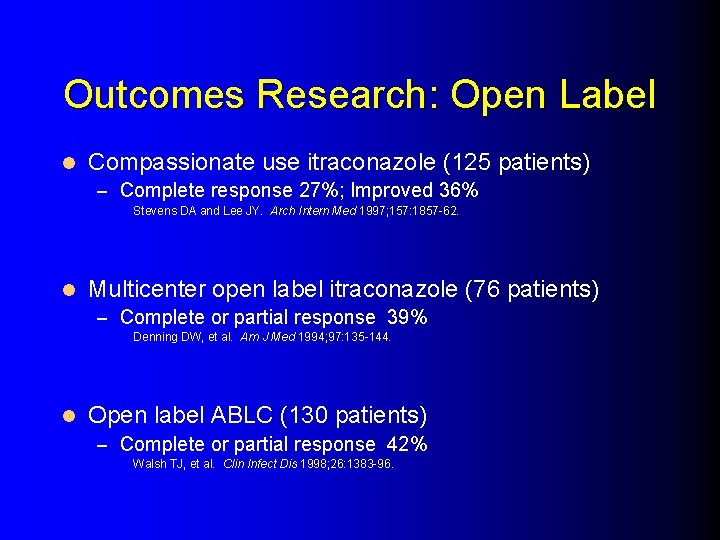
Outcomes Research: Open Label l Compassionate use itraconazole (125 patients) – Complete response 27%; Improved 36% Stevens DA and Lee JY. Arch Intern Med 1997; 157: 1857 -62. l Multicenter open label itraconazole (76 patients) – Complete or partial response 39% Denning DW, et al. Am J Med 1994; 97: 135 -144. l Open label ABLC (130 patients) – Complete or partial response 42% Walsh TJ, et al. Clin Infect Dis 1998; 26: 1383 -96.

Antifungal Pre-Exposure l Serial passages of 10 clinical isolates to fluconazole (x 4) – 4 -fold increase in MFC (but not MIC) of Itraconazole and Voriconazole – Fluconazole pre-exposure attenuates Itraconazole/ Voriconazole fungicidal activity, but no effect in Am. B – XTT growth rates pre-exposed/no fluconazole were same Liu W, et al. Antimicrob Agents Chemother 2003; 47: 3592 -7. l In vitro pre-exposure of A. fumigatus to Itraconazole or Caspofungin resulted in enhanced activity for either, in contrast to antagonistic effect of sequential itraconazole then Am. B – Suggests a preferential role for azole-Caspofungin sequential combinations over azole-Am. B regimens Kontoyiannis DP, et al. Diag Microbiol Infect Dis 2003; 47: 415 -9.

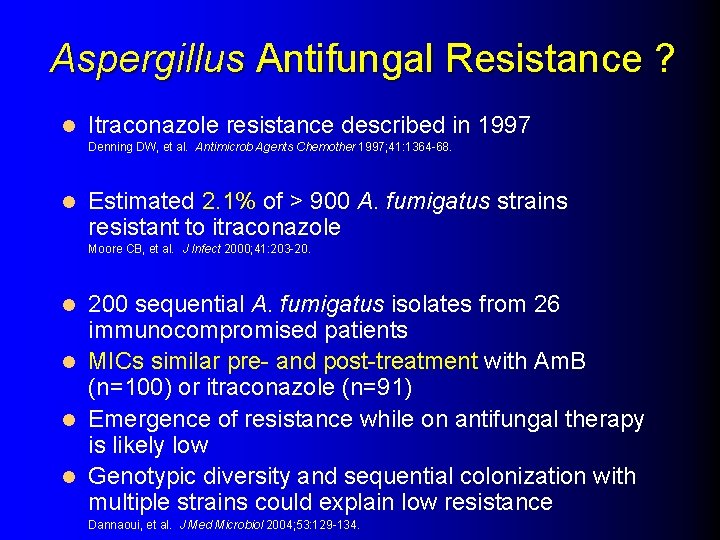
Aspergillus Antifungal Resistance ? l Itraconazole resistance described in 1997 Denning DW, et al. Antimicrob Agents Chemother 1997; 41: 1364 -68. l Estimated 2. 1% of > 900 A. fumigatus strains resistant to itraconazole Moore CB, et al. J Infect 2000; 41: 203 -20. 200 sequential A. fumigatus isolates from 26 immunocompromised patients l MICs similar pre- and post-treatment with Am. B (n=100) or itraconazole (n=91) l Emergence of resistance while on antifungal therapy is likely low l Genotypic diversity and sequential colonization with multiple strains could explain low resistance l Dannaoui, et al. J Med Microbiol 2004; 53: 129 -134.

Voriconazole Fungicidal Activity on Hyphae Previous in vitro studies examined killing of conidia and germinated conidia (sporelings) l But patients have hyphae growing l l Voriconazole killed hyphae in both time- and concentration-dependent fashions – Kill curve and MTT cell wall viability testing l Voriconazole had better fungicidal activity against A. fumigatus hyphae than Am. B at 48 hours – VCZ 1 ug/ml >95% killed on agar (Am. B 1 ug/ml 70% killed) – VCZ 1 ug/ml 99% killed in broth (Am. B 1 ug/ml 82% killed) Krishnan S, et al. J Antimicrob Chemother e. Pub April 20005

Only Three Randomized Clinical Trials ever completed for the Treatment of Invasive Aspergillosis

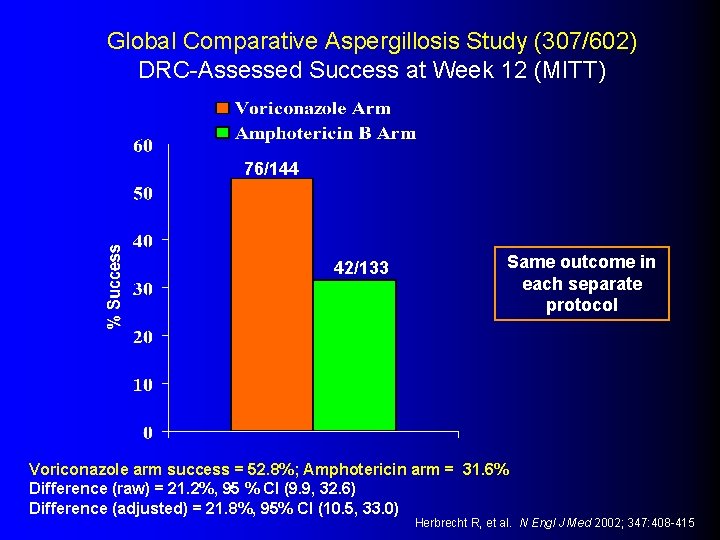
Global Comparative Aspergillosis Study (307/602) DRC-Assessed Success at Week 12 (MITT) 76/144 42/133 Same outcome in each separate protocol Voriconazole arm success = 52. 8%; Amphotericin arm = 31. 6% Difference (raw) = 21. 2%, 95 % CI (9. 9, 32. 6) Difference (adjusted) = 21. 8%, 95% CI (10. 5, 33. 0) Herbrecht R, et al. N Engl J Med 2002; 347: 408 -415

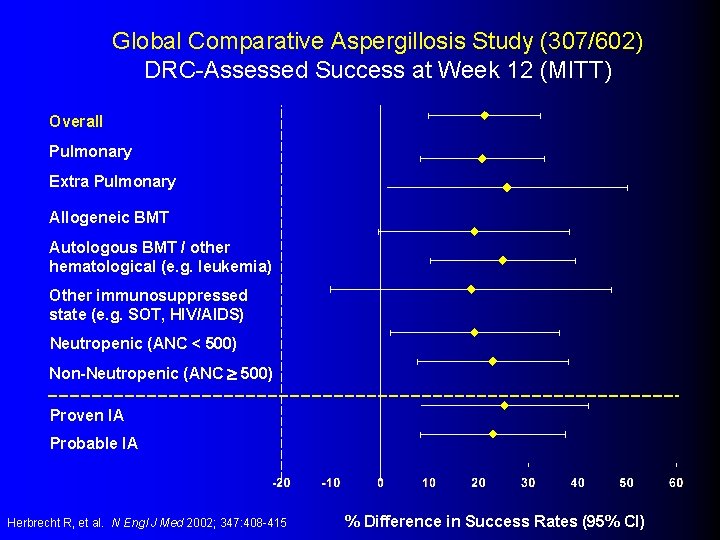
Global Comparative Aspergillosis Study (307/602) DRC-Assessed Success at Week 12 (MITT) Overall Pulmonary Extra Pulmonary Allogeneic BMT Autologous BMT / other hematological (e. g. leukemia) Other immunosuppressed state (e. g. SOT, HIV/AIDS) Neutropenic (ANC < 500) Non-Neutropenic (ANC 500) Proven IA Probable IA Herbrecht R, et al. N Engl J Med 2002; 347: 408 -415 % Difference in Success Rates (95% CI)

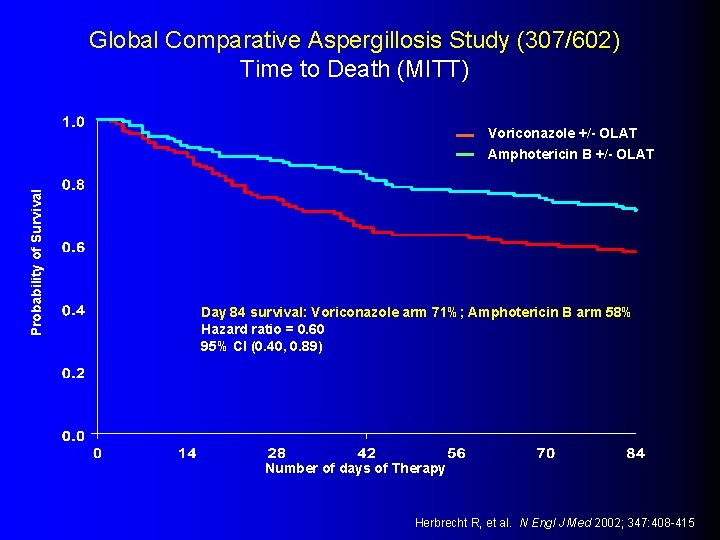
Global Comparative Aspergillosis Study (307/602) Time to Death (MITT) Probability of Survival Voriconazole +/- OLAT Amphotericin B +/- OLAT Day 84 survival: Voriconazole arm 71%; Amphotericin B arm 58% Hazard ratio = 0. 60 95% CI (0. 40, 0. 89) Number of days of Therapy Herbrecht R, et al. N Engl J Med 2002; 347: 408 -415

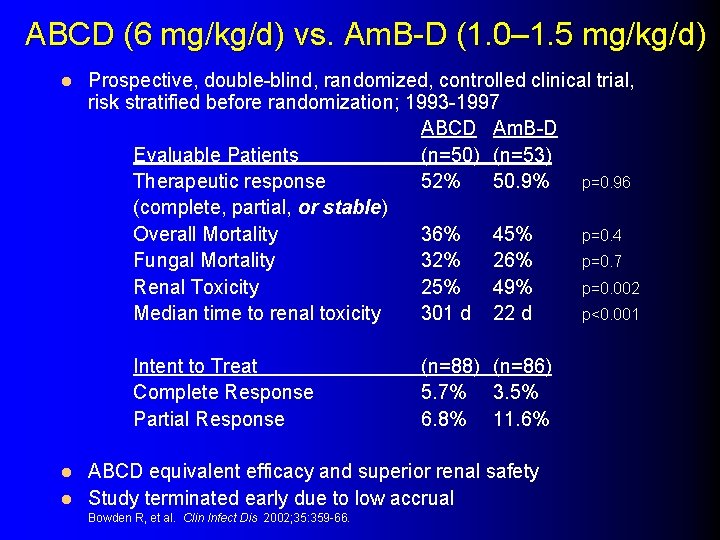
ABCD (6 mg/kg/d) vs. Am. B-D (1. 0– 1. 5 mg/kg/d) l Prospective, double-blind, randomized, controlled clinical trial, risk stratified before randomization; 1993 -1997 ABCD Am. B-D Evaluable Patients (n=50) (n=53) Therapeutic response 52% 50. 9% p=0. 96 (complete, partial, or stable) Overall Mortality 36% 45% p=0. 4 Fungal Mortality 32% 26% p=0. 7 Renal Toxicity 25% 49% p=0. 002 Median time to renal toxicity 301 d 22 d p<0. 001 Intent to Treat Complete Response Partial Response (n=88) (n=86) 5. 7% 3. 5% 6. 8% 11. 6% ABCD equivalent efficacy and superior renal safety l Study terminated early due to low accrual l Bowden R, et al. Clin Infect Dis 2002; 35: 359 -66.

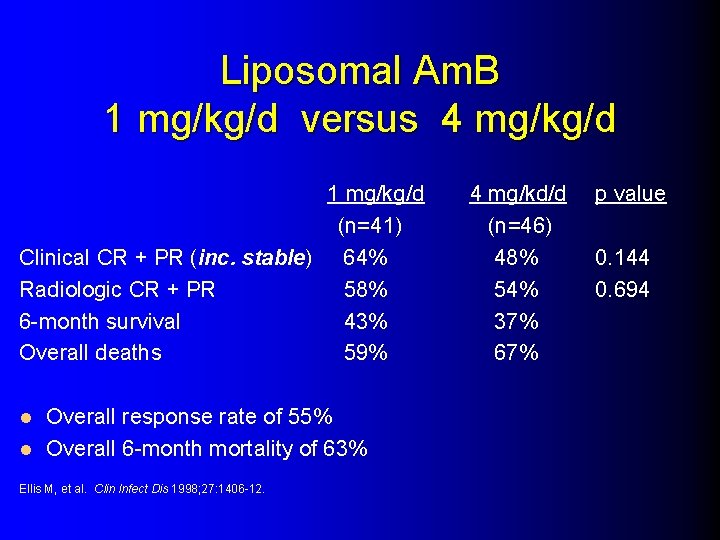
Liposomal Am. B 1 mg/kg/d versus 4 mg/kg/d 1 mg/kg/d (n=41) Clinical CR + PR (inc. stable) 64% Radiologic CR + PR 58% 6 -month survival 43% Overall deaths 59% Overall response rate of 55% l Overall 6 -month mortality of 63% l Ellis M, et al. Clin Infect Dis 1998; 27: 1406 -12. 4 mg/kd/d (n=46) 48% 54% 37% 67% p value 0. 144 0. 694

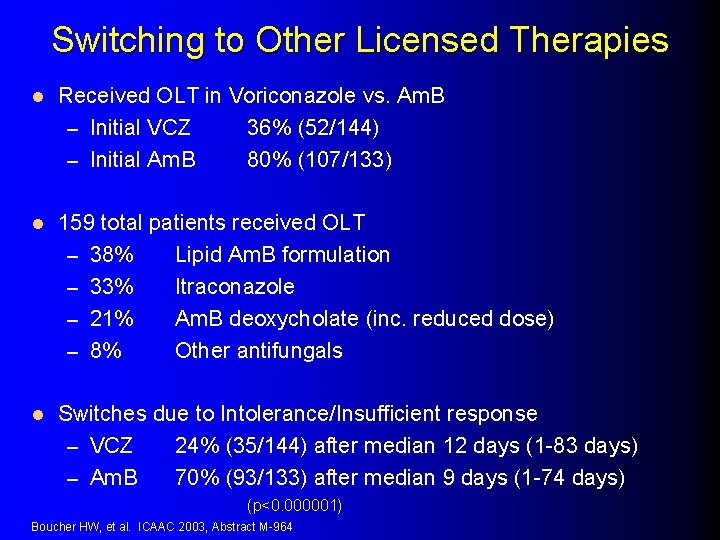
Switching to Other Licensed Therapies l Received OLT in Voriconazole vs. Am. B – Initial VCZ 36% (52/144) – Initial Am. B 80% (107/133) l 159 total patients received OLT – 38% Lipid Am. B formulation – 33% Itraconazole – 21% Am. B deoxycholate (inc. reduced dose) – 8% Other antifungals l Switches due to Intolerance/Insufficient response – VCZ 24% (35/144) after median 12 days (1 -83 days) – Am. B 70% (93/133) after median 9 days (1 -74 days) (p<0. 000001) Boucher HW, et al. ICAAC 2003, Abstract M-964

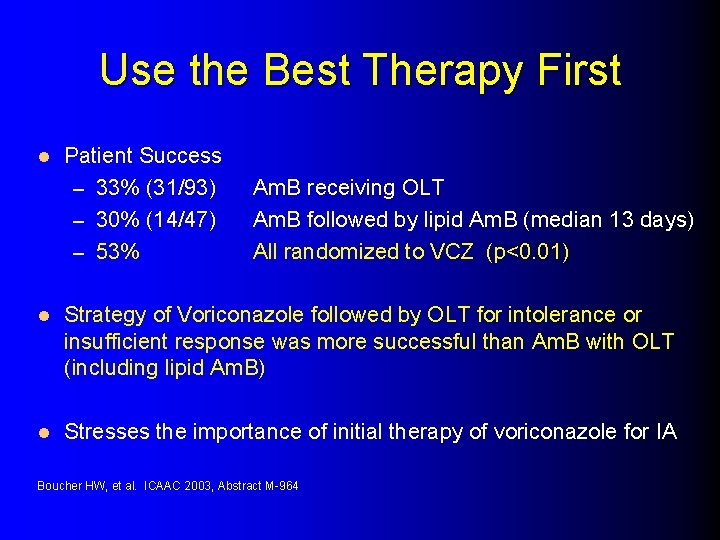
Use the Best Therapy First l Patient Success – 33% (31/93) – 30% (14/47) – 53% Am. B receiving OLT Am. B followed by lipid Am. B (median 13 days) All randomized to VCZ (p<0. 01) l Strategy of Voriconazole followed by OLT for intolerance or insufficient response was more successful than Am. B with OLT (including lipid Am. B) l Stresses the importance of initial therapy of voriconazole for IA Boucher HW, et al. ICAAC 2003, Abstract M-964

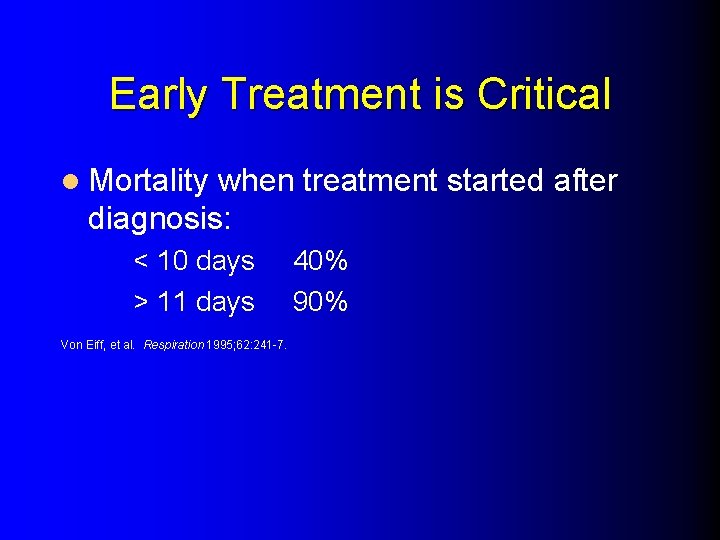
Early Treatment is Critical l Mortality when treatment started after diagnosis: < 10 days > 11 days Von Eiff, et al. Respiration 1995; 62: 241 -7. 40% 90%

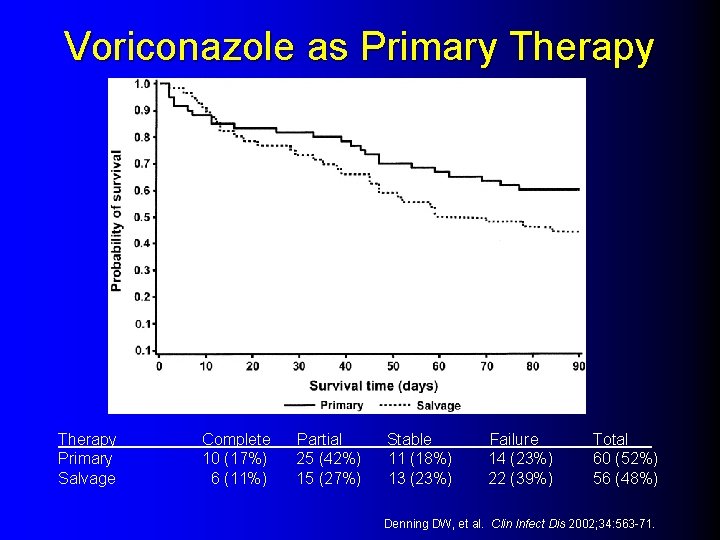
Voriconazole as Primary Therapy Primary Salvage Complete 10 (17%) 6 (11%) Partial 25 (42%) 15 (27%) Stable 11 (18%) 13 (23%) Failure 14 (23%) 22 (39%) Total 60 (52%) 56 (48%) Denning DW, et al. Clin Infect Dis 2002; 34: 563 -71.

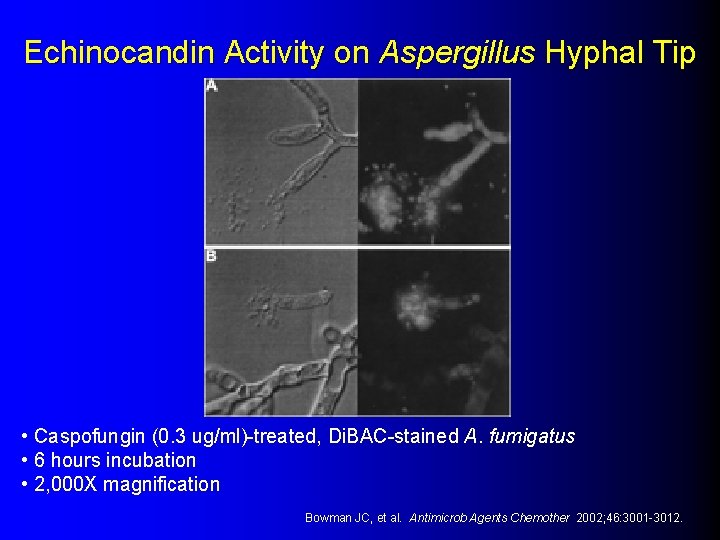
Echinocandin Activity on Aspergillus Hyphal Tip • Caspofungin (0. 3 ug/ml)-treated, Di. BAC-stained A. fumigatus • 6 hours incubation • 2, 000 X magnification Bowman JC, et al. Antimicrob Agents Chemother 2002; 46: 3001 -3012.

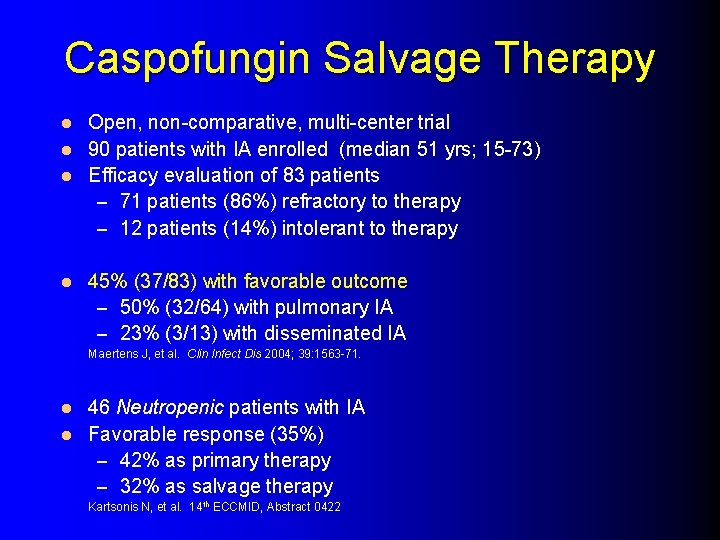
Caspofungin Salvage Therapy Open, non-comparative, multi-center trial l 90 patients with IA enrolled (median 51 yrs; 15 -73) l Efficacy evaluation of 83 patients – 71 patients (86%) refractory to therapy – 12 patients (14%) intolerant to therapy l l 45% (37/83) with favorable outcome – 50% (32/64) with pulmonary IA – 23% (3/13) with disseminated IA Maertens J, et al. Clin Infect Dis 2004; 39: 1563 -71. 46 Neutropenic patients with IA l Favorable response (35%) – 42% as primary therapy – 32% as salvage therapy l Kartsonis N, et al. 14 th ECCMID, Abstract 0422

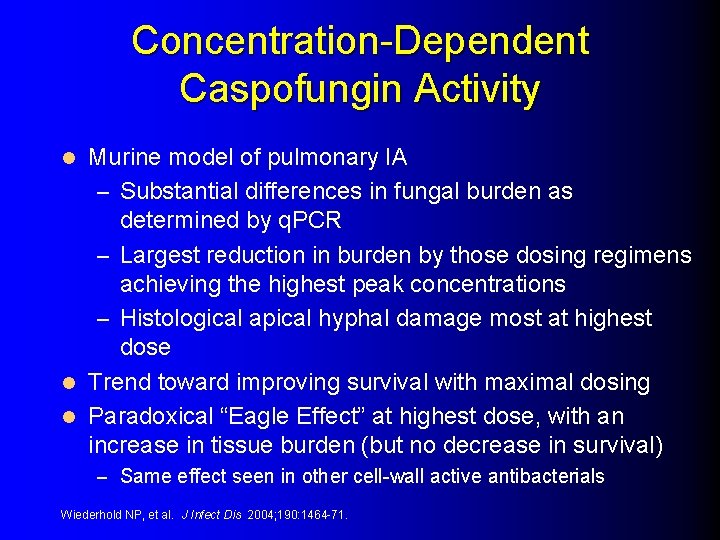
Concentration-Dependent Caspofungin Activity Murine model of pulmonary IA – Substantial differences in fungal burden as determined by q. PCR – Largest reduction in burden by those dosing regimens achieving the highest peak concentrations – Histological apical hyphal damage most at highest dose l Trend toward improving survival with maximal dosing l Paradoxical “Eagle Effect” at highest dose, with an increase in tissue burden (but no decrease in survival) l – Same effect seen in other cell-wall active antibacterials Wiederhold NP, et al. J Infect Dis 2004; 190: 1464 -71.

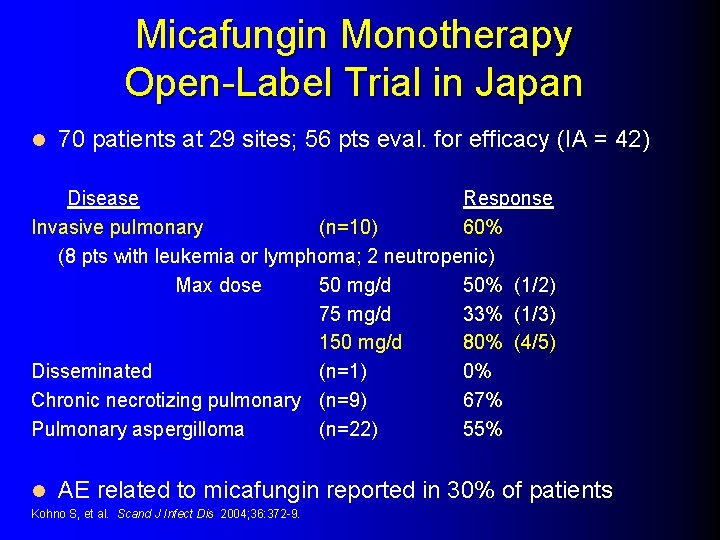
Micafungin Monotherapy Open-Label Trial in Japan l 70 patients at 29 sites; 56 pts eval. for efficacy (IA = 42) Disease Response Invasive pulmonary (n=10) 60% (8 pts with leukemia or lymphoma; 2 neutropenic) Max dose 50 mg/d 50% (1/2) 75 mg/d 33% (1/3) 150 mg/d 80% (4/5) Disseminated (n=1) 0% Chronic necrotizing pulmonary (n=9) 67% Pulmonary aspergilloma (n=22) 55% l AE related to micafungin reported in 30% of patients Kohno S, et al. Scand J Infect Dis 2004; 36: 372 -9.

Posaconazole Monotherapy l Multicenter study for salvage therapy – Included 25 pts with IA – Effective in 53% (8/15) at week 4 – Effective in 85% (6/7) at week 8 – No mention of patients without complete follow-up Hachem RY, et al. ICAAC 2000, Abstract 1109 l Multi-center study of patients with IA refractory to or intolerant of Am. B formulations and itraconazole – 107 posaconazole, 86 controls – Global response rate at end of treatment l Posaconazole 42% l Controls 26% Walsh TJ, et al. ASH 2003, Abstract 682

Cerebral Aspergillosis 86 patients (9 mo - 81 yo) with proven or probable CNS aspergillosis – A. fumigatus (n=34); A. nidulans (n=5); Aspergillus spp. (n=24) l Underlying disease – BMT (n=33); Hem malignancy (n=14) – SOT (n=12); Acquired/Cong immunosuppression (n=15) – Other (n=12) l Only 13/86 received VCZ primary therapy (others with previous antifungal therapy before VCZ use) l Global Clinical Outcome – Complete / Partial Response 34% – Stable / Failed response 66% – BMT Recipient Response 15% – All Others Response 42 -50% l Troke PF, et al. ICAAC 2003, Abstract M-1755

Bone Aspergillosis 20 patients from Clinical trials and Compassionate use l Bone Involvement – Spondylodiscitis (n=9); Sternum/Rib (n=6); Peripheral (n=5) l Immunocompromised (n=14) l – Largest population: Chronic Granulomatous Disease (n=5) Bone was the only infection site in 10 patients l Salvage voriconazole therapy in 18/20 patients l Median duration of voriconazole 83. 5 days (4 -395 days) l Global Clinical Outcome l Complete / Partial Response 55% (11/20) – Complete (n=4); Partial (n=7), Failure (n=9) l Mouas H, et al. Clin Infect Dis 2005; 40: 1141 -7.

Combination Antifungal Therapy in Invasive Aspergillosis

Combination Therapy Rationale Widened spectrum and potency More rapid antifungal effect Additive or synergistic efficacy effects Lowered dosing or less toxicity Reduce risk of emerging resistance Historic poor outcomes with monotherapy Increased penetration / transport Inhibit different stages of the same biochemical pathway l Simultaneous inhibition of different fungal targets l Creation of a fungicidal combination l l l l

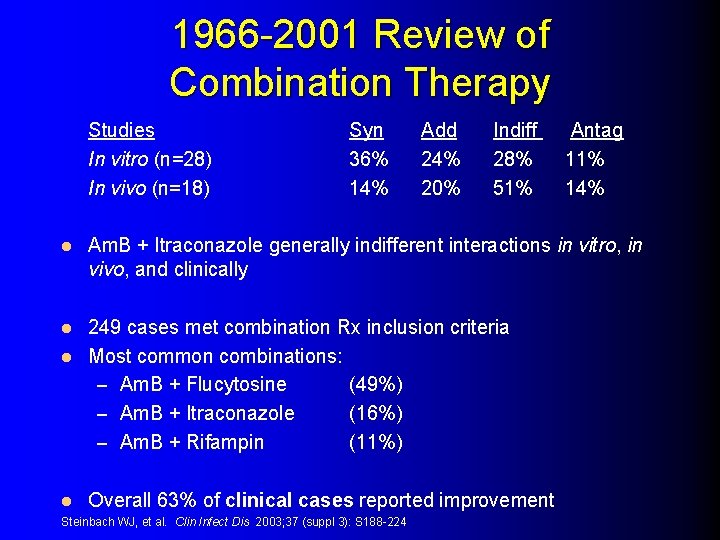
1966 -2001 Review of Combination Therapy Studies In vitro (n=28) In vivo (n=18) l Syn 36% 14% Add 24% 20% Indiff 28% 51% Am. B + Itraconazole generally indifferent interactions in vitro, in vivo, and clinically 249 cases met combination Rx inclusion criteria l Most common combinations: – Am. B + Flucytosine (49%) – Am. B + Itraconazole (16%) – Am. B + Rifampin (11%) l l Antag 11% 14% Overall 63% of clinical cases reported improvement Steinbach WJ, et al. Clin Infect Dis 2003; 37 (suppl 3): S 188 -224

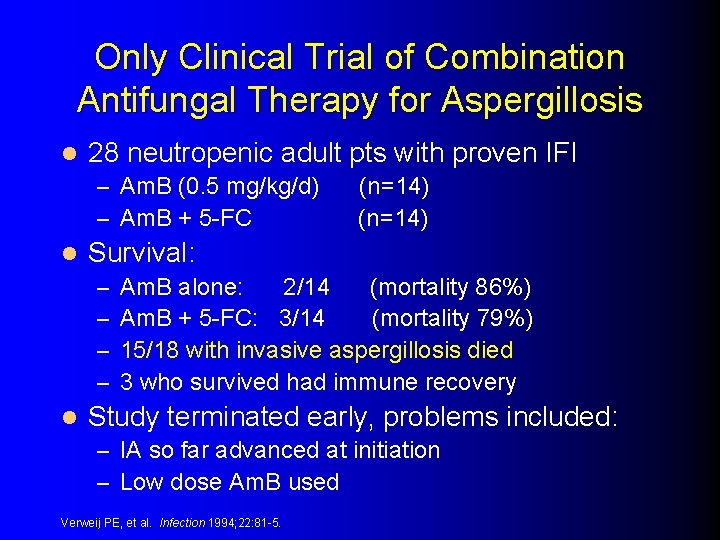
Only Clinical Trial of Combination Antifungal Therapy for Aspergillosis l 28 neutropenic adult pts with proven IFI – Am. B (0. 5 mg/kg/d) – Am. B + 5 -FC l Survival: – – l (n=14) Am. B alone: 2/14 (mortality 86%) Am. B + 5 -FC: 3/14 (mortality 79%) 15/18 with invasive aspergillosis died 3 who survived had immune recovery Study terminated early, problems included: – IA so far advanced at initiation – Low dose Am. B used Verweij PE, et al. Infection 1994; 22: 81 -5.

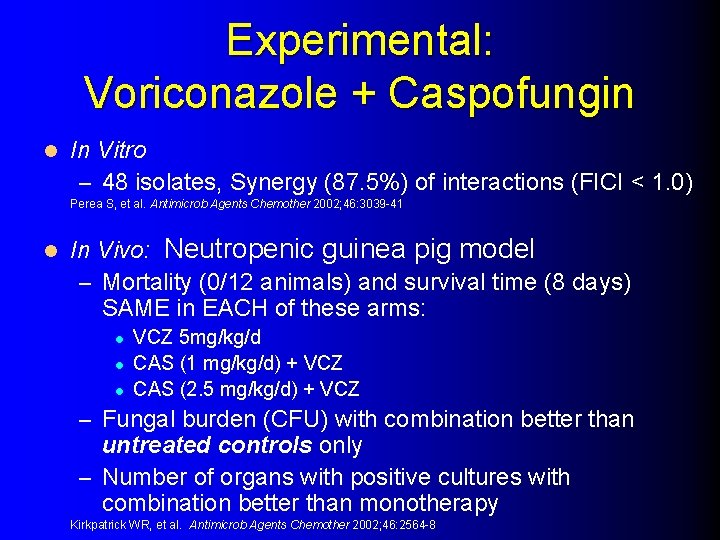
Experimental: Voriconazole + Caspofungin l In Vitro – 48 isolates, Synergy (87. 5%) of interactions (FICI < 1. 0) Perea S, et al. Antimicrob Agents Chemother 2002; 46: 3039 -41 l In Vivo: Neutropenic guinea pig model – Mortality (0/12 animals) and survival time (8 days) SAME in EACH of these arms: l l l VCZ 5 mg/kg/d CAS (1 mg/kg/d) + VCZ CAS (2. 5 mg/kg/d) + VCZ – Fungal burden (CFU) with combination better than untreated controls only – Number of organs with positive cultures with combination better than monotherapy Kirkpatrick WR, et al. Antimicrob Agents Chemother 2002; 46: 2564 -8

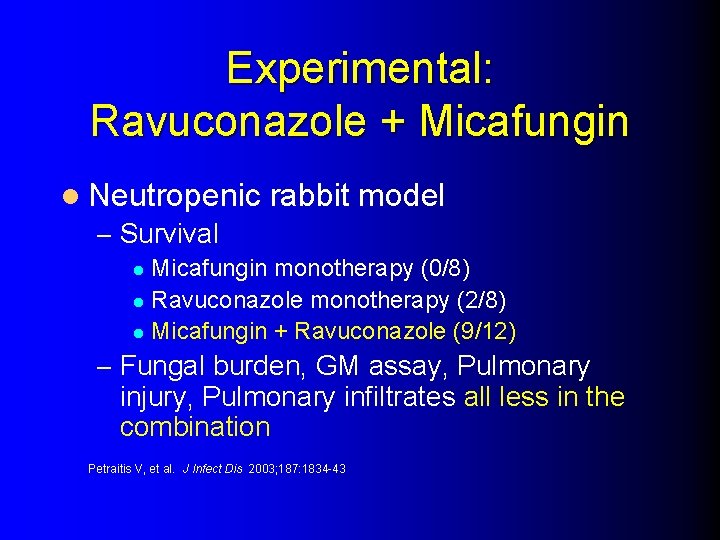
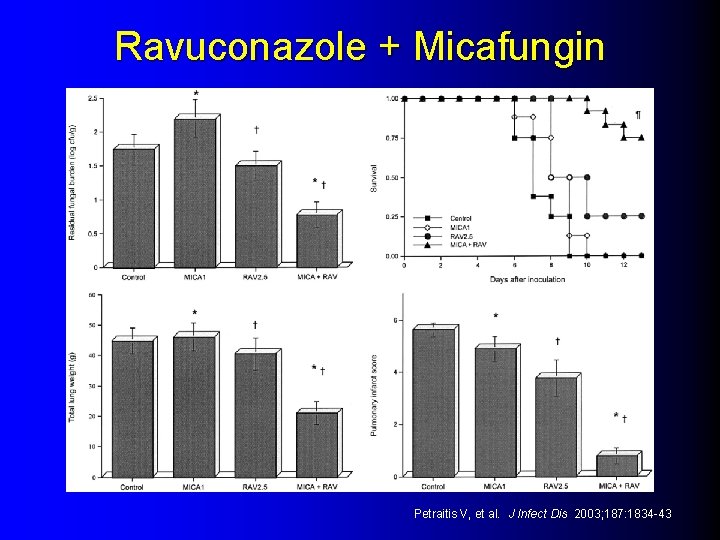
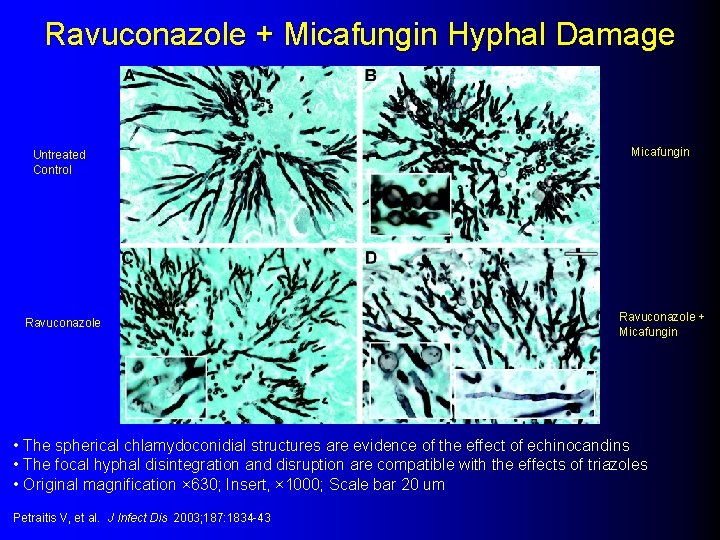
Experimental: Ravuconazole + Micafungin l Neutropenic – Survival rabbit model Micafungin monotherapy (0/8) l Ravuconazole monotherapy (2/8) l Micafungin + Ravuconazole (9/12) l – Fungal burden, GM assay, Pulmonary injury, Pulmonary infiltrates all less in the combination Petraitis V, et al. J Infect Dis 2003; 187: 1834 -43

Ravuconazole + Micafungin Petraitis V, et al. J Infect Dis 2003; 187: 1834 -43

Ravuconazole + Micafungin Hyphal Damage Untreated Control Ravuconazole Micafungin Ravuconazole + Micafungin • The spherical chlamydoconidial structures are evidence of the effect of echinocandins • The focal hyphal disintegration and disruption are compatible with the effects of triazoles • Original magnification × 630; Insert, × 1000; Scale bar 20 um Petraitis V, et al. J Infect Dis 2003; 187: 1834 -43

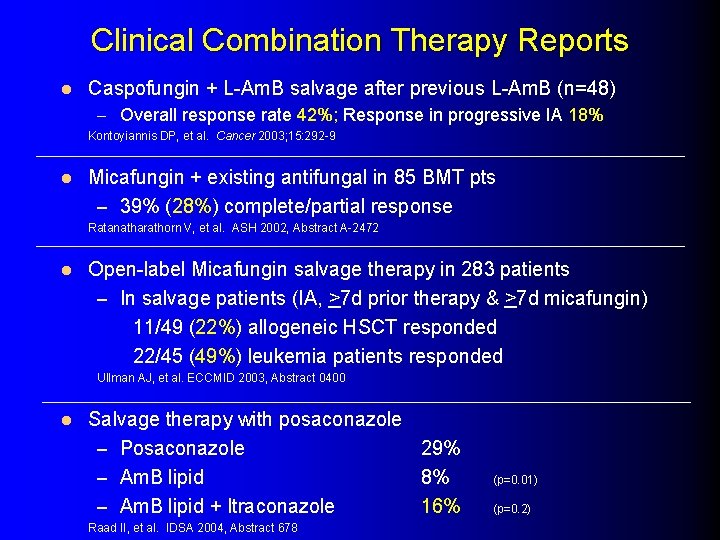
Clinical Combination Therapy Reports l Caspofungin + L-Am. B salvage after previous L-Am. B (n=48) – Overall response rate 42%; Response in progressive IA 18% Kontoyiannis DP, et al. Cancer 2003; 15: 292 -9 l Micafungin + existing antifungal in 85 BMT pts – 39% (28%) complete/partial response Ratanatharathorn V, et al. ASH 2002, Abstract A-2472 l Open-label Micafungin salvage therapy in 283 patients – In salvage patients (IA, >7 d prior therapy & >7 d micafungin) 11/49 (22%) allogeneic HSCT responded 22/45 (49%) leukemia patients responded Ullman AJ, et al. ECCMID 2003, Abstract 0400 l Salvage therapy with posaconazole – Posaconazole 29% – Am. B lipid 8% – Am. B lipid + Itraconazole 16% Raad II, et al. IDSA 2004, Abstract 678 (p=0. 01) (p=0. 2)

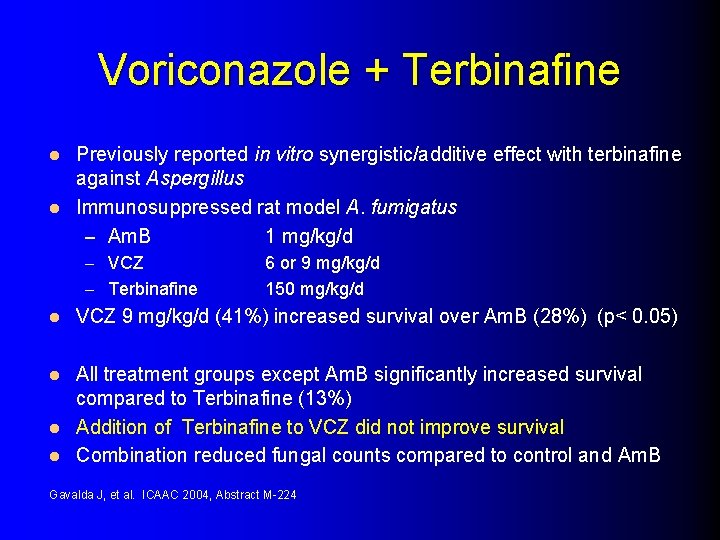
Voriconazole + Terbinafine Previously reported in vitro synergistic/additive effect with terbinafine against Aspergillus l Immunosuppressed rat model A. fumigatus – Am. B 1 mg/kg/d l – VCZ – Terbinafine l 6 or 9 mg/kg/d 150 mg/kg/d VCZ 9 mg/kg/d (41%) increased survival over Am. B (28%) (p< 0. 05) All treatment groups except Am. B significantly increased survival compared to Terbinafine (13%) l Addition of Terbinafine to VCZ did not improve survival l Combination reduced fungal counts compared to control and Am. B l Gavalda J, et al. ICAAC 2004, Abstract M-224

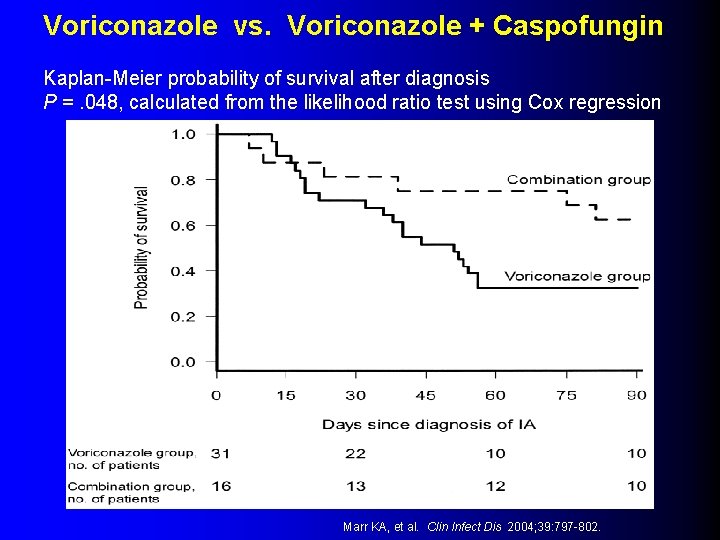
New Data: Combination Therapy for IA l l l 47 patients with proven/probable IA from 1997 -2001 Patients experienced failure of initial therapy with Am. B formulations Received either voriconazole (n=31) or voriconazole + caspofungin (n=16) as salvage therapy Voriconazole + Caspofungin with improved 3 -month survival rate compared to voriconazole monotherapy (HR 0. 42; 95% CI 0. 17 -1. 1; p=0. 048) Multivariate model, combination with reduced mortality (HR 0. 28; 95% CI 0. 28 -0. 92; p=0. 11) Marr KA, et al. Clin Infect Dis 2004; 39: 797 -802.

Voriconazole vs. Voriconazole + Caspofungin Kaplan-Meier probability of survival after diagnosis P =. 048, calculated from the likelihood ratio test using Cox regression Marr KA, et al. Clin Infect Dis 2004; 39: 797 -802.

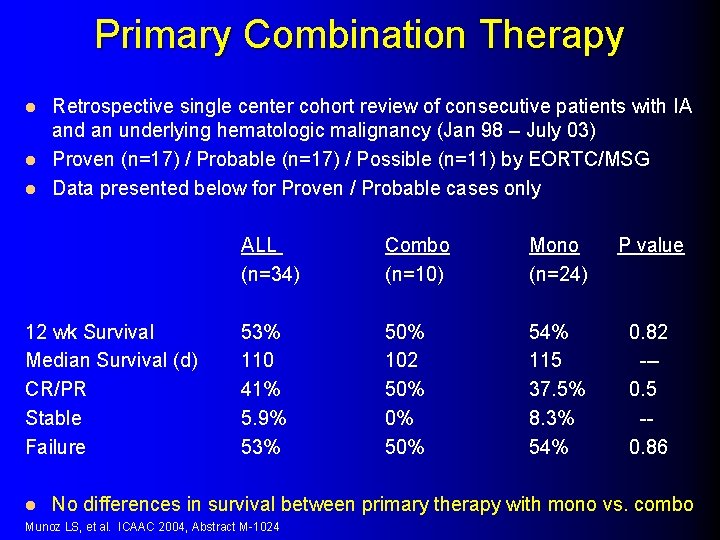
Primary Combination Therapy Retrospective single center cohort review of consecutive patients with IA and an underlying hematologic malignancy (Jan 98 – July 03) l Proven (n=17) / Probable (n=17) / Possible (n=11) by EORTC/MSG l Data presented below for Proven / Probable cases only l 12 wk Survival Median Survival (d) CR/PR Stable Failure l ALL (n=34) Combo (n=10) Mono (n=24) P value 53% 110 41% 5. 9% 53% 50% 102 50% 0% 54% 115 37. 5% 8. 3% 54% 0. 82 --0. 5 -0. 86 No differences in survival between primary therapy with mono vs. combo Munoz LS, et al. ICAAC 2004, Abstract M-1024

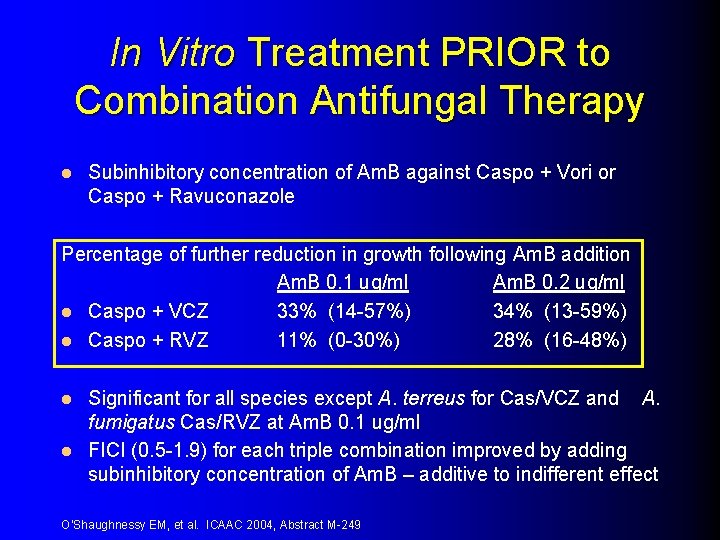
In Vitro Treatment PRIOR to Combination Antifungal Therapy l Subinhibitory concentration of Am. B against Caspo + Vori or Caspo + Ravuconazole Percentage of further reduction in growth following Am. B addition Am. B 0. 1 ug/ml Am. B 0. 2 ug/ml l Caspo + VCZ 33% (14 -57%) 34% (13 -59%) l Caspo + RVZ 11% (0 -30%) 28% (16 -48%) Significant for all species except A. terreus for Cas/VCZ and A. fumigatus Cas/RVZ at Am. B 0. 1 ug/ml l FICI (0. 5 -1. 9) for each triple combination improved by adding subinhibitory concentration of Am. B – additive to indifferent effect l O’Shaughnessy EM, et al. ICAAC 2004, Abstract M-249

Pediatric Antifungal Data

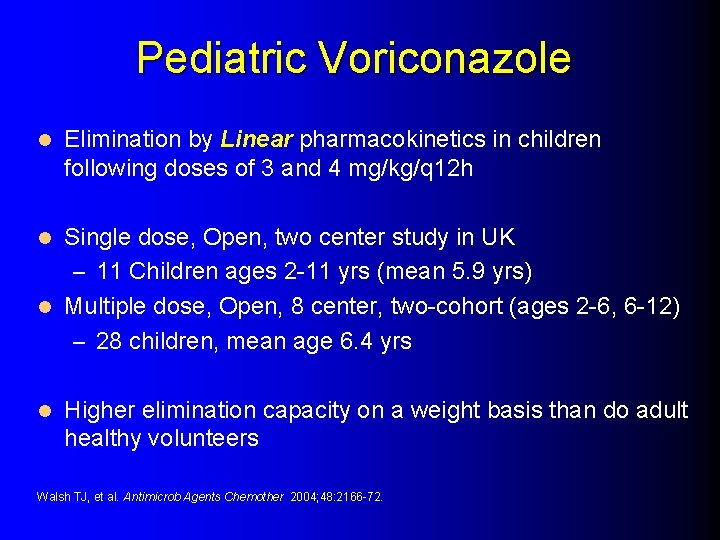
Pediatric Voriconazole l Elimination by Linear pharmacokinetics in children following doses of 3 and 4 mg/kg/q 12 h Single dose, Open, two center study in UK – 11 Children ages 2 -11 yrs (mean 5. 9 yrs) l Multiple dose, Open, 8 center, two-cohort (ages 2 -6, 6 -12) – 28 children, mean age 6. 4 yrs l l Higher elimination capacity on a weight basis than do adult healthy volunteers Walsh TJ, et al. Antimicrob Agents Chemother 2004; 48: 2166 -72.

Pediatric Voriconazole l Extrapolated plasma pharmacokinetics of pediatric doses (5 -12 mg/kg/q 12 h) vs. adult (4 mg/kg/q 12 h) – Pediatric dose of approx. 11 mg/kg/q 12 h is equivalent to adult dose of 4 mg/kg/q 12 h by AUC and plasma concentration – This is only valid if linear pharmacokinetics maintained throughout full dosage range Walsh TJ, et al. Antimicrob Agents Chemother 2004; 48: 2166 -72. l Correct pediatric dosing not fully established, but clearly higher than adult dosing – prompted a second PK study

2 nd Pediatric Voriconazole Pharmacokinetic Study l Study completed, data analyses ongoing l PK study (2 -12 yo) to evaluate > 4 mg/kg BID dosing – Enrolled 48 (39 completed all three PK periods) – Doses of 4, 6, 8 mg/kg/q 12 h – Each child received at least two different doses in escalating order, then switched to PO l Oral Suspension (40 mg/ml) – FDA approved 12/24/03, orange flavor

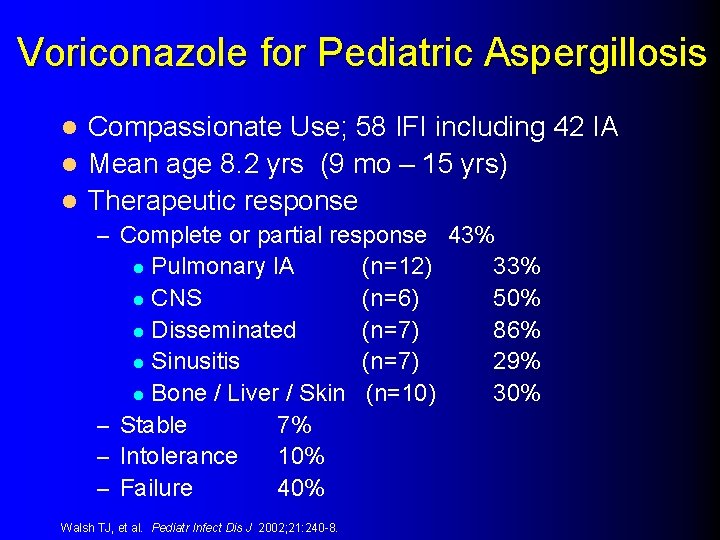
Voriconazole for Pediatric Aspergillosis Compassionate Use; 58 IFI including 42 IA l Mean age 8. 2 yrs (9 mo – 15 yrs) l Therapeutic response l – Complete or partial response 43% Pulmonary IA l CNS l Disseminated l Sinusitis l Bone / Liver / Skin – Stable 7% – Intolerance 10% – Failure 40% l Walsh TJ, et al. Pediatr Infect Dis J 2002; 21: 240 -8. (n=12) (n=6) (n=7) (n=10) 33% 50% 86% 29% 30%

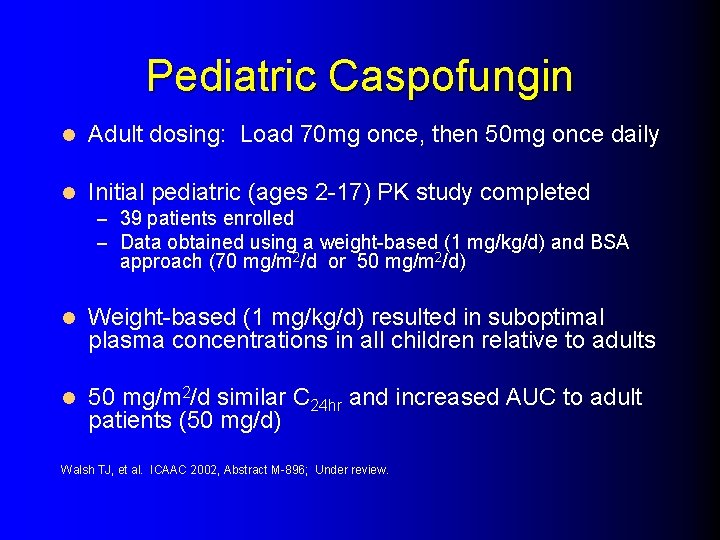
Pediatric Caspofungin l Adult dosing: Load 70 mg once, then 50 mg once daily l Initial pediatric (ages 2 -17) PK study completed – 39 patients enrolled – Data obtained using a weight-based (1 mg/kg/d) and BSA approach (70 mg/m 2/d or 50 mg/m 2/d) l Weight-based (1 mg/kg/d) resulted in suboptimal plasma concentrations in all children relative to adults l 50 mg/m 2/d similar C 24 hr and increased AUC to adult patients (50 mg/d) Walsh TJ, et al. ICAAC 2002, Abstract M-896; Under review.

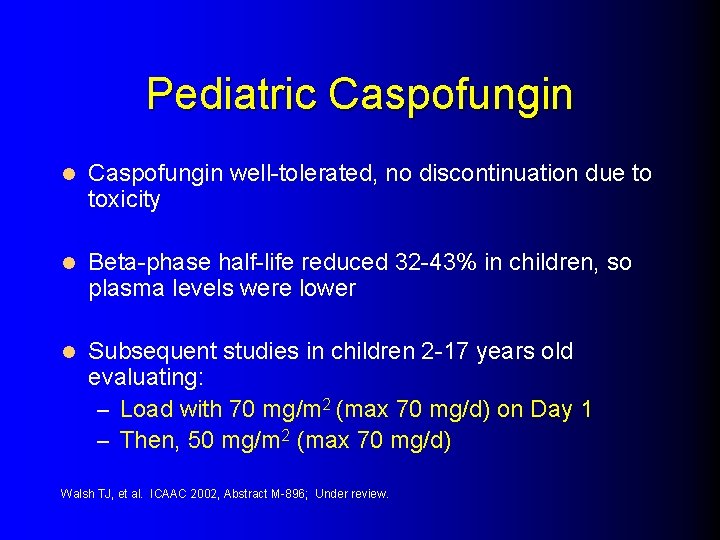
Pediatric Caspofungin l Caspofungin well-tolerated, no discontinuation due to toxicity l Beta-phase half-life reduced 32 -43% in children, so plasma levels were lower l Subsequent studies in children 2 -17 years old evaluating: – Load with 70 mg/m 2 (max 70 mg/d) on Day 1 – Then, 50 mg/m 2 (max 70 mg/d) Walsh TJ, et al. ICAAC 2002, Abstract M-896; Under review.

Summary l l l Aspergillus epidemiology changing GM assay interpretations different in specific populations Aspergillus q. PCR still debated for diagnosis Echinocandins unlikely to be best monotherapy (fungistatic against Aspergillus) l Voriconazole is clearly the best monotherapy l Voriconzole primary therapy better than salvage therapy l Voriconazole has linear pharmacokinetics in children l l Combination therapy – unproven – Reports are often contradictory – Potentially would be best if used as primary therapy

 Doc for invasive aspergillosis
Doc for invasive aspergillosis Aspergillosis symptoms
Aspergillosis symptoms Aspergillosis symptoms
Aspergillosis symptoms Aspergillosis
Aspergillosis Aspergillosis
Aspergillosis Invasive ductal carcinoma with medullary features
Invasive ductal carcinoma with medullary features Non invasive cgm
Non invasive cgm Non invasive health monitor
Non invasive health monitor Invasive candidiasis
Invasive candidiasis Indiana invasive species council
Indiana invasive species council Etetp
Etetp Sesleria autumnalis spacing
Sesleria autumnalis spacing What is the aim of antt for all invasive procedures
What is the aim of antt for all invasive procedures Invasive species characteristics
Invasive species characteristics Exponential growth of invasive species
Exponential growth of invasive species Invasive plant atlas
Invasive plant atlas Invasive beatmung über tracheostoma
Invasive beatmung über tracheostoma Invasive species laws
Invasive species laws Invasive species investigator worksheet
Invasive species investigator worksheet 100 worst invasive species
100 worst invasive species Invasive species characteristics
Invasive species characteristics Lerman non invasive halo
Lerman non invasive halo Are invasive species always bad
Are invasive species always bad Invasive meningococcal disease
Invasive meningococcal disease Non invasive ventilation
Non invasive ventilation Lerman halo
Lerman halo Exotic species definition biology
Exotic species definition biology Ontario invasive fish
Ontario invasive fish Invasive species investigator worksheet
Invasive species investigator worksheet Invasive species characteristics
Invasive species characteristics Invasive species act ontario
Invasive species act ontario Minimally invasive surgery
Minimally invasive surgery Invasive species compendium
Invasive species compendium Non invasive ventilation
Non invasive ventilation Andrew heywood comparative politics
Andrew heywood comparative politics Irpwm new
Irpwm new Upstu
Upstu Citect pty ltd
Citect pty ltd D
D Reported commands
Reported commands Latest subject integrity
Latest subject integrity Florence and the machine lyrics
Florence and the machine lyrics Ubbl 2012
Ubbl 2012 Latest relocation trends
Latest relocation trends Oracle apex latest version features
Oracle apex latest version features Electronics and information technology department odisha
Electronics and information technology department odisha Prayer items for the philippines
Prayer items for the philippines Letter writing formal and informal
Letter writing formal and informal Dd form 577 latest version
Dd form 577 latest version Is 10262 : 2019
Is 10262 : 2019 Sgrrits dehradun
Sgrrits dehradun Nbc
Nbc Sspc-pa 2 latest edition
Sspc-pa 2 latest edition Illustrate the organizational structure of the pnp
Illustrate the organizational structure of the pnp Wco data model
Wco data model Latest trands
Latest trands Sbm assessment tool 2021 excel
Sbm assessment tool 2021 excel Ms word latest version
Ms word latest version