Kinetika i kataliza Osnovni pojmovi u hemijskoj kinetici

















- Slides: 21

Kinetika i kataliza Osnovni pojmovi u hemijskoj kinetici ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Osnovni pojmovi u hemijskoj kinetici • Hemijska reakcija • Hemijska termodinamika • Hemiska kinetika je nauka koja proucava kvantitativne zakonitosti hemijskih reakcija i procesa, koje se odvijaju u vremenu i prostoru. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

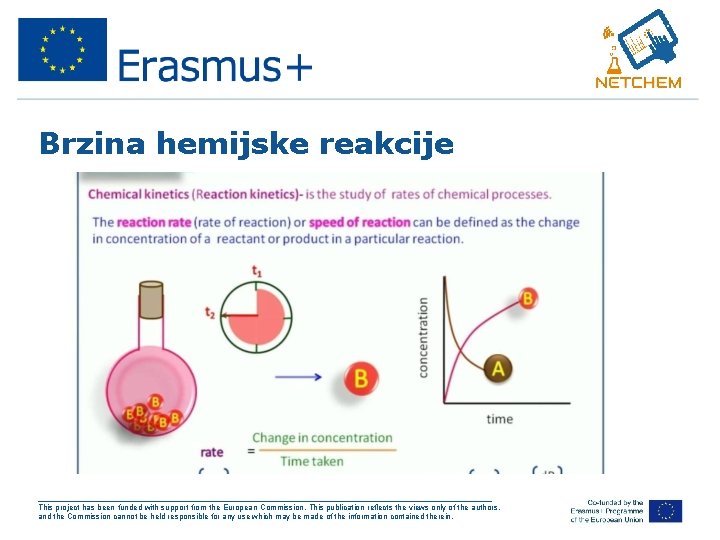
Brzina hemijske reakcije ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Postavlja se pitanje: koliko traju hemijske ili koliko su hemijske reakcije brze i koji tip hemijskog reagovanja ima najvecu a koji najmanju brzinu? Posmatramo reakciju prenosa elektrona: Na + Cl →Na+ + Cl. Minimalno vreme za koje elektron predje sa jona Na na jon Cl dobija se iz relacije: t=d/v d-rastojanje izmedju dva atoma v- brzina elektrona ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Osnovni pojmovi u hemijskoj kinetici Ako se zna vrednost Ek elektrona koja je jednaka prosecnoj energiji koja se oslobadja pri formiranju neke hemijske veze (300 -400 k. J/mol), onda se moze brzina izracunati. Vreme potrebno da e predje sa Na na Cl je 5 x 10 -16 s. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Podela hemijske kinetike • Hemijska kinetika je nauka koja proucava kvantitativne zakonitosti hemijskih reakcija i procesa, koje se odvijaju u vremenu i prostoru. 1. Kinetika homogenih hemijskih reakcija 2. Kinetika katalitickih hemijskih reakcija 3. Kinetika heterogenih topohemijskih reakcija 4. Neravnotezna hemijska kinetika 5. Termodinamika neravnoteznih hemijskih reakcija ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Hemijska energija, kinetička energija ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

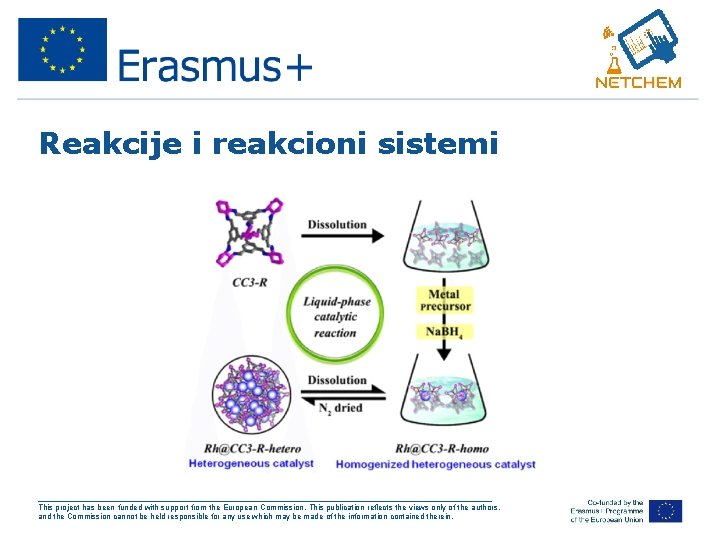
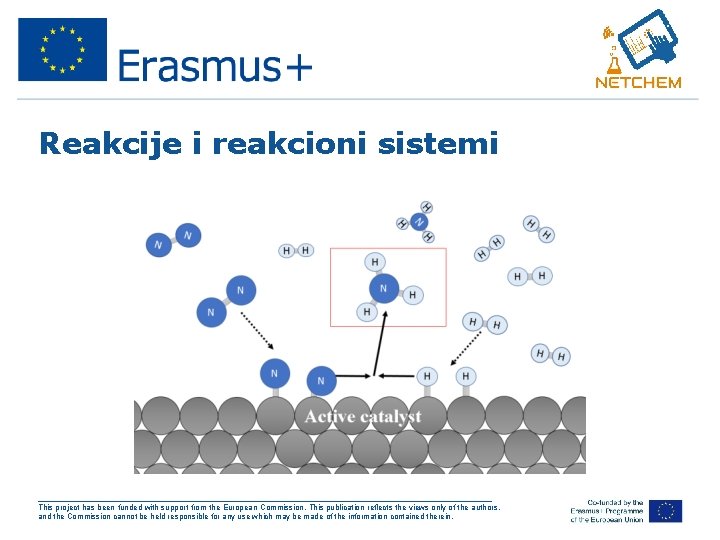
Reakcije i reakcioni sistemi • Otvoreni reakcioni sistem • Zatvoreni reakcioni sistem • Homogena hemijska reakcija • Heterogena hemijska reakcija ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Reakcije i reakcioni sistemi ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Reakcije i reakcioni sistemi ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Reakcija građenja amonijaka • N 2 +3 H 2 → 2 NH 3 Poznato je da se gradjenje amonijaka odvija vrlo brzo u prisustvu katalizatora (gvozdja) ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

• Reakcioni sistem u kome postoji vise reakcionih stupnjeva –slozeni reakcioni sistem • Oksidacija Fe 2+ u kiseloj sredini atmosferskim kiseonikom: 4 Fe 2++4 H+ +O 2 → 4 Fe 3+ + 2 H 2 O Ova oksido-redukcija odvija se u vise stupnjeva ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Oksido-redukcija u vise stupnjeva • ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Elementarna reakcija • Elementarna reakcijahemijsku promenu kaja se odvija u jednom stupnju u kome nije moguce eksperimentalno identifikovati intermedijere tj. međuproduktne reakcije. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Molekularnost • Monomolekulske - jedan molekul se transformiše u produkt • Bimolekulske reakcije - predstavljaju sudar dva molekula istovremeno, a redje su tri i višemolekulske ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Reakcije ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Monomolekulske reakcije O 3 → O 2 + O C 2 H 6 → 2 CH 3 ( reakcija termičkog razlaganja etana na dva metalradikala) 2 C 2 H 5 = n-C 4 H 10 ( reakcija asocijacije ili rekombinacije u kojima dolazi do stvaranja jedne veze – dva etil radikala u nbutanu ). ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Bimolekulske reakcije CO + Cl 2 → COCl 2 CH 4 + Cl → CH 3 + HCl C 2 H 4 + HI → C 2 H 5 I O 3 + NO → O 2 + NO 2 Br + CHCl 3 → HBr + CCl 3( reakcije sjedinjavanja, reakcije izmene…) ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Trimolekulske reakcije • 2 NO + O 2 → 2 NO 2 • 2 NO + Cl 2 → 2 NOCl • 2 J + H 2 → 2 HJ • Potrebno je da se sudare tri cestice istovremeno sto je veoma retko ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Author, Editor and Referee References This remote access laboratory was created thanks to work done primarily at University of Niš. Contributors to this material were: _Emilija Pecev-Marinkovic________ Refereeing of this material was done by: ___________ Editing into NETCHEM Format and onto NETCHEM platform was completed by: __________________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, ___________________________________________________ andhas the been Commission be held responsible for any use which may made of the information contained This project funded cannot with support from the European Commission. Thisbe publication reflects the views only of therein. the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

References and Supplemental Material The NETCHEM platform was established at the University of Nis in 2016 -2019 through the Erasmus Programme. Please contact a NETCHEM representatives at your institution or visit our website for an expanded contact list. The work included had been led by the NETCHEM staff at your institution. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, ___________________________________________________ andhas the been Commission be held responsible for any use which may made of the information contained This project funded cannot with support from the European Commission. Thisbe publication reflects the views only of therein. the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.
 Pojam informatike
Pojam informatike Excel osnovni pojmovi
Excel osnovni pojmovi Hemija osnovni pojmovi
Hemija osnovni pojmovi Koncentrične kružnice
Koncentrične kružnice Opisna geometrija
Opisna geometrija Osnovni pojmovi u programiranju
Osnovni pojmovi u programiranju Osnove informatike
Osnove informatike Osnovni pojmovi u informatici
Osnovni pojmovi u informatici Pravila ponasanja u laboratoriji
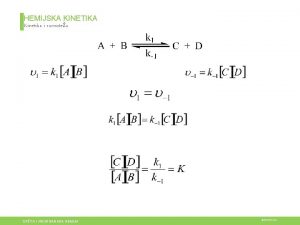
Pravila ponasanja u laboratoriji Hemijska kinetika
Hemijska kinetika Hukum laju terintegrasi
Hukum laju terintegrasi Procesy katalityczne
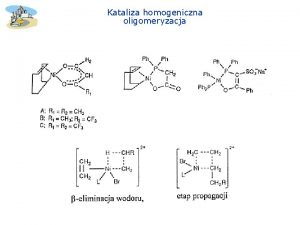
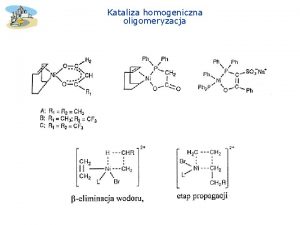
Procesy katalityczne Katalizator homogeniczny
Katalizator homogeniczny Crtanje logičkih sklopova
Crtanje logičkih sklopova Test književnost 6 razred
Test književnost 6 razred Grad drzava pojmovi
Grad drzava pojmovi Književni rodovi i vrste 5 razred
Književni rodovi i vrste 5 razred Likovni pojmovi
Likovni pojmovi Informatički pojmovi
Informatički pojmovi Medijska kultura 8 razred pojmovi
Medijska kultura 8 razred pojmovi Pojmovi za crtanje
Pojmovi za crtanje Pjeni se more analiza pjesme
Pjeni se more analiza pjesme