Kinetics Followup Average Rate Instantaneous rate of reactant


![Order of Reaction A + B → C • Rate = k[A]n [B]m • Order of Reaction A + B → C • Rate = k[A]n [B]m •](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-9.jpg)
![If you plot the concentration versus time of [N 2 O 5], you can If you plot the concentration versus time of [N 2 O 5], you can](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-11.jpg)
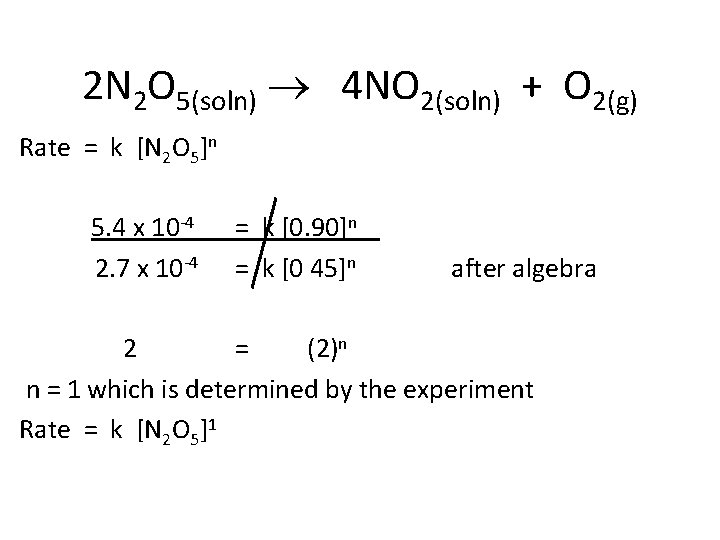

![Answer: • • • Rate = k[I-]x[OCl-]y 7. 91 x 10 -2 = k(0. Answer: • • • Rate = k[I-]x[OCl-]y 7. 91 x 10 -2 = k(0.](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-21.jpg)
![Integrated Law - Zero Order Rate = - [A] = k t Set up Integrated Law - Zero Order Rate = - [A] = k t Set up](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-24.jpg)
![Integrated Rate Law – First Order Rate = [A] = k [A] n t Integrated Rate Law – First Order Rate = [A] = k [A] n t](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-25.jpg)
![Why? If Rate = - [A] = k [A] 1 t Then you set Why? If Rate = - [A] = k [A] 1 t Then you set](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-26.jpg)
![Integrated Rate Law ln[A] = -kt + ln[A]0 • The equation shows the [A] Integrated Rate Law ln[A] = -kt + ln[A]0 • The equation shows the [A]](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-27.jpg)

![Zero Rate Law Rate = K[A]0 First Second Rate = K[A]1 Rate = K[A]2 Zero Rate Law Rate = K[A]0 First Second Rate = K[A]1 Rate = K[A]2](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-33.jpg)
![___1___ [C 4 H 6] Ln [C 4 H 6] Graphical Analysis ___1___ [C 4 H 6] Ln [C 4 H 6] Graphical Analysis](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-36.jpg)
![Experimental Derivation of Reaction Order • Arrange data in the form 1/[A] or ln Experimental Derivation of Reaction Order • Arrange data in the form 1/[A] or ln](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-37.jpg)

![Half-Life Algebraic Determination Half-life Zero First Second t 1/2 = [A]0 2 k t Half-Life Algebraic Determination Half-life Zero First Second t 1/2 = [A]0 2 k t](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-40.jpg)
- Slides: 40

Kinetics Follow-up

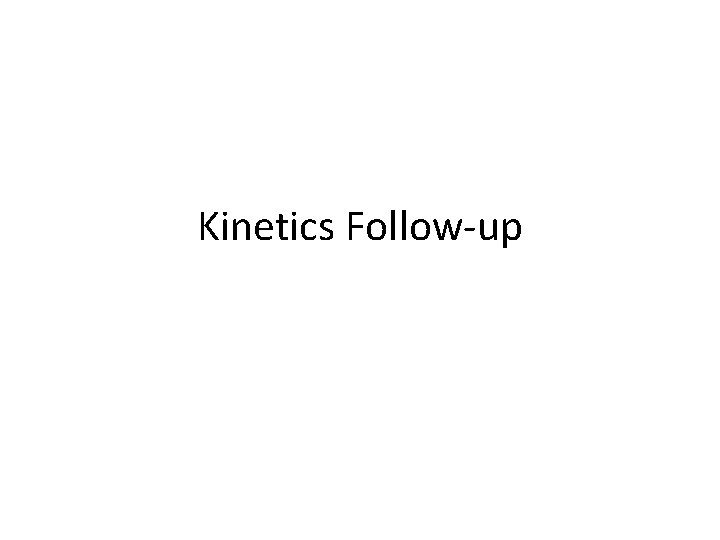
Average Rate Instantaneous rate of reactant disappearance Instantaneous rate of product formation

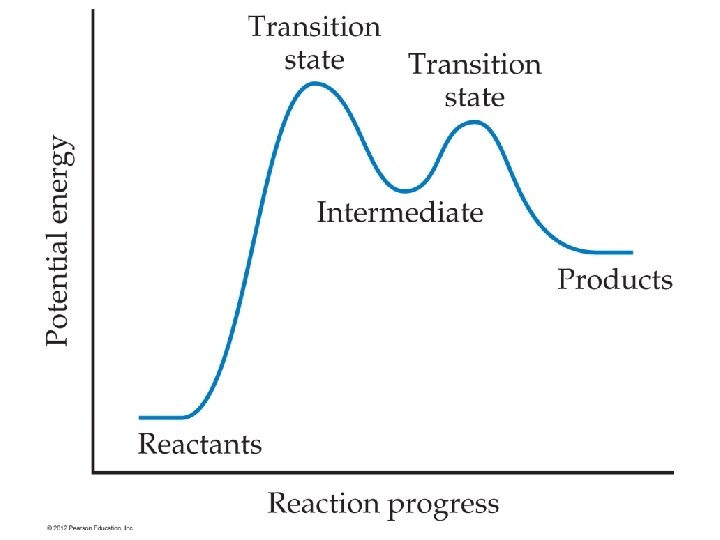
Mechanisms • Reactions take place over the course of several steps. • In some cases pieces of particles with unpaired electrons called radicals form as transition states before temporarily forming intermediates. • The different steps have different rates. • The overall rate of the reaction is closest to the rate of the slowest step. • This is why the order is not exactly matching the stoichiometric coefficients for most reactions.


Slow first steps • Step 1: NO 2 + NO 2 → NO 3 + NO (slow) • Step 2: NO 3 +CO → NO 2 + CO 2 (fast) • Overall reaction • NO 2 + CO → NO + CO 2 • Rate = k[NO 2]2 (matches all reactants needed for the slow step)

Fast First Steps • • • Reaction : NO + Br 2 → 2 NOBr Step 1: NO + Br 2 → NOBr 2 (fast) Step 2: NOBr 2 + NO → 2 NOBr (slow) Rate = k[NO]2[Br 2] All reactants necessary for the first reaction and the second reaction are in the rate law, and all intermediates are removed.

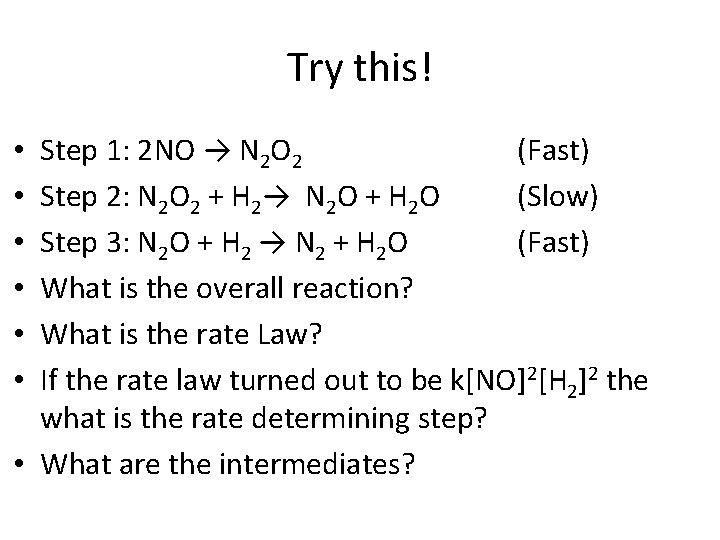
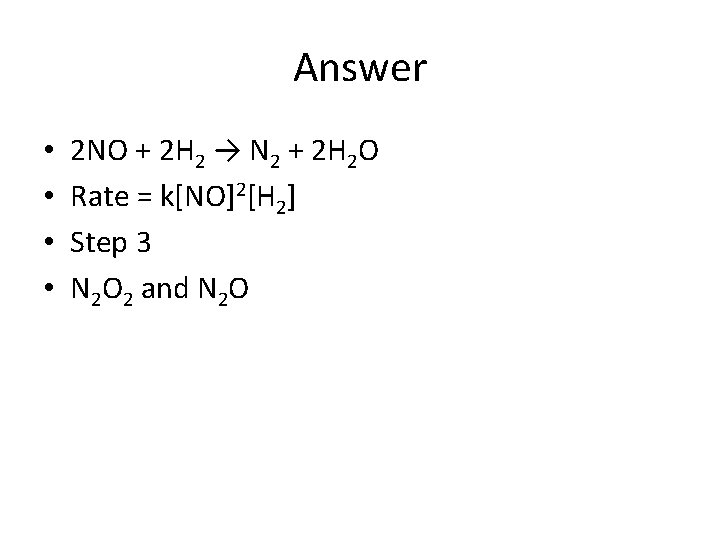
Try this! Step 1: 2 NO → N 2 O 2 (Fast) Step 2: N 2 O 2 + H 2→ N 2 O + H 2 O (Slow) Step 3: N 2 O + H 2 → N 2 + H 2 O (Fast) What is the overall reaction? What is the rate Law? If the rate law turned out to be k[NO]2[H 2]2 the what is the rate determining step? • What are the intermediates? • • •

Answer • • 2 NO + 2 H 2 → N 2 + 2 H 2 O Rate = k[NO]2[H 2] Step 3 N 2 O 2 and N 2 O
![Order of Reaction A B C Rate kAn Bm Order of Reaction A + B → C • Rate = k[A]n [B]m •](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-9.jpg)
Order of Reaction A + B → C • Rate = k[A]n [B]m • (n + m) = order of the reaction = 1 unimolecular =2 bimolecular =3 trimolecular This means how many particles are involved in the rate determining step

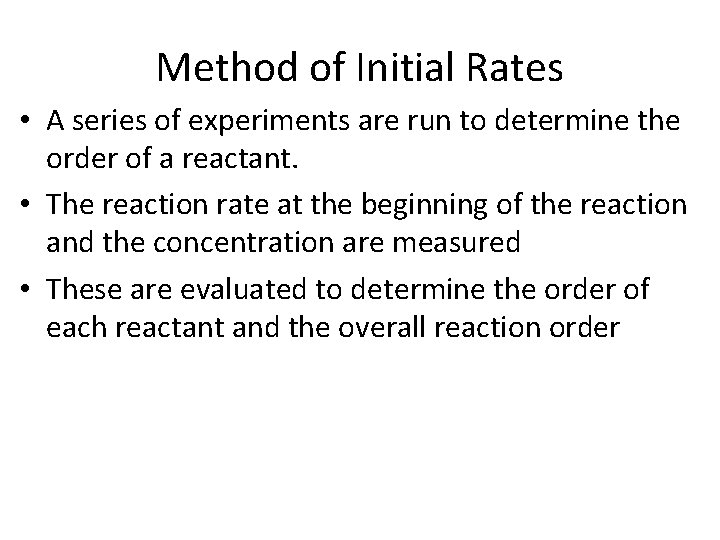
Method of Initial Rates • A series of experiments are run to determine the order of a reactant. • The reaction rate at the beginning of the reaction and the concentration are measured • These are evaluated to determine the order of each reactant and the overall reaction order
![If you plot the concentration versus time of N 2 O 5 you can If you plot the concentration versus time of [N 2 O 5], you can](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-11.jpg)
If you plot the concentration versus time of [N 2 O 5], you can determine the rate at 0. 90 M and 0. 45 M. What is the rate law for this reaction? Rate = k [N 2 O 5]n n = the order. It is determined experimentally.

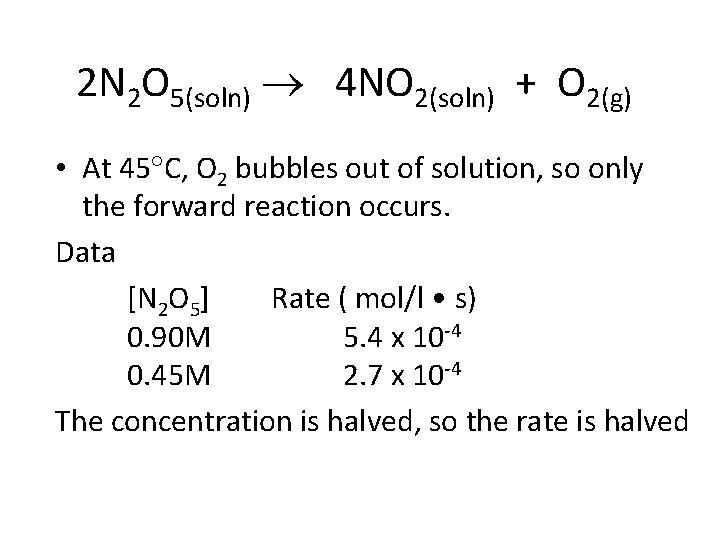
2 N 2 O 5(soln) 4 NO 2(soln) + O 2(g) • At 45 C, O 2 bubbles out of solution, so only the forward reaction occurs. Data [N 2 O 5] Rate ( mol/l • s) 0. 90 M 5. 4 x 10 -4 0. 45 M 2. 7 x 10 -4 The concentration is halved, so the rate is halved
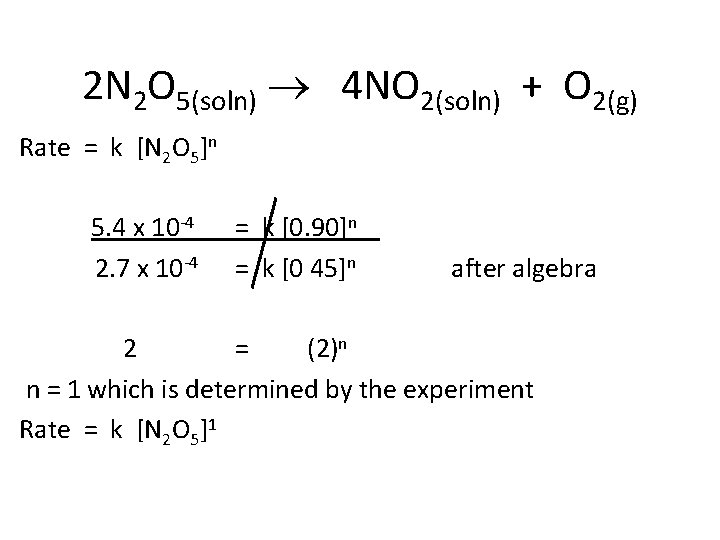
2 N 2 O 5(soln) 4 NO 2(soln) + O 2(g) Rate = k [N 2 O 5]n 5. 4 x 10 -4 2. 7 x 10 -4 = k [0. 90]n = k [0 45]n after algebra 2 = (2)n n = 1 which is determined by the experiment Rate = k [N 2 O 5]1

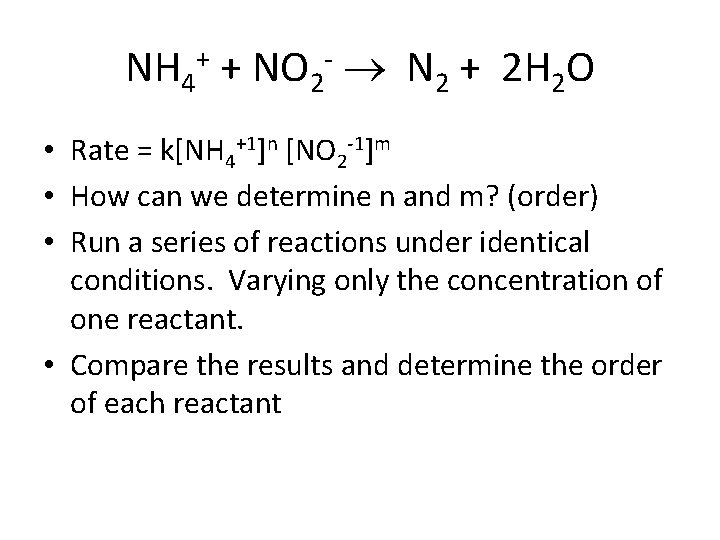
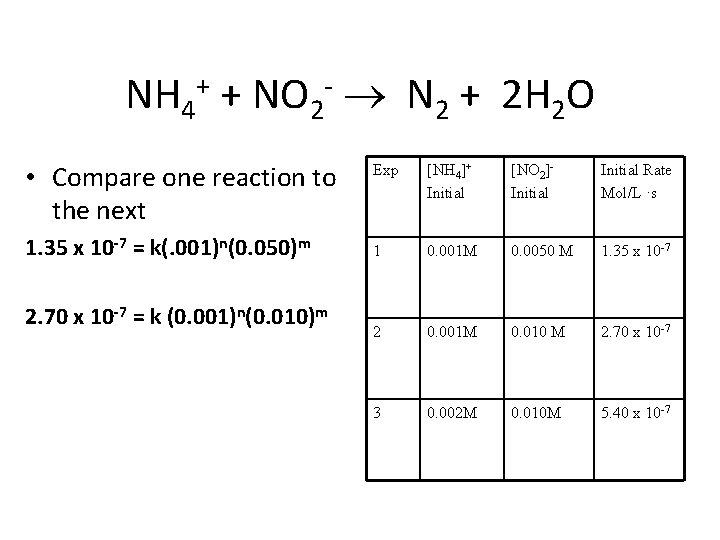
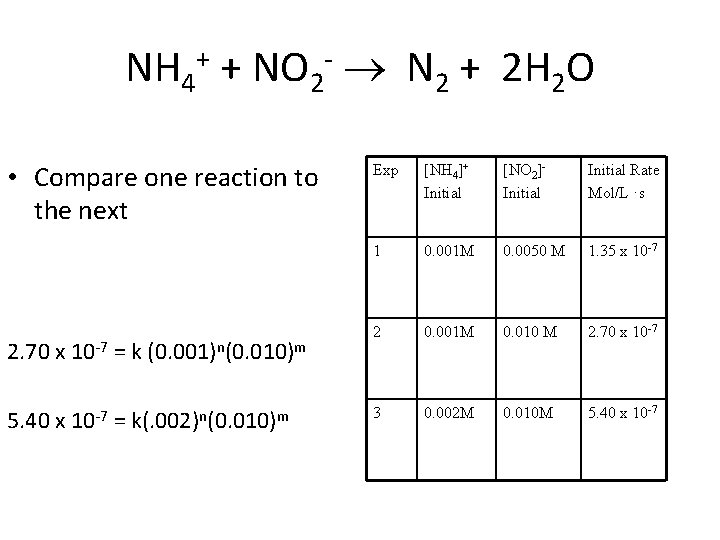
NH 4+ + NO 2 - N 2 + 2 H 2 O • Rate = k[NH 4+1]n [NO 2 -1]m • How can we determine n and m? (order) • Run a series of reactions under identical conditions. Varying only the concentration of one reactant. • Compare the results and determine the order of each reactant

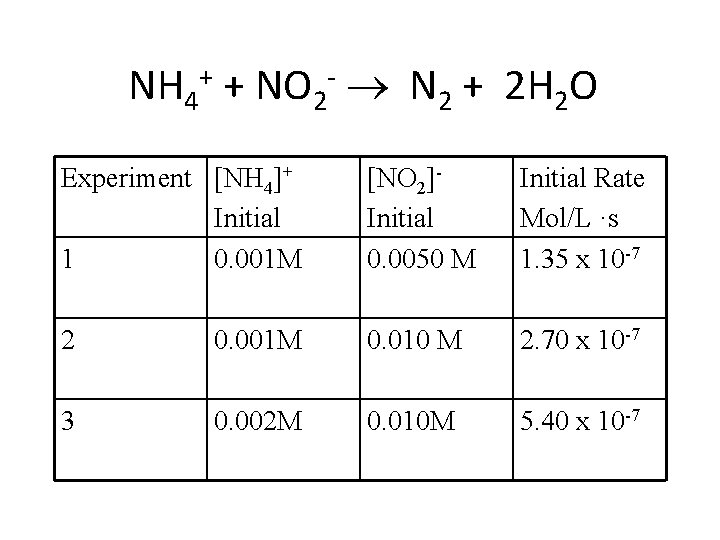
NH 4+ + NO 2 - N 2 + 2 H 2 O Experiment [NH 4]+ Initial 1 0. 001 M [NO 2]Initial 0. 0050 M Initial Rate Mol/L ·s 1. 35 x 10 -7 2 0. 001 M 0. 010 M 2. 70 x 10 -7 3 0. 002 M 0. 010 M 5. 40 x 10 -7

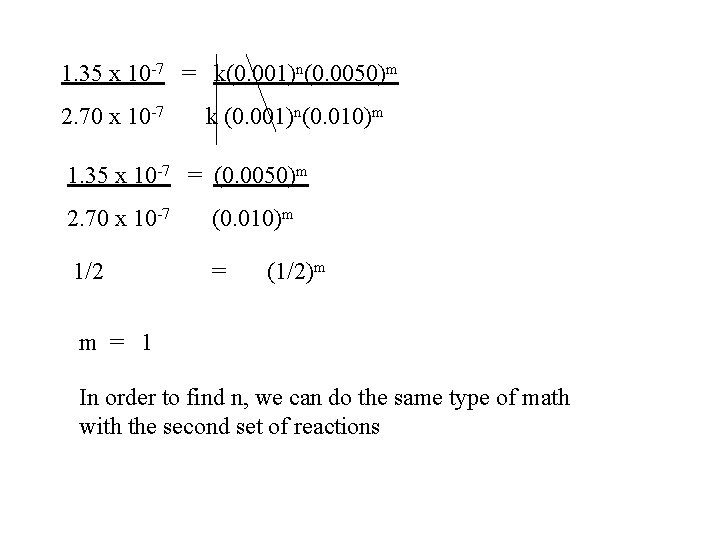
NH 4+ + NO 2 - N 2 + 2 H 2 O • Compare one reaction to the next 1. 35 x 10 -7 = k(. 001)n(0. 050)m 2. 70 x 10 -7 = k (0. 001)n(0. 010)m Exp [NH 4]+ Initial [NO 2]Initial Rate Mol/L ·s 1 0. 001 M 0. 0050 M 1. 35 x 10 -7 2 0. 001 M 0. 010 M 2. 70 x 10 -7 3 0. 002 M 0. 010 M 5. 40 x 10 -7

1. 35 x 10 -7 = k(0. 001)n(0. 0050)m 2. 70 x 10 -7 k (0. 001)n(0. 010)m 1. 35 x 10 -7 = (0. 0050)m 2. 70 x 10 -7 (0. 010)m 1/2 = (1/2)m m = 1 In order to find n, we can do the same type of math with the second set of reactions

NH 4+ + NO 2 - N 2 + 2 H 2 O • Compare one reaction to the next 2. 70 x 10 -7 = k (0. 001)n(0. 010)m 5. 40 x 10 -7 = k(. 002)n(0. 010)m Exp [NH 4]+ Initial [NO 2]Initial Rate Mol/L ·s 1 0. 001 M 0. 0050 M 1. 35 x 10 -7 2 0. 001 M 0. 010 M 2. 70 x 10 -7 3 0. 002 M 0. 010 M 5. 40 x 10 -7

2. 70 x 10 -7 = k (0. 001)n(0. 010)m 5. 40 x 10 -7 k(. 002)n(0. 010)m 0. 5 = (0. 5)n n = 1 n + m = order of the reaction 1 + 1 = 2 or second order

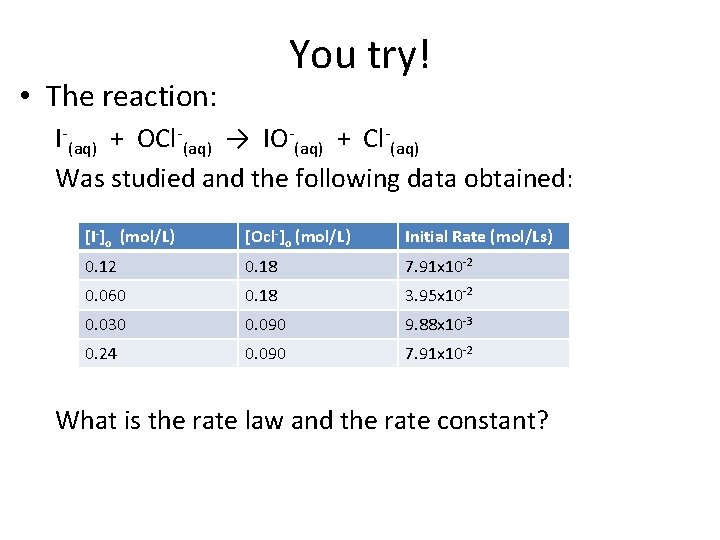
You try! • The reaction: I-(aq) + OCl-(aq) → IO-(aq) + Cl-(aq) Was studied and the following data obtained: [I-]o (mol/L) [Ocl-]o (mol/L) Initial Rate (mol/Ls) 0. 12 0. 18 7. 91 x 10 -2 0. 060 0. 18 3. 95 x 10 -2 0. 030 0. 090 9. 88 x 10 -3 0. 24 0. 090 7. 91 x 10 -2 What is the rate law and the rate constant?
![Answer Rate kIxOCly 7 91 x 10 2 k0 Answer: • • • Rate = k[I-]x[OCl-]y 7. 91 x 10 -2 = k(0.](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-21.jpg)
Answer: • • • Rate = k[I-]x[OCl-]y 7. 91 x 10 -2 = k(0. 12)x(0. 18)y 3. 95 x 10 -2 k(0. 060)x(0. 18)y 2. 00 = 2. 0 x x=1 3. 95 x 10 -2 = k(0. 060)1(0. 18)y 9. 88 x 10 -3 k(0. 030)1(0. 090)y 4. 00 = (2)(2 y) y=1 Rate = k[I-][OCl-] 7. 91 x 10 -2 mol/Ls = k(0. 12 M)(0. 18 M) = 3. 7 L/mol s

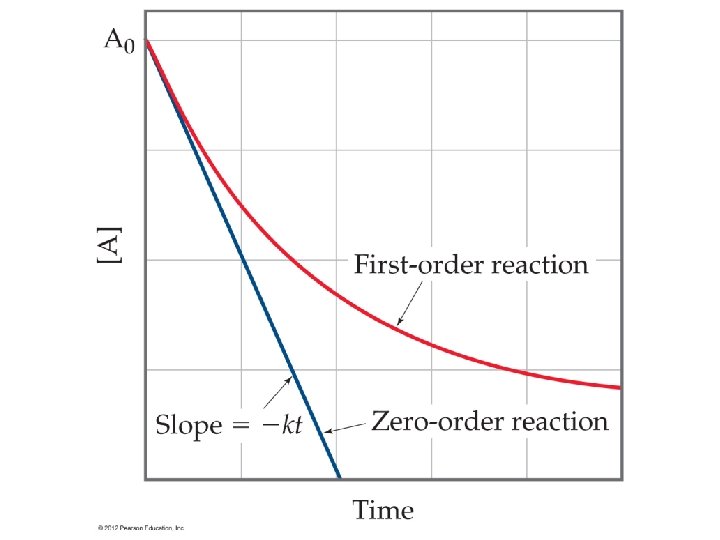
The Integrated Rate Law • Expresses how concentrations depend on time • Depends on the order of the reaction Remember • Rate = k[A]n[B]m Order = n + m • Integrated Rate law takes the form by “integrating” the rate function. (calculus used to determine) – The value of n and m change the order of the reaction – The form of the integrated rate depends on the value of n – You get a different equation for zero, first and second order equations.

Reaction Order • Order of the reaction determines or affects our calculations. • Zero order indicates the use of a catalyst or enzyme. The surface area of catalyst is the rate determining factor. • First or second order is more typical (of college problems)
![Integrated Law Zero Order Rate A k t Set up Integrated Law - Zero Order Rate = - [A] = k t Set up](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-24.jpg)
Integrated Law - Zero Order Rate = - [A] = k t Set up the differential equation d[A] = -kt Integral of 1 with respect to A is [A]
![Integrated Rate Law First Order Rate A k A n t Integrated Rate Law – First Order Rate = [A] = k [A] n t](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-25.jpg)
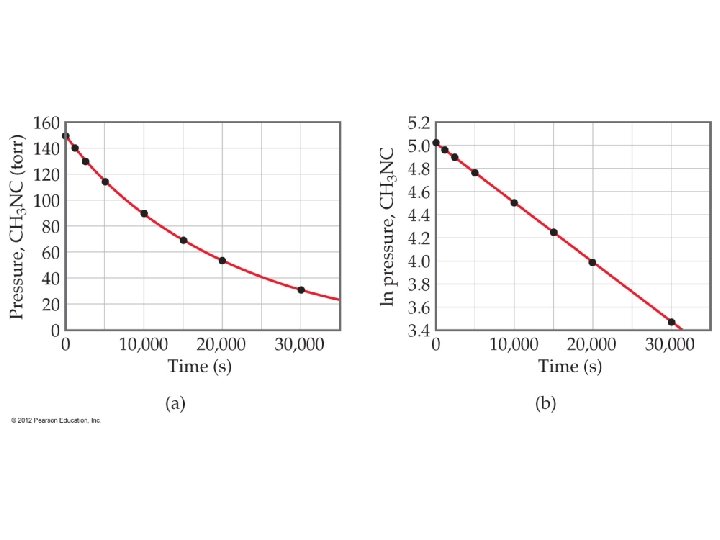
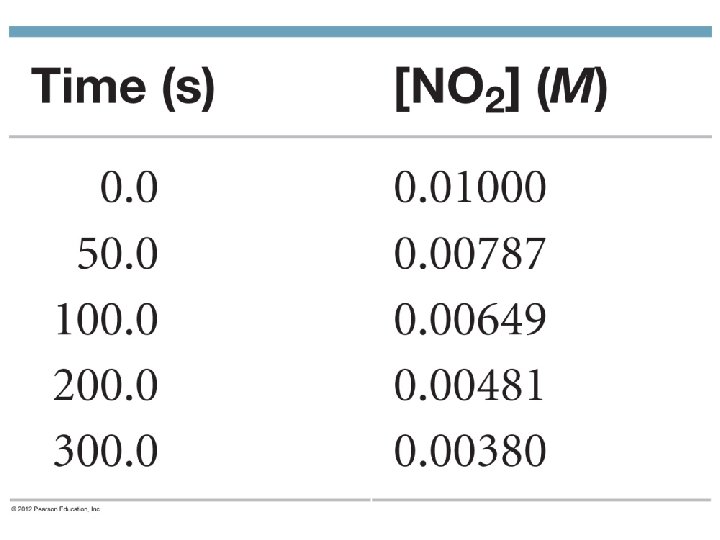
Integrated Rate Law – First Order Rate = [A] = k [A] n t If n = 1, this is a first order reaction. If we “integrate” this equation we get a new form. Ln[A] = -kt + ln[A 0] where A 0 is the initial concentration
![Why If Rate A k A 1 t Then you set Why? If Rate = - [A] = k [A] 1 t Then you set](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-26.jpg)
Why? If Rate = - [A] = k [A] 1 t Then you set up the differential equation: d[A] = -kdt [A] Integral of 1/[A] with respect to [A] is the ln[A].
![Integrated Rate Law lnA kt lnA0 The equation shows the A Integrated Rate Law ln[A] = -kt + ln[A]0 • The equation shows the [A]](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-27.jpg)
Integrated Rate Law ln[A] = -kt + ln[A]0 • The equation shows the [A] depends on time • If you know k and A 0, you can calculate the concentration at any time. • Is in the form y = mx +b Y = ln[A] m = -k b = ln[A]0 Can be rewritten ln( [A]0/[A] ) = kt • This equation is only good for first order reactions!





![Zero Rate Law Rate KA0 First Second Rate KA1 Rate KA2 Zero Rate Law Rate = K[A]0 First Second Rate = K[A]1 Rate = K[A]2](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-33.jpg)
Zero Rate Law Rate = K[A]0 First Second Rate = K[A]1 Rate = K[A]2 Integrated [A] = -kt + [A]0 Ln[A] = -kt +ln[A]0 1 = [A] Rate Law Line Slope = Half-life [A] vs t -k t 1/2 = [A]0 2 k ln[A] vs t -k t 1/2 = 0. 693 k 1 [A] kt + vs t k T 1/2 = 1 k[A]0 1 [A]0

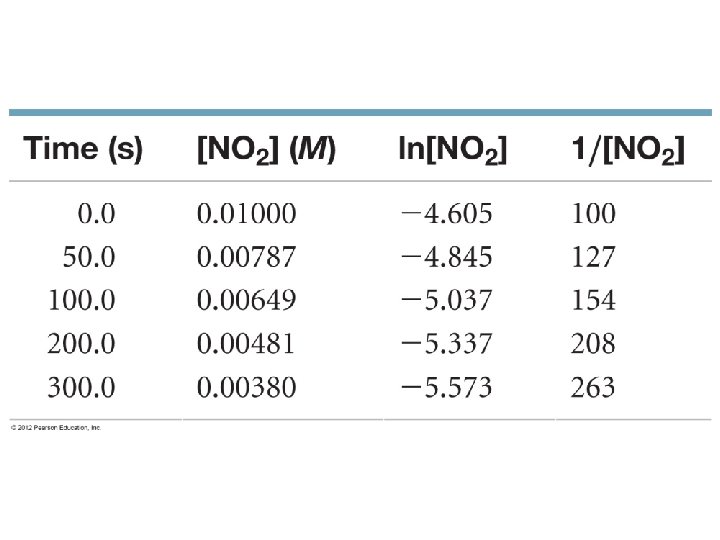
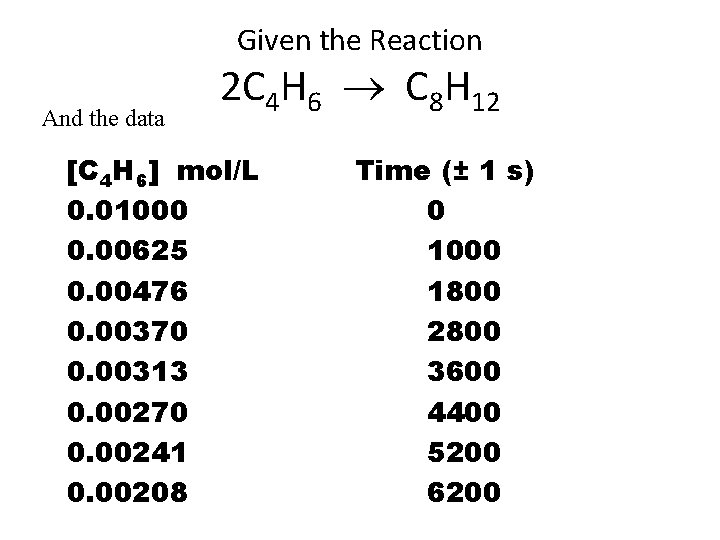
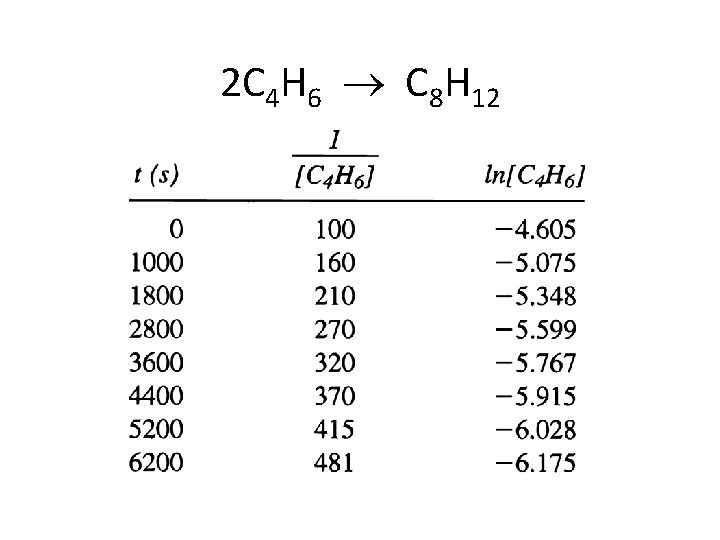
Given the Reaction And the data 2 C 4 H 6 C 8 H 12 [C 4 H 6] mol/L 0. 01000 0. 00625 0. 00476 0. 00370 0. 00313 0. 00270 0. 00241 0. 00208 Time (± 1 s) 0 1000 1800 2800 3600 4400 5200 6200

2 C 4 H 6 C 8 H 12
![1 C 4 H 6 Ln C 4 H 6 Graphical Analysis ___1___ [C 4 H 6] Ln [C 4 H 6] Graphical Analysis](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-36.jpg)
___1___ [C 4 H 6] Ln [C 4 H 6] Graphical Analysis
![Experimental Derivation of Reaction Order Arrange data in the form 1A or ln Experimental Derivation of Reaction Order • Arrange data in the form 1/[A] or ln](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-37.jpg)
Experimental Derivation of Reaction Order • Arrange data in the form 1/[A] or ln [A] • Plot the data vs time • Choose the straight line y = mx + b or [A] 1/[A] = kt + b → 2 nd ln[A] = kt + b → 1 st [A] = kt + b → zero • Determine the k value from the slope • Graphical rate laws

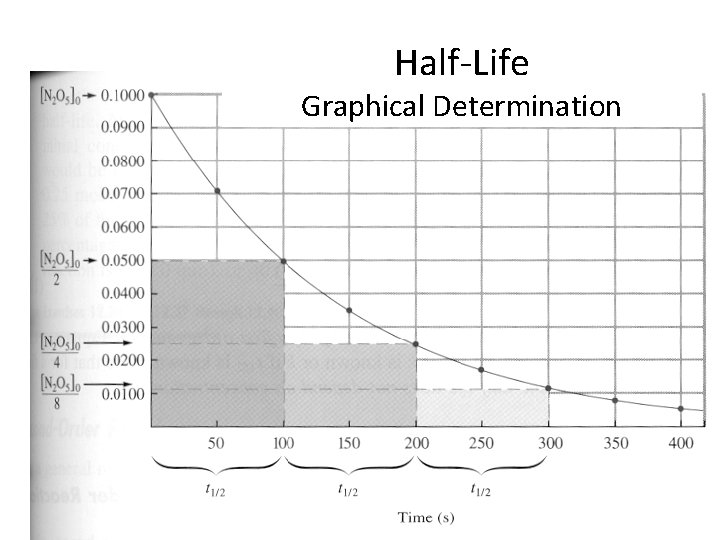
Half-life • The time it takes 1/2 of the reactant to be consumed • This can be determined – Graphically – Calculate from the integrated rate law

Half-Life Graphical Determination
![HalfLife Algebraic Determination Halflife Zero First Second t 12 A0 2 k t Half-Life Algebraic Determination Half-life Zero First Second t 1/2 = [A]0 2 k t](https://slidetodoc.com/presentation_image_h2/ffac24f25076b5a6f347953c079d09b3/image-40.jpg)
Half-Life Algebraic Determination Half-life Zero First Second t 1/2 = [A]0 2 k t 1/2 = 0. 693 k T 1/2 = Equations are derived from the Integrated Rate Laws. 1 k[A]0
 Instantaneous vs average rate of change
Instantaneous vs average rate of change Followup:actionitems
Followup:actionitems Followup visit
Followup visit Followup edge
Followup edge Instantaneous velocity vs average velocity
Instantaneous velocity vs average velocity How to calculate the instantaneous rate of reaction
How to calculate the instantaneous rate of reaction Math calculus
Math calculus Refers to the instantaneous rate of change of profit
Refers to the instantaneous rate of change of profit Formula of average rate of change
Formula of average rate of change Motion in a straight line formula
Motion in a straight line formula Instantaneous gas flows
Instantaneous gas flows Instantaneous current
Instantaneous current Si unit for angular displacement
Si unit for angular displacement Drag force ap physics c
Drag force ap physics c Lwwr
Lwwr The analysis of ac circuits uses a rotating vector called a
The analysis of ac circuits uses a rotating vector called a Instantaneous dipole
Instantaneous dipole Instantaneous velocity example
Instantaneous velocity example Instantaneous description of pda
Instantaneous description of pda Instantaneous voltage formula
Instantaneous voltage formula Instantaneous voltage formula
Instantaneous voltage formula Fall vectors
Fall vectors What meaning of tm
What meaning of tm Kinematics formulas
Kinematics formulas The instantaneous description is pda shows
The instantaneous description is pda shows Instantaneous acceleration
Instantaneous acceleration Curvilinear translation
Curvilinear translation Xxxz0
Xxxz0 Instantaneous codes
Instantaneous codes Induced dipole
Induced dipole Instantaneous velocity circular motion
Instantaneous velocity circular motion Rate of change ratio
Rate of change ratio The fundamental theorem of calculus
The fundamental theorem of calculus Pulse rate normal range by age
Pulse rate normal range by age Formula for average rate of change
Formula for average rate of change I have to present
I have to present Average rate of change exponential function
Average rate of change exponential function Cara menghitung room revenue hotel
Cara menghitung room revenue hotel Purpose of circuit training
Purpose of circuit training Average tax rate example
Average tax rate example Units for average rate of change
Units for average rate of change