Jayanti Tokas 1 Puneet Tokas 2 Shailini Jain









- Slides: 33

Jayanti Tokas 1, Puneet Tokas 2, Shailini Jain 3, Hariom Yadav 3 1 Department of Biotechnology, JMIT, Radaur, Haryana, India 2 KITM, Kurukshetra, Haryana, India 3 NIDDK, National Institute of Health, Bethesda, MD 20892, USA Email: yadavhariom@gmail. com

Stem Cells • Stem cells are master cells with two important characteristics – Unspecialized cells capable of their own renewal – Ability to differentiate into different cell types • The stem cells may have various differentiation potentials • • Totipotent Pluripotent Multipotent Unipotent

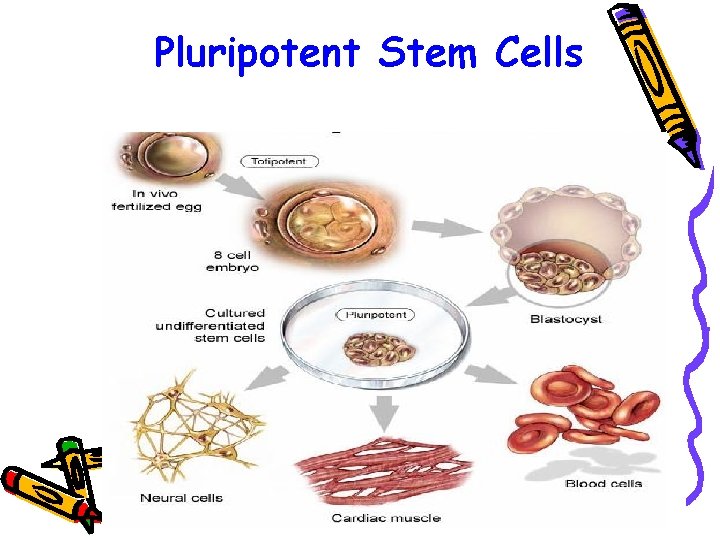
Pluripotent Stem Cells Pluri derived from latin word plures – means several or many Ø Most commonly the term is used to describe stem cells that give rise to cells derived from all three embryonic germ Ø

Pluripotent Stem Cells

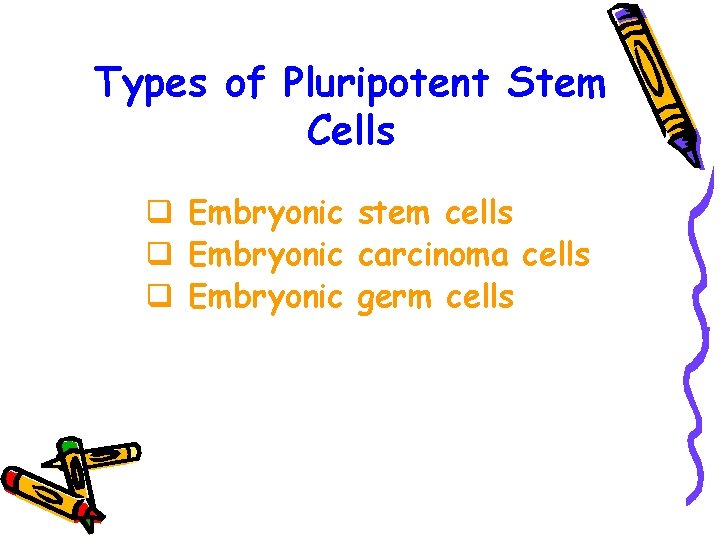
Types of Pluripotent Stem Cells q Embryonic stem cells q Embryonic carcinoma cells q Embryonic germ cells

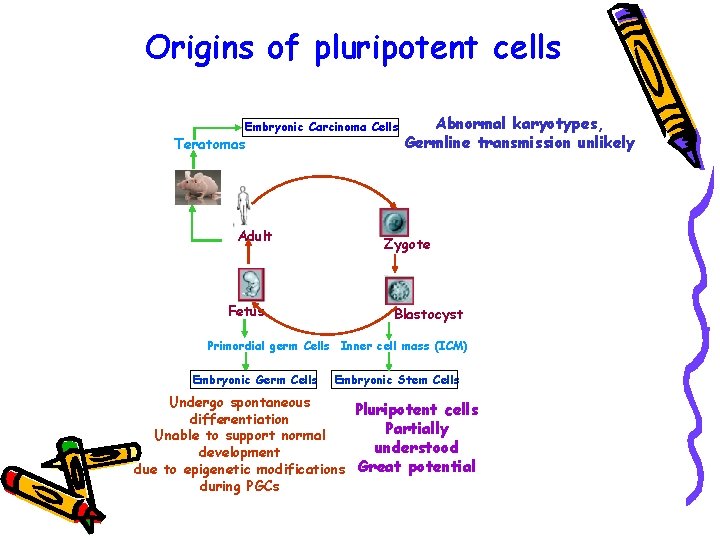
Origins of pluripotent cells Embryonic Carcinoma Cells Teratomas Adult Fetus Abnormal karyotypes, Germline transmission unlikely Zygote Blastocyst Primordial germ Cells Inner cell mass (ICM) Embryonic Germ Cells Embryonic Stem Cells Undergo spontaneous Pluripotent cells differentiation Partially Unable to support normal understood development due to epigenetic modifications Great potential during PGCs

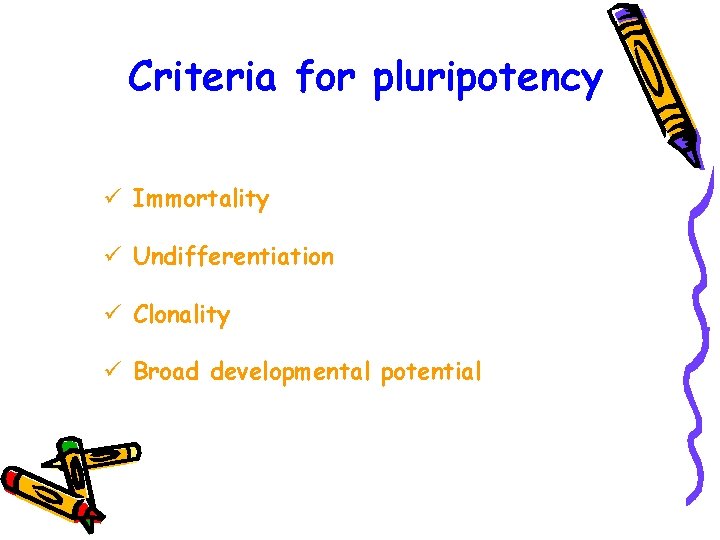
Criteria for pluripotency ü Immortality ü Undifferentiation ü Clonality ü Broad developmental potential

Demonstration of pluripotency

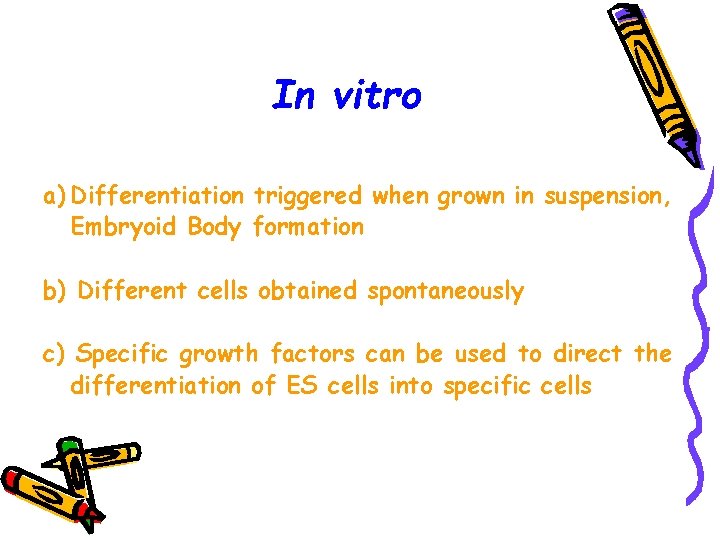
In vitro a) Differentiation triggered when grown in suspension, Embryoid Body formation b) Different cells obtained spontaneously c) Specific growth factors can be used to direct the differentiation of ES cells into specific cells

In vivo a) Teratoma formation when injected into nude mouse b) When injected into host blastocysts, the ES cells integrate, proliferate and differentiate into all germ lineages including germ cells

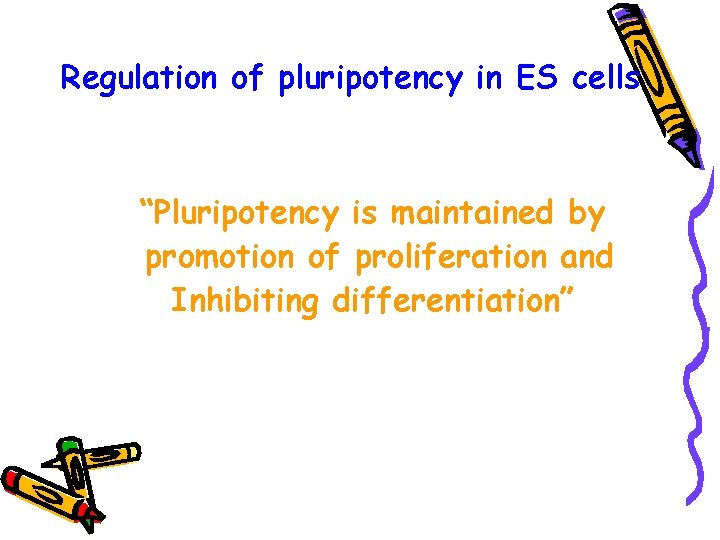
Regulation of pluripotency in ES cells “Pluripotency is maintained by promotion of proliferation and Inhibiting differentiation”

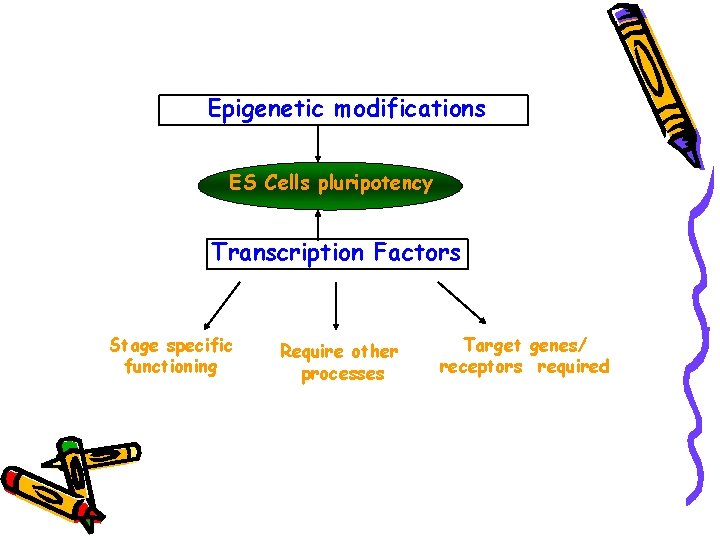
Epigenetic modifications ES Cells pluripotency Transcription Factors Stage specific functioning Require other processes Target genes/ receptors required

Factors Required Ø Nanog Ø Oct 3/4 Ø Sox 2 Ø LIF Ø c-Myc Ø Klf 4 Ø Zic 3

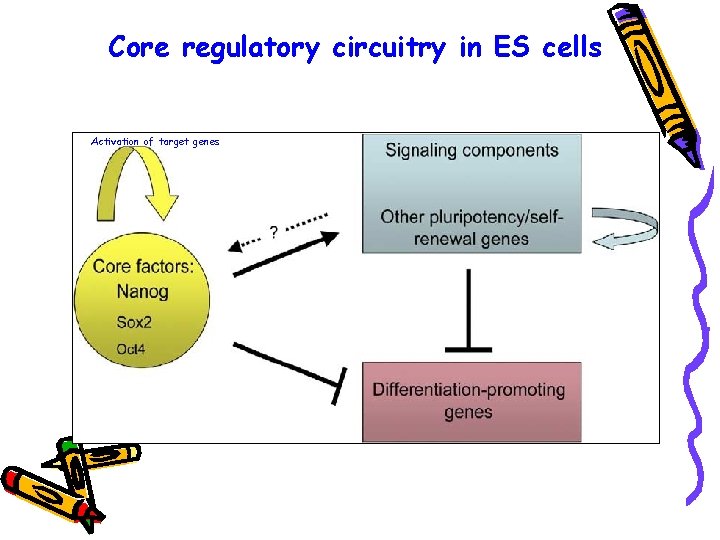
Core regulatory circuitry in ES cells Activation of target genes

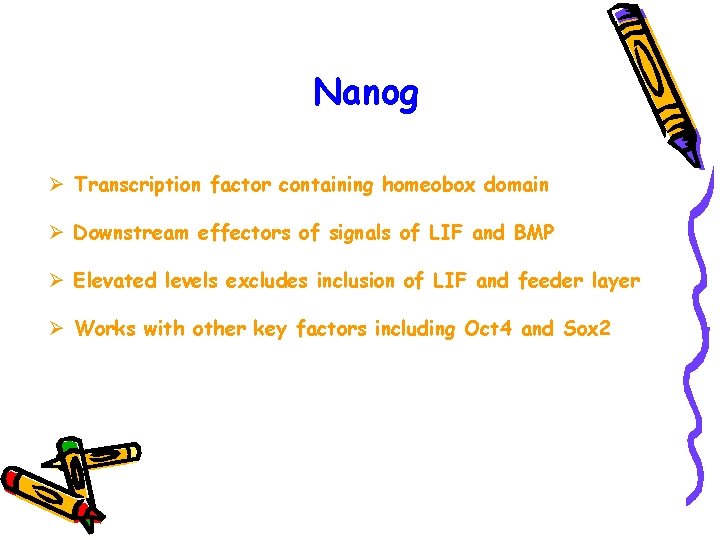
Nanog Ø Transcription factor containing homeobox domain Ø Downstream effectors of signals of LIF and BMP Ø Elevated levels excludes inclusion of LIF and feeder layer Ø Works with other key factors including Oct 4 and Sox 2

Oct 3/4 q POU-domain transcription factor q Maintains pluripotency (ESCs, EGCs, ECCs, GSCs) q Tightly regulated transcription factor, associated with a number of target genes implicated in pluripotency maintenance q Regulatory elements in target genes are in close vicinity of Sox 2 binding sites q Key factor in the transcriptional framework of self-renewing stem cells

Sox 2 Ø Member of HMG-domain DNA-BP family Ø Necessary for embryonal development and to prevent ES cell differentiation Ø Many ES cell pluripotency-associated genes are co-regulated by Sox 2 and Oct 3/4 Ø A ternary complex formed with Oct 4 or Oct 1 on enhancer sequence of Fgf 4 is must for functioning Ø Cooperate with other TFs, e. g. Nanog to activate transcription of pluripotency markers

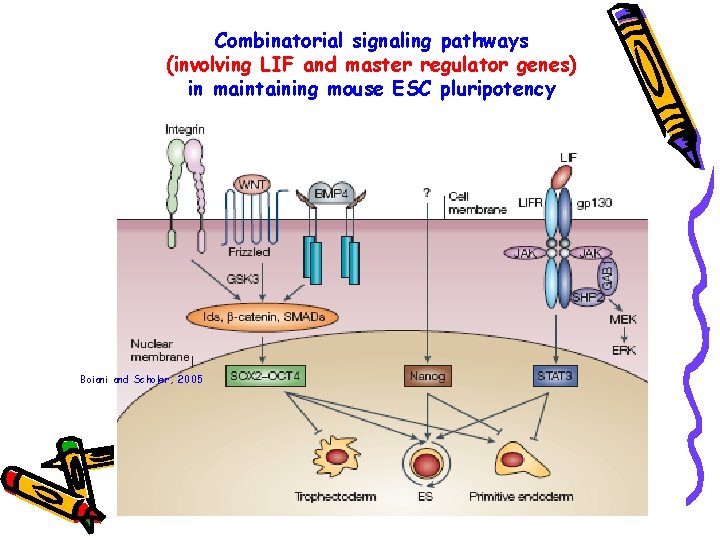
Leukaemia inhibitory factor, LIF Ø Interleukin-6 cytokine family Ø Essential for maintaining pluripotency in vitro in the presence of serum Ø Binds to a heterodimeric receptor comprising of LIF-receptor (LIFR) and gp 130 on cell membrane Ø Binding results in the activation of Jak/ Stat signal transduction pathway Ø Activated Stat 3 maintains pluripotency

Combinatorial signaling pathways (involving LIF and master regulator genes) in maintaining mouse ESC pluripotency Boiani and Scholer, 2005

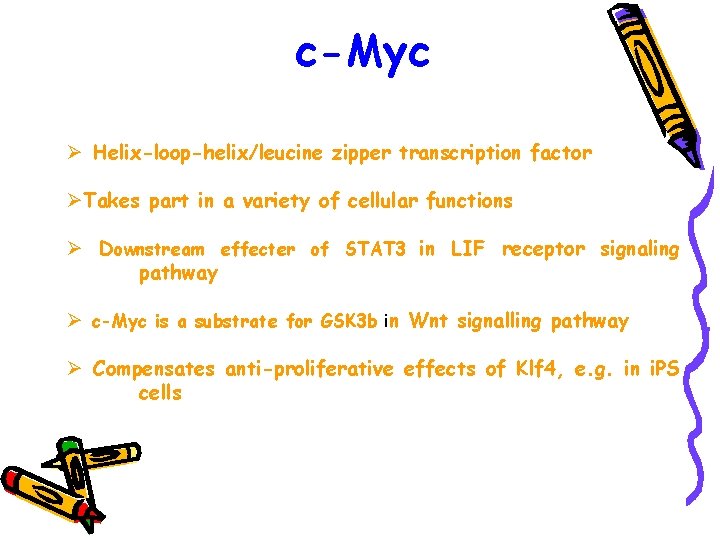
c-Myc Ø Helix-loop-helix/leucine zipper transcription factor ØTakes part in a variety of cellular functions Ø Downstream effecter of STAT 3 in LIF receptor signaling pathway Ø c-Myc is a substrate for GSK 3 b in Wnt signalling pathway Ø Compensates anti-proliferative effects of Klf 4, e. g. in i. PS cells

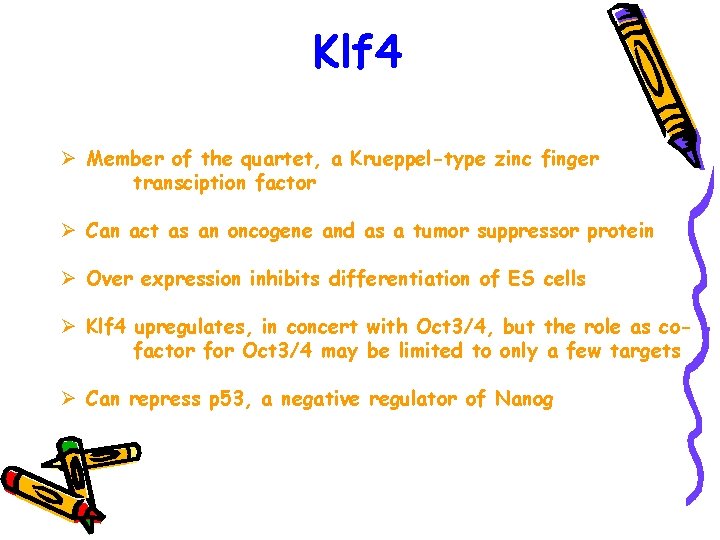
Klf 4 Ø Member of the quartet, a Krueppel-type zinc finger transciption factor Ø Can act as an oncogene and as a tumor suppressor protein Ø Over expression inhibits differentiation of ES cells Ø Klf 4 upregulates, in concert with Oct 3/4, but the role as cofactor for Oct 3/4 may be limited to only a few targets Ø Can repress p 53, a negative regulator of Nanog

Pluripotent lineages in the mouse embryo ll

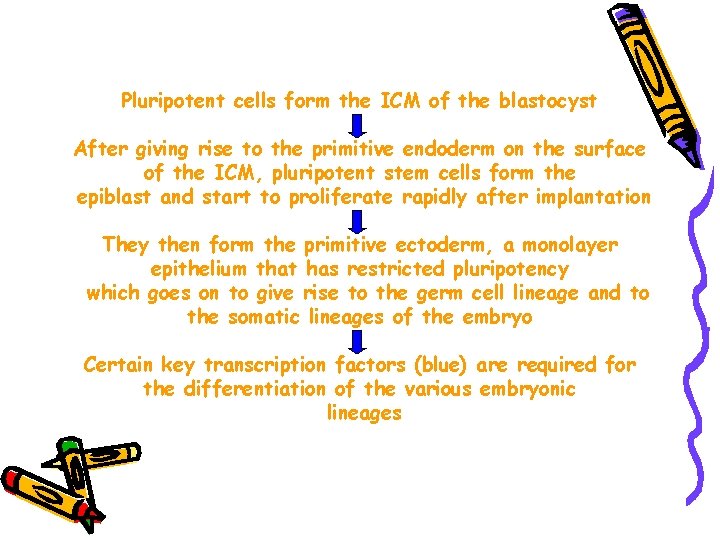
Pluripotent cells form the ICM of the blastocyst After giving rise to the primitive endoderm on the surface of the ICM, pluripotent stem cells form the epiblast and start to proliferate rapidly after implantation They then form the primitive ectoderm, a monolayer epithelium that has restricted pluripotency which goes on to give rise to the germ cell lineage and to the somatic lineages of the embryo Certain key transcription factors (blue) are required for the differentiation of the various embryonic lineages

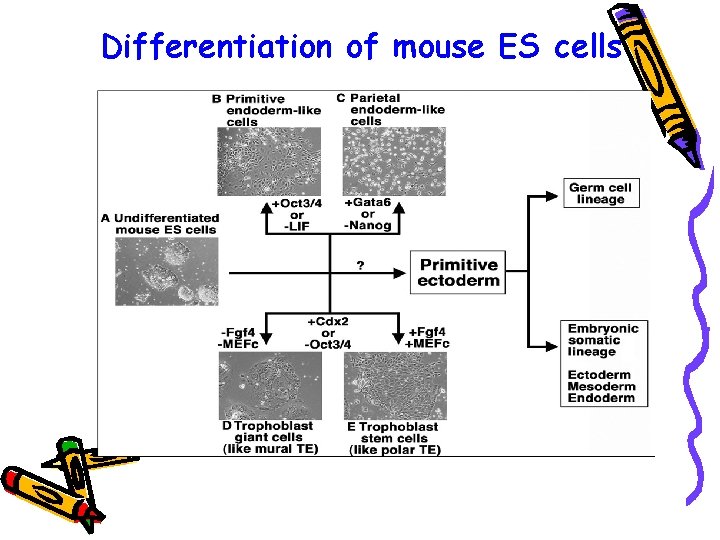
Differentiation of mouse ES cells

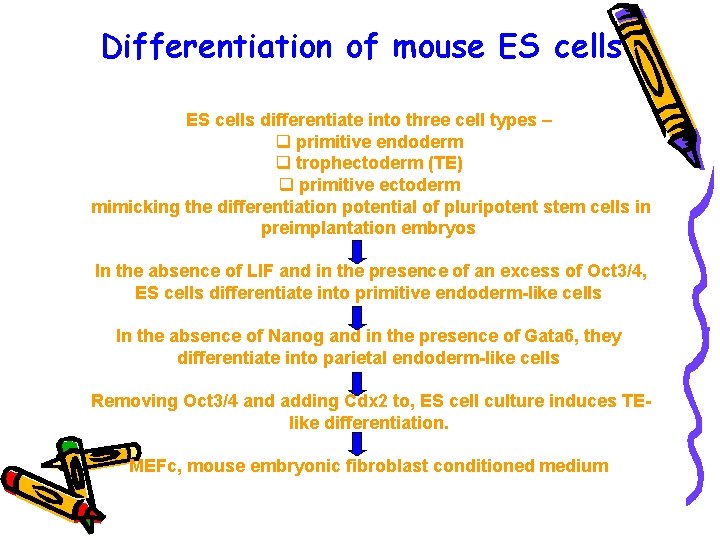
Differentiation of mouse ES cells differentiate into three cell types – q primitive endoderm q trophectoderm (TE) q primitive ectoderm mimicking the differentiation potential of pluripotent stem cells in preimplantation embryos In the absence of LIF and in the presence of an excess of Oct 3/4, ES cells differentiate into primitive endoderm-like cells In the absence of Nanog and in the presence of Gata 6, they differentiate into parietal endoderm-like cells Removing Oct 3/4 and adding Cdx 2 to, ES cell culture induces TElike differentiation. MEFc, mouse embryonic fibroblast conditioned medium

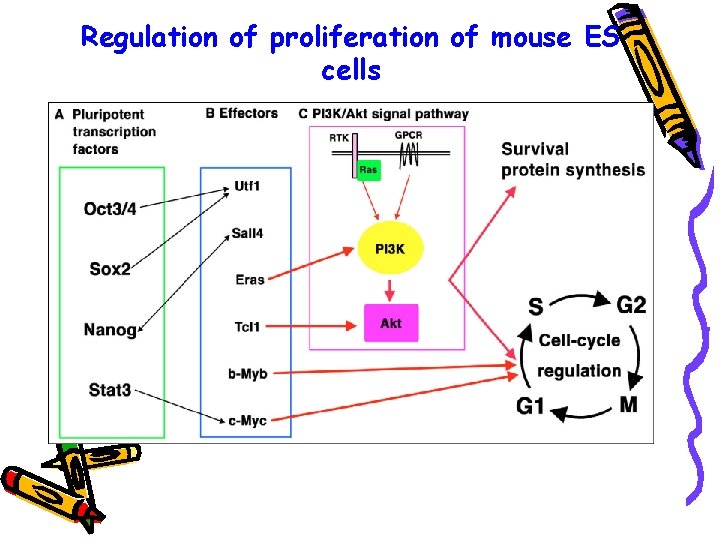
Regulation of proliferation of mouse ES cells

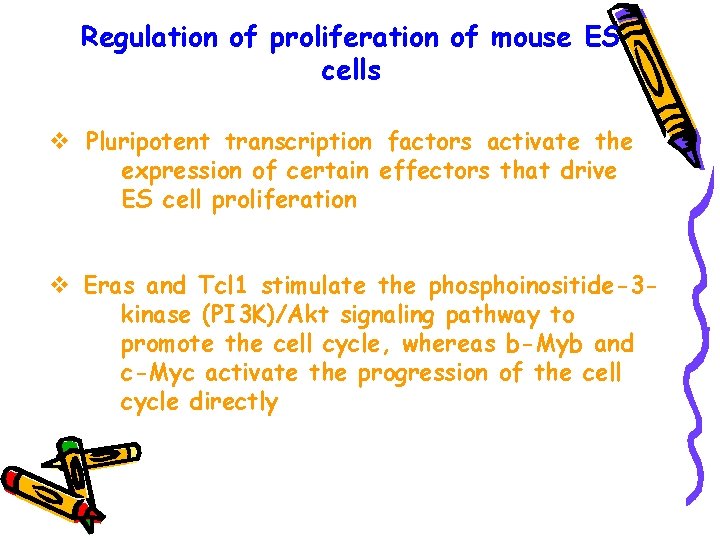
Regulation of proliferation of mouse ES cells v Pluripotent transcription factors activate the expression of certain effectors that drive ES cell proliferation v Eras and Tcl 1 stimulate the phosphoinositide-3 kinase (PI 3 K)/Akt signaling pathway to promote the cell cycle, whereas b-Myb and c-Myc activate the progression of the cell cycle directly

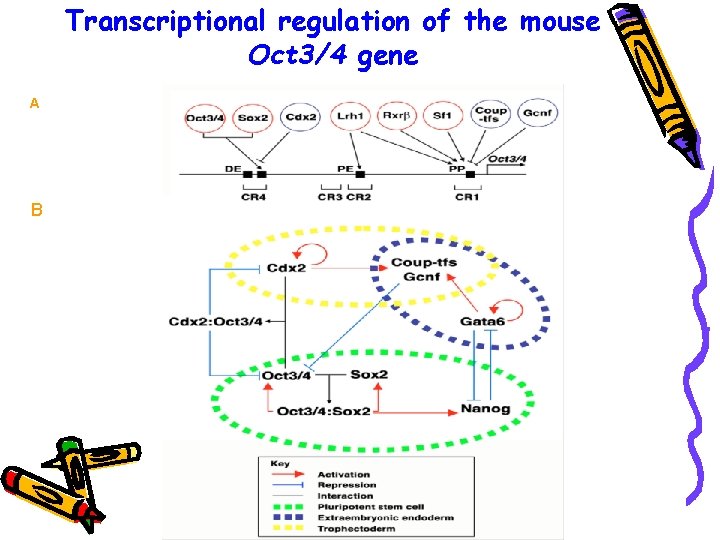
Transcriptional regulation of the mouse Oct 3/4 gene A B

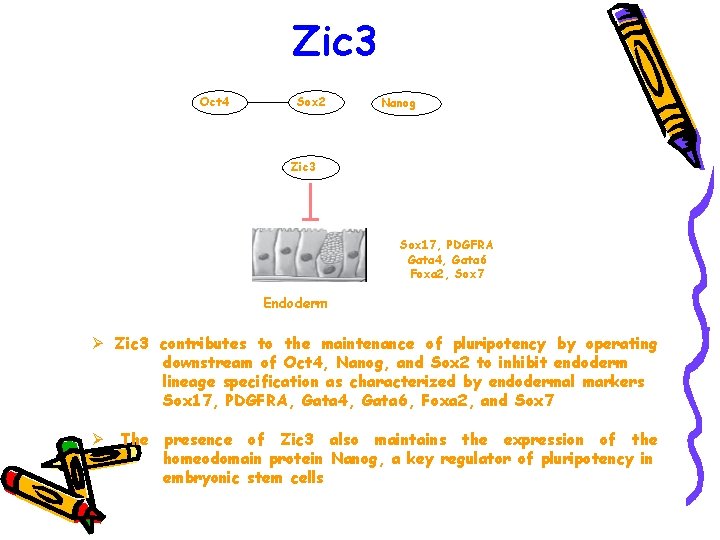
Zic 3 Oct 4 Sox 2 Nanog Zic 3 Sox 17, PDGFRA Gata 4, Gata 6 Foxa 2, Sox 7 Endoderm Ø Zic 3 contributes to the maintenance of pluripotency by operating downstream of Oct 4, Nanog, and Sox 2 to inhibit endoderm lineage specification as characterized by endodermal markers Sox 17, PDGFRA, Gata 4, Gata 6, Foxa 2, and Sox 7 Ø The presence of Zic 3 also maintains the expression of the homeodomain protein Nanog, a key regulator of pluripotency in embryonic stem cells

Epigenetic regulations of ES cells pluripotency

Characteristics of the pluripotent epigenome The nucleus shrinks and the distribution of electrondense areas (mainly heterochromatin) changes dramatically when ES cells are induced to differentiate into primitive endoderm by the ectopic expression of Gata 6

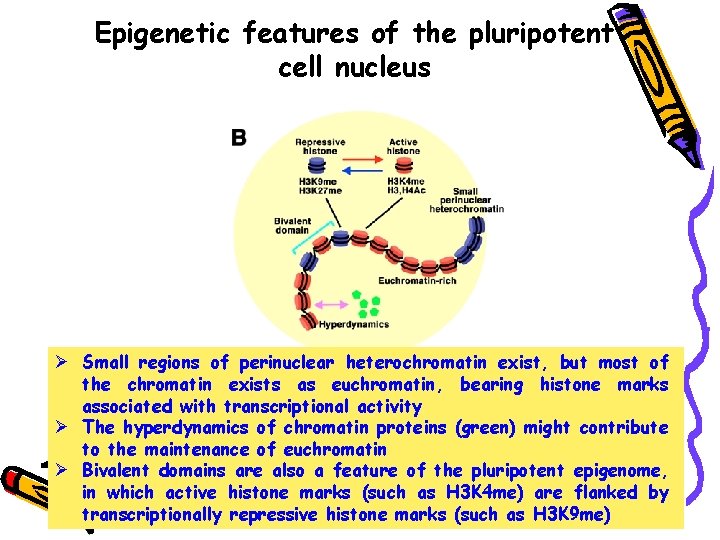
Epigenetic features of the pluripotent cell nucleus Ø Small regions of perinuclear heterochromatin exist, but most of the chromatin exists as euchromatin, bearing histone marks associated with transcriptional activity Ø The hyperdynamics of chromatin proteins (green) might contribute to the maintenance of euchromatin Ø Bivalent domains are also a feature of the pluripotent epigenome, in which active histone marks (such as H 3 K 4 me) are flanked by transcriptionally repressive histone marks (such as H 3 K 9 me)

 Arka puneet is the progeny from the cross of
Arka puneet is the progeny from the cross of Tcs kristu jayanti
Tcs kristu jayanti Vivek jain
Vivek jain Mitthu jain
Mitthu jain Lunawat & co
Lunawat & co Jain
Jain Seamless mpls architecture
Seamless mpls architecture Ashish jain microsoft
Ashish jain microsoft Jainendra k. jain
Jainendra k. jain Ratna karand shravakachar
Ratna karand shravakachar Cherry hill jain sangh
Cherry hill jain sangh Pankaj jain iitk
Pankaj jain iitk Prateek jain
Prateek jain Atap vs iip
Atap vs iip Main beliefs of jainism
Main beliefs of jainism Preet jain
Preet jain Conclusion of jainism
Conclusion of jainism Eric jain
Eric jain Jsne
Jsne Dr tulika jain
Dr tulika jain Anu jain
Anu jain Vimal vasahi temple plan
Vimal vasahi temple plan Rajeev jain md
Rajeev jain md Jain way of life
Jain way of life Pravin k shah
Pravin k shah Jain society of central florida
Jain society of central florida Ambuj tewari
Ambuj tewari Jain bcn
Jain bcn Jain portal
Jain portal Vinod jain ca
Vinod jain ca Jain center of greater boston
Jain center of greater boston Vaibhav.14v
Vaibhav.14v Dr salil jain
Dr salil jain Geisel student government
Geisel student government