Investigation of electrode materials with 3 DOM structures










- Slides: 19

Investigation of electrode materials with 3 DOM structures Antony Han Chem 750/7530

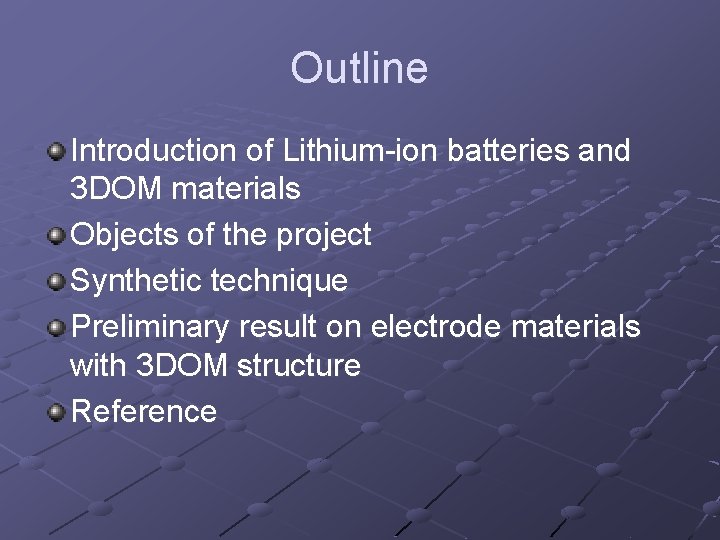
Outline Introduction of Lithium-ion batteries and 3 DOM materials Objects of the project Synthetic technique Preliminary result on electrode materials with 3 DOM structure Reference

Applications of Li-ion batteries

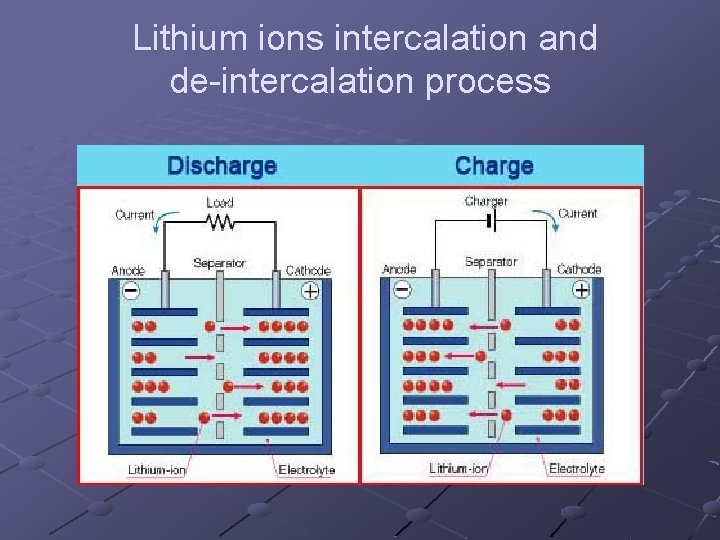
Lithium ions intercalation and de-intercalation process

Parameters to evaluate electrode materials First charge/discharge capacities Irreversible capacities between each charge/discharge cycle Charge/discharge cycleabilities Charge/discharge rate capacity Volumetric charge/discharge capacities Electrical conductivities

What is 3 DOM? 3 DOM structure = 3 dimensional ordered macroporous structure Replicas of their colloidal-crystal templates Nanometer-sized walls Well-interconnected close-packed spherical voids with sub-micron diameters Both cathode and anode materials can be fabricated into 3 DOM structure

Comparison Conventional electrode materials n n Volumetric capacities Stable cycleability 3 DOM electrode materials n n Solid-state diffusion distance Electrode–electrolyte interface and Li-ion conduction through the electrolyte.

Objects of the project Preparation of the colloidal crystal templates used for the generation of 3 DOM materials; Methods of integration of the precursors of electrode materials into the colloidal crystal templates; Methods of removal of the colloidal crystal templates according to the different properties of electrode materials; Electrochemistry performance of these asprepared 3 DOM electrode materials.

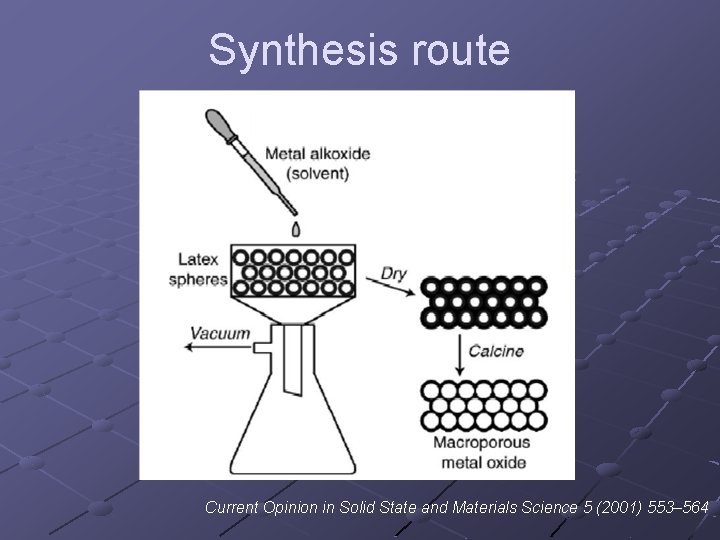
Synthesis route Current Opinion in Solid State and Materials Science 5 (2001) 553– 564

Template Desired properties n n n Easier template removal Possibility of providing additional functionality Max the precursor loading (easy access of the voids) Preparation methods n n n n Gravity sedimentation Centrifugation Vertical deposition Templated deposition Electrophoresis Patterning Controlled drying

Loading technique Methods to load the fluid precursors n n n n n Sol-gel chemistry Polymerization Salt-precipitation and chemical conversion Chemical vapour deposition (CVD) Spraying techniques Nanocrystal deposition and sintering Oxide and salt reduction Electrodeposition Electroless deposition,

Template removal technique Polymer templates n n Calcination simultaneously with conversion of the precursor to a solid in the desired phase. If the solidification is feasible at low temperatures, spheres can also be extracted with appropriate solvents, such as toluene or tetrahydrofuran (THF)/acetone mixtures. Silica sphere templates are removed by dissolution in aqueous HF solutions.

Characterization Powder X-ray diffractometer (PXRD) Scanning electron microscope (SEM) Brunauer-Emmett-Teller (BET) Energy dispersive spectroscopy (EDS) Electrochemical characterization (coin cell type batteries)

Some of preliminary results J. of Electro. Soc. , 152 10 A 1989 2005

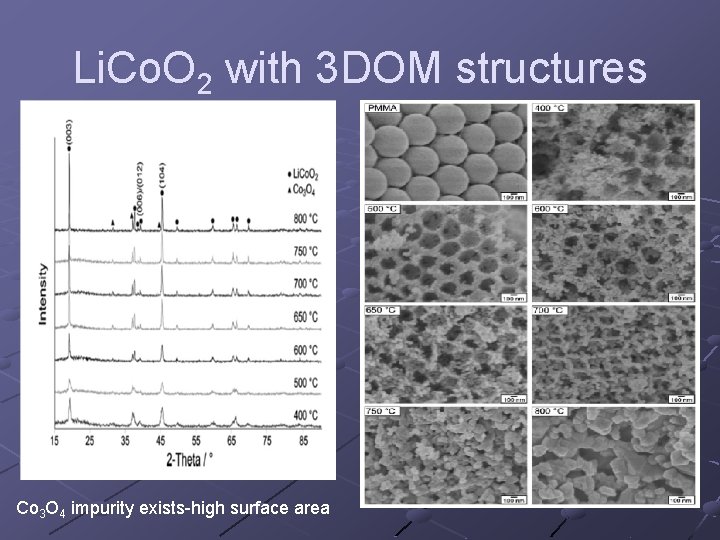
Li. Co. O 2 with 3 DOM structures Co 3 O 4 impurity exists-high surface area

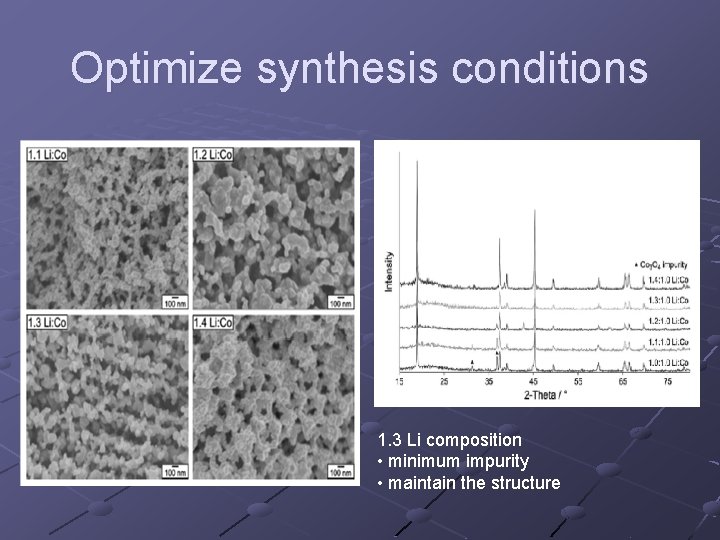
Optimize synthesis conditions 1. 3 Li composition • minimum impurity • maintain the structure

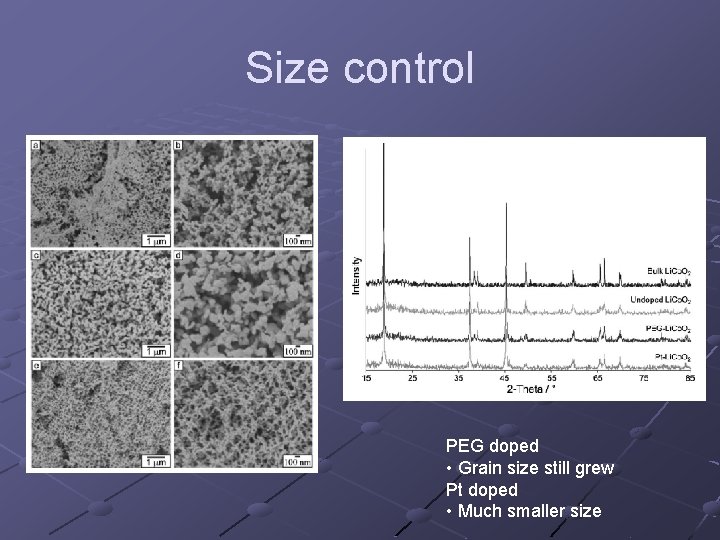
Size control PEG doped • Grain size still grew Pt doped • Much smaller size

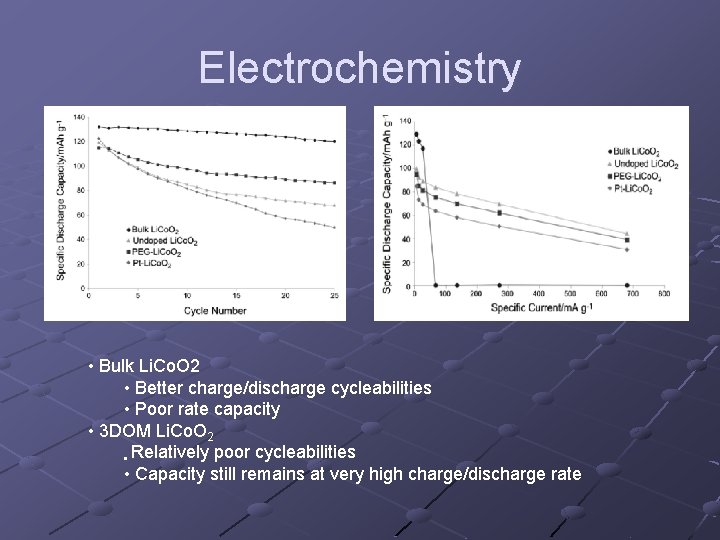
Electrochemistry • Bulk Li. Co. O 2 • Better charge/discharge cycleabilities • Poor rate capacity • 3 DOM Li. Co. O 2 • Relatively poor cycleabilities • Capacity still remains at very high charge/discharge rate

Reference Ergang, N. S. ; Lytle, J. C. ; Yan, H. ; Stein, A. ; “The Effect of a Macropore Structure on Cycling Rates of Li. Co. O 2” J. Electrochem. Soc. 2005, 152, A 1989 -A 1995. Lee, K. T. ; Lytle, J. C. ; Ergang, N. S. ; Oh, S. M. ; Stein, A. ; “Synthesis and Rate Performance of Monolithic Macroporous Carbon Electrodes for Lithium Secondary Batteries”, Adv. Funct. Mater. 2005, 15, 547 -556. Lytle, J. C. ; Yan, H. ; Ergang, N. S. ; Smyrl, W. H. ; Stein, A. ; “Structural and electrochemical properties of three-dimensionally ordered macroporous tin(IV) oxide films”, J. Mater. Chem. 2004, 1616 -1622. Yan, H. ; Sokolov, S. ; Lytle, J. C. ; Stein, A. ; Zhang, F. ; Smyrl, W. H. ; "Colloidal-Crystal-Templated Synthesis of Ordered Macroporous Electrode Materials for Lithium Secondary Batteries", J. Electrochem. Soc. 2003, 150, A 1102 -A 1107.
 Dom materials
Dom materials Homologous structures
Homologous structures Air supported pneumatic structures
Air supported pneumatic structures Natural materials and man made materials
Natural materials and man made materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Household materials useful
Household materials useful Natural man made
Natural man made Adopting and adapting teaching materials
Adopting and adapting teaching materials Weld slag chippers and needle scalers are typically
Weld slag chippers and needle scalers are typically Electrode movement
Electrode movement Spontaneity of redox reaction
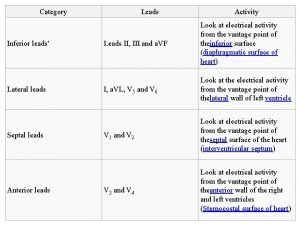
Spontaneity of redox reaction Anterior lateral leads
Anterior lateral leads Glass electrode
Glass electrode Drop mercury electrode
Drop mercury electrode N
N Alloy steel electrode
Alloy steel electrode Smaw personal protective equipment
Smaw personal protective equipment Low medium and high frequency currents
Low medium and high frequency currents Ideal polarized electrode
Ideal polarized electrode Monode electrode
Monode electrode