Introduction to Minerals And you thought you were


























- Slides: 27

Introduction to Minerals And you thought you were finished with chemistry

Photos of really cool places! Ú As we study rocks and minerals, remember these spectacular sites Ú Think about how they formed Ú Travel and ask questions!

Why should we care? Ú Salar de Uyini, Bolivia Ú World’s largest salt flat

Antelope Canyon, AZ

Zhangye Danxia Landform, Gansu, China

Fly Geyser, NV

Naica Mine, Mexico

What is a mineral? 1. Naturally occurring 2. Homogeneous solid 3. Definite, but generally not fixed, composition 4. Ordered atomic arrangement 5. Inorganic processes

Mineral Examples: How are compounds related to minerals? 3. Definite composition: Si. O 2 OR Fe. S 2

Mineral Crystal Shapes 4. Crystalline: 3 D periodic arrays of precise geometric arrangement of atoms Ú Cut on the solid lines and fold on the dotted lines

Mineral or Not? At your table discuss whether or not the following materials are minerals. Be ready to justify your answers! Ú Snowflake Ú Coal Ú Rock salt Ú Window glass Ú Graphite Ú Oxygen

Mineral or Not…answers ÚSnowflake - Mineral ÚCoal - Not ÚTable salt - Mineral ÚWindow glass - Not ÚGraphite – Mineral ÚOxygen- Not

How do minerals form? Ú There are four ways that minerals form: 1. Crystallization within magma (i. e. tourmaline, olivine, quartz, feldspar) 2. Precipitation from solution (salt flats) 3. Changes in pressure or temperature 4. Formation from hydrothermal solutions

How are minerals classified? Ú 4, 000 known minerals Ú International Mineralogical Association uses chemical composition to classify minerals Ú 8 mineral classes that are categorized by their anion group

8 mineral classes 1. 2. 3. 4. 5. 6. 7. 8. Silicate (Si. O 2) Carbonate (CO 3) Sulfate (SO 4) Halide (F, Cl, I, Br are most common) Oxide (usually a single O or OH) Sulfide (S is usually bonded to a metal = ores) Phosphate (AO 4 where A can be P, Sb, As, V Element

How do you identify minerals? Ú Geologists rely on several simple tests to identify minerals. Ú These are based on physical and chemical properties. Ú How would you use the 8 identifying properties to identify the minerals at your table?

8 Identifying Properties: 1. Color: This is the most noticeable characteristics of a mineral and the least helpful - This is determined by the presence of trace elements in certain compounds. Ex: Quartz: these are all the same

2. Luster: The way that a mineral reflects light from its surface. - Described as Metallic or Non-Metallic - Metallic – Reflect light like Gold, Copper, Silver - Non-Metallic – Calcite, Gypsum, Sulfur

Non-Metallic Luster continued… Most common Non-metallic Luster Descriptions: 1. Adamantine = brilliant like a polished diamond 2. Vitreous = glassy, like glazed porcelain or quartz 3. Resinous = yellow, dark orange, or brown like tree sap 4. Pearly = Exhibiting a luster similar to the inside of a mollusk shell or shirt button 5. Silky = minerals that have a very fine fibrous structure 6. Earthy = dull, little reflection

3. Texture: Describes how a mineral feels to the touch. - Words to describe: Smooth, Rough, Ragged, Greasy, Glassy. Smooth Rough

4. Streak: The color of a mineral when it is broken up or in powdered form. - Usually rubbed against a porcelain plate, which leaves a powder behind. - Sometimes the external color doesn’t match streak color. - Streak hardly ever changes, even though the colors of a mineral do Pyrite: Streaks greenish Gold: Streaks yellow

5. Hardness: a measure of how easily a mineral can be scratched. - Measured on the Mohs Hardness Scale – Ranges from 1 – 10 1 = Softest 10 = Hardest Talc = 1 Diamond = 10

Mohs Hardness Scale Name Hardness Talc Gypsum 1 2 Calcite Fluorite Apatite Feldspar Quartz Topaz Corundum Diamond 3 4 5 6 7 8 9 10 Hardness of Common Objects Softest Scratched by Fingernail Scratch by Penny Scratched by Nail Scratched by Glass Scratched by Steel File Scratches quartz Scratches Topaz Hardest – Scratches Everything


6. Cleavage and 7. Fracture: How a mineral will break. - Cleavage – a mineral that splits relatively easily and evenly along one or more flat planes. - Fracture – Minerals that break with rough or jagged edges. Fracture Cleavage

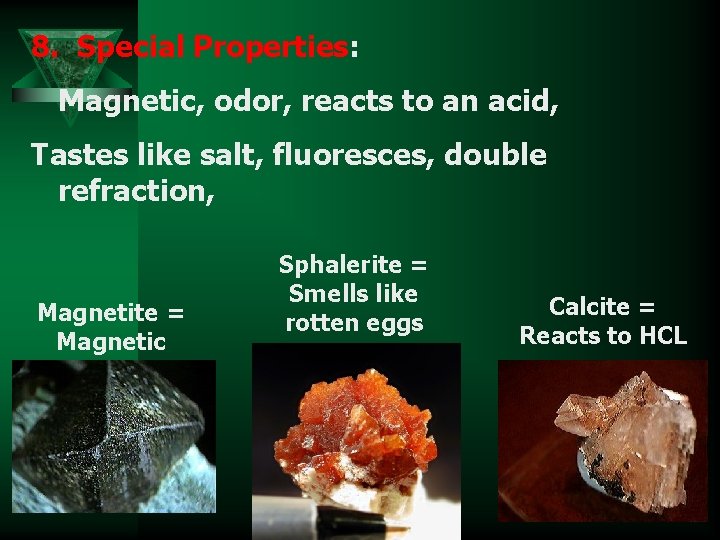
8. Special Properties: Magnetic, odor, reacts to an acid, Tastes like salt, fluoresces, double refraction, Magnetite = Magnetic Sphalerite = Smells like rotten eggs Calcite = Reacts to HCL

Double Refraction- Calcite
 Complete and incomplete thinking
Complete and incomplete thinking Nageeb thought all nurses were young females
Nageeb thought all nurses were young females How were european rulers guided by enlightenment thought
How were european rulers guided by enlightenment thought You thought that i was altogether like you
You thought that i was altogether like you Was/were going to future in the past
Was/were going to future in the past What are vitamins functions
What are vitamins functions A long narrow coiled tube where digestion is completed
A long narrow coiled tube where digestion is completed Have you ever looked in the mirror and thought
Have you ever looked in the mirror and thought Soldier island devon uk
Soldier island devon uk Rocks and minerals song
Rocks and minerals song Rock cycle
Rock cycle Chapter 8 vitamins and minerals
Chapter 8 vitamins and minerals Major and minor elements
Major and minor elements Difference between minerals and rocks
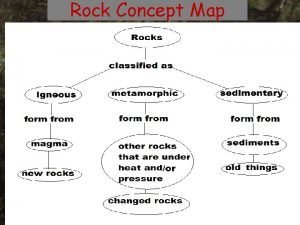
Difference between minerals and rocks Concept map for igneous rock
Concept map for igneous rock Suzanna socked me sunday poem
Suzanna socked me sunday poem Absorbs water and minerals
Absorbs water and minerals Whats the difference between rocks and minerals
Whats the difference between rocks and minerals Padma mines and minerals corporation
Padma mines and minerals corporation Stores minerals and anchors muscles
Stores minerals and anchors muscles Cumbria minerals and waste local plan
Cumbria minerals and waste local plan Resource that can be replaced
Resource that can be replaced Minerals and fuels
Minerals and fuels Deficiency chart of macronutrients
Deficiency chart of macronutrients Minerals and fuels
Minerals and fuels Kyanite formula
Kyanite formula Vitamin flowchart
Vitamin flowchart Rock type
Rock type