I nuovi anticoagulanti orali ed il dilemma delle









- Slides: 37

I nuovi anticoagulanti orali ed il dilemma delle copatologie Bruno Trimarco Dipartimento di Scienze Biomediche Avanzate Università degli Studi di Napoli “Federico II”


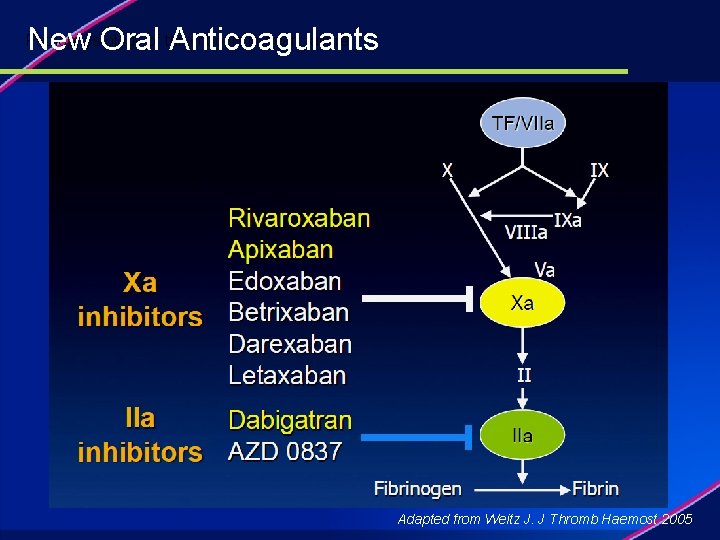
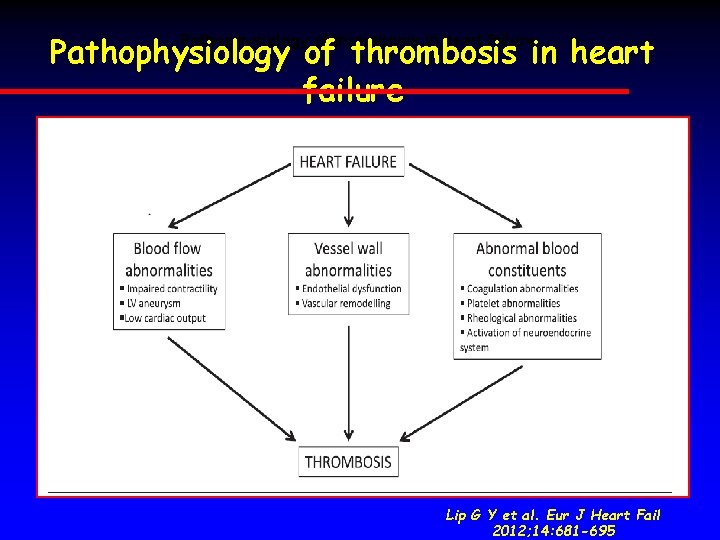
New Oral Anticoagulants Pathophysiology of thrombosis in heart failure. Adapted from Weitz J. J Thromb Haemost 2005

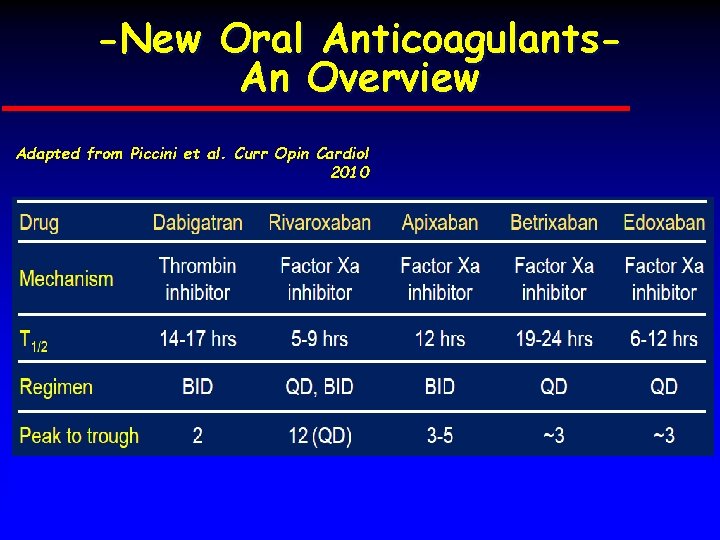
-New Oral Anticoagulants. An Overview Adapted from Piccini et al. Curr Opin Cardiol 2010 DIVISION OF CARDIOLOGY - UNIVERSITY OF NAPLES

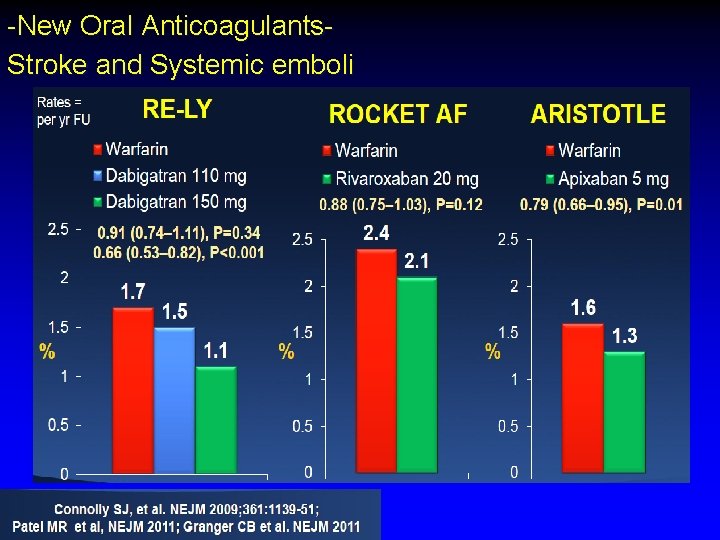
-New Oral Anticoagulants. Stroke and Systemic emboli

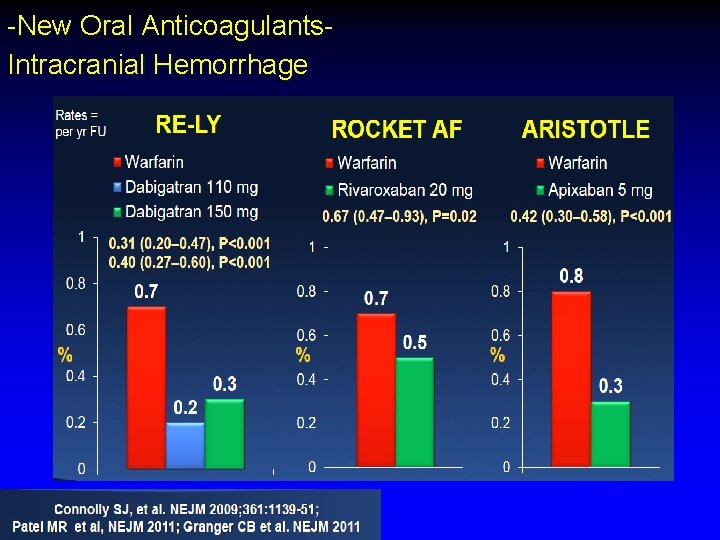
-New Oral Anticoagulants. Intracranial Hemorrhage

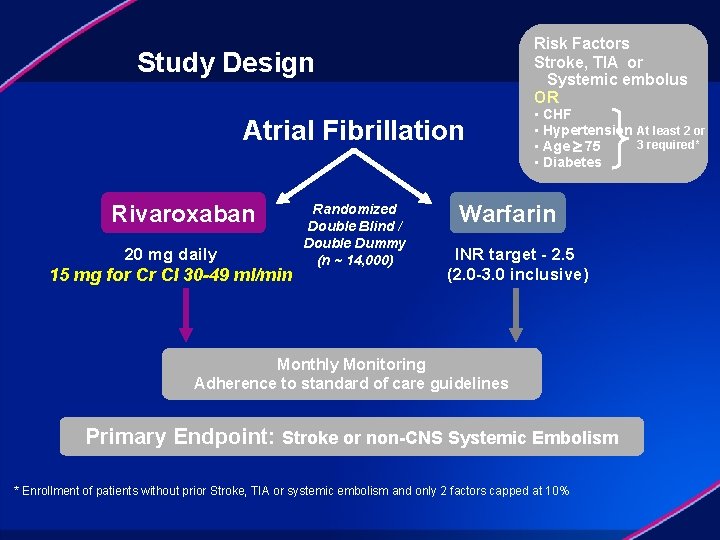
Risk Factors Stroke, TIA or Systemic embolus OR Study Design Atrial Fibrillation Rivaroxaban 20 mg daily 15 mg for Cr Cl 30 -49 ml/min Randomized Double Blind / Double Dummy (n ~ 14, 000) • CHF • Hypertension At least 2 or 3 required* • Age 75 • Diabetes Warfarin INR target - 2. 5 (2. 0 -3. 0 inclusive) Monthly Monitoring Adherence to standard of care guidelines Primary Endpoint: Stroke or non-CNS Systemic Embolism * Enrollment of patients without prior Stroke, TIA or systemic embolism and only 2 factors capped at 10%

Pathophysiology of thrombosis in heart failure. Lip G Y et al. Eur J Heart Fail 2012; 14: 681 -695

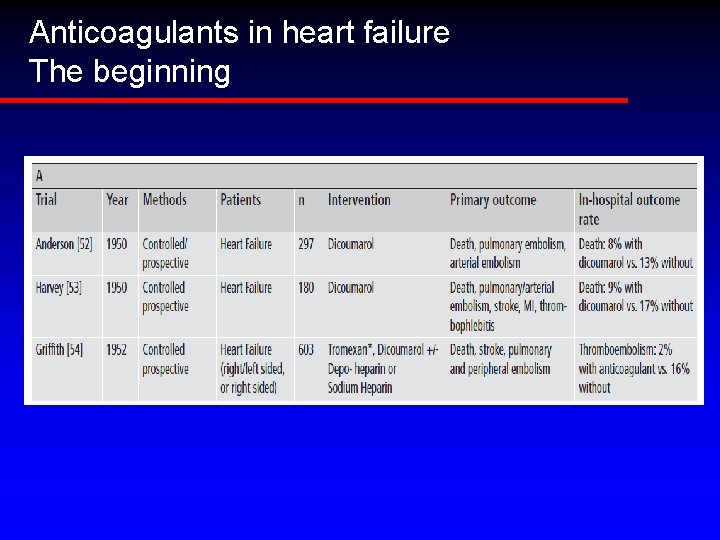
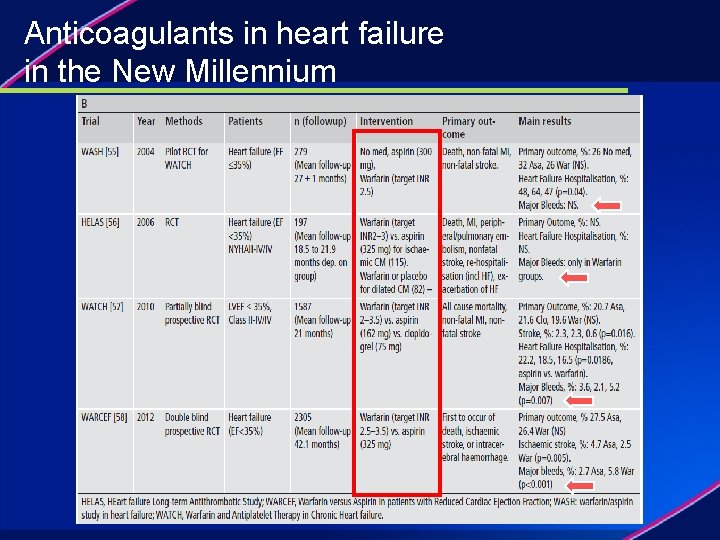
Anticoagulants in heart failure The beginning

Anticoagulants in heart failure in the New Millennium

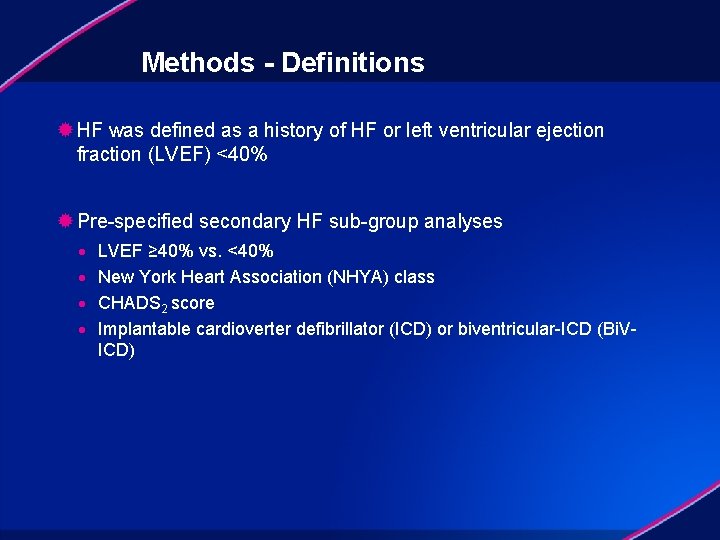
Methods - Definitions ® HF was defined as a history of HF or left ventricular ejection fraction (LVEF) <40% ® Pre-specified secondary HF sub-group analyses · · LVEF ≥ 40% vs. <40% New York Heart Association (NHYA) class CHADS 2 score Implantable cardioverter defibrillator (ICD) or biventricular-ICD (Bi. VICD)

Methods - Outcomes ® Efficacy endpoints (intention-to-treat population) · Primary: Stroke or systemic embolism · Secondary – All-cause death – Stroke, systemic embolism, or vascular death ® Safety endpoints (safety population) · Primary : Major or non-major clinically relevant (NMCR) bleeding · Secondary – Intracranial hemorrhage (ICH) – Hemorrhagic stroke *All outcomes reported as adjusted hazard ratios (HR) per 100 patient-years (pt-yrs)

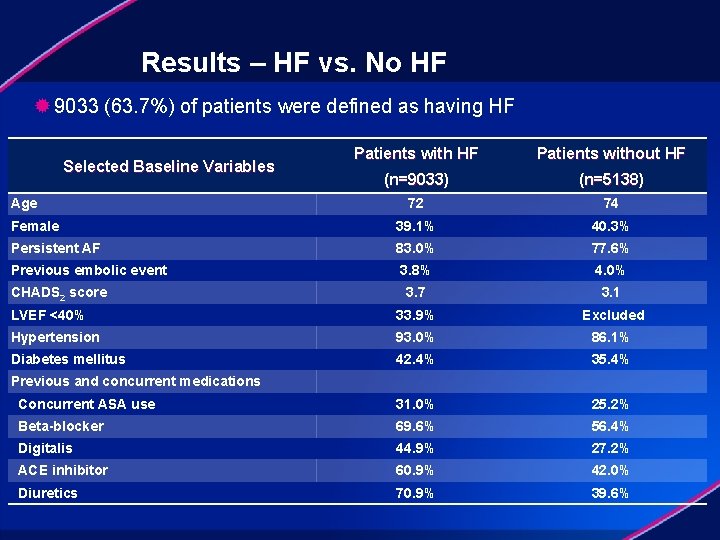
Results – HF vs. No HF ® 9033 (63. 7%) of patients were defined as having HF Patients without HF (n=9033) (n=5138) 72 74 Female 39. 1% 40. 3% Persistent AF 83. 0% 77. 6% Previous embolic event 3. 8% 4. 0% 3. 7 3. 1 LVEF <40% 33. 9% Excluded Hypertension 93. 0% 86. 1% Diabetes mellitus 42. 4% 35. 4% Concurrent ASA use 31. 0% 25. 2% Beta-blocker 69. 6% 56. 4% Digitalis 44. 9% 27. 2% ACE inhibitor 60. 9% 42. 0% Diuretics 70. 9% 39. 6% Selected Baseline Variables Age CHADS 2 score Previous and concurrent medications

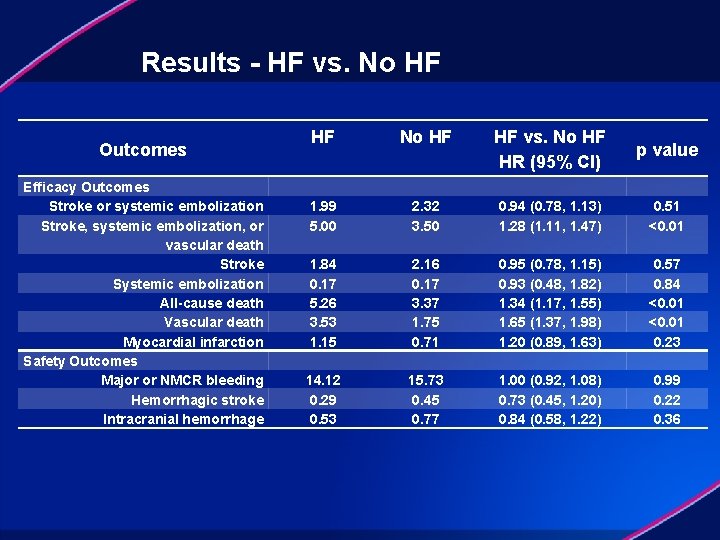
Results - HF vs. No HF Outcomes Efficacy Outcomes Stroke or systemic embolization Stroke, systemic embolization, or vascular death Stroke Systemic embolization All-cause death Vascular death Myocardial infarction Safety Outcomes Major or NMCR bleeding Hemorrhagic stroke Intracranial hemorrhage HF No HF vs. No HF HR (95% CI) p value 1. 99 5. 00 2. 32 3. 50 0. 94 (0. 78, 1. 13) 1. 28 (1. 11, 1. 47) 0. 51 <0. 01 1. 84 0. 17 5. 26 3. 53 1. 15 14. 12 0. 29 0. 53 2. 16 0. 17 3. 37 1. 75 0. 71 15. 73 0. 45 0. 77 0. 95 (0. 78, 1. 15) 0. 93 (0. 48, 1. 82) 1. 34 (1. 17, 1. 55) 1. 65 (1. 37, 1. 98) 1. 20 (0. 89, 1. 63) 1. 00 (0. 92, 1. 08) 0. 73 (0. 45, 1. 20) 0. 84 (0. 58, 1. 22) 0. 57 0. 84 <0. 01 0. 23 0. 99 0. 22 0. 36

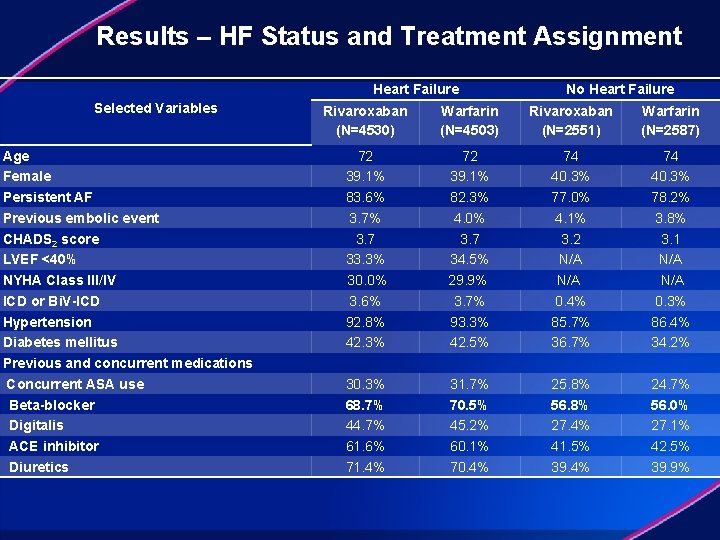
Results – HF Status and Treatment Assignment Selected Variables Age Female Persistent AF Previous embolic event CHADS 2 score LVEF <40% NYHA Class III/IV ICD or Bi. V-ICD Hypertension Diabetes mellitus Previous and concurrent medications Concurrent ASA use Beta-blocker Digitalis ACE inhibitor Diuretics Heart Failure No Heart Failure Rivaroxaban (N=4530) Warfarin (N=4503) Rivaroxaban (N=2551) Warfarin (N=2587) 72 39. 1% 83. 6% 3. 7 33. 3% 30. 0% 3. 6% 92. 8% 42. 3% 30. 3% 68. 7% 44. 7% 61. 6% 71. 4% 72 39. 1% 82. 3% 4. 0% 3. 7 34. 5% 29. 9% 3. 7% 93. 3% 42. 5% 31. 7% 70. 5% 45. 2% 60. 1% 70. 4% 74 40. 3% 77. 0% 4. 1% 3. 2 N/A 0. 4% 85. 7% 36. 7% 25. 8% 56. 8% 27. 4% 41. 5% 39. 4% 74 40. 3% 78. 2% 3. 8% 3. 1 N/A 0. 3% 86. 4% 34. 2% 24. 7% 56. 0% 27. 1% 42. 5% 39. 9%



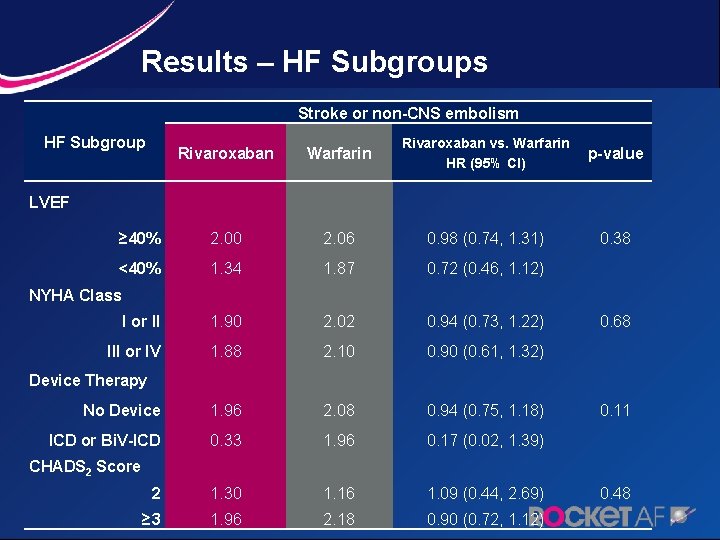
Results – HF Subgroups Stroke or non-CNS embolism HF Subgroup Rivaroxaban LVEF Rivaroxaban vs. Warfarin HR (95% CI) Warfarin p-value ≥ 40% 2. 00 2. 06 0. 98 (0. 74, 1. 31) 0. 38 <40% 1. 34 1. 87 0. 72 (0. 46, 1. 12) I or II 1. 90 2. 02 0. 94 (0. 73, 1. 22) 0. 68 III or IV 1. 88 2. 10 0. 90 (0. 61, 1. 32) No Device 1. 96 2. 08 0. 94 (0. 75, 1. 18) 0. 11 ICD or Bi. V-ICD 0. 33 1. 96 0. 17 (0. 02, 1. 39) 2 1. 30 1. 16 1. 09 (0. 44, 2. 69) 0. 48 ≥ 3 1. 96 2. 18 0. 90 (0. 72, 1. 12) NYHA Class Device Therapy CHADS 2 Score

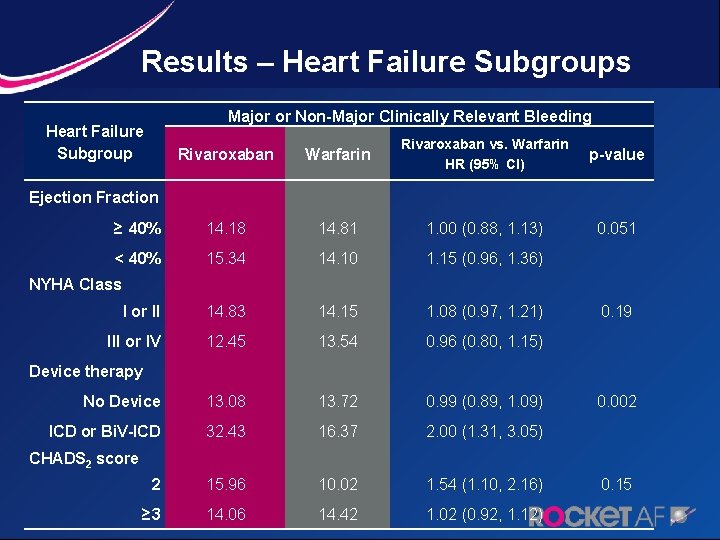
Results – Heart Failure Subgroups Major or Non-Major Clinically Relevant Bleeding Heart Failure Subgroup Rivaroxaban Ejection Fraction Rivaroxaban vs. Warfarin HR (95% CI) Warfarin p-value ≥ 40% 14. 18 14. 81 1. 00 (0. 88, 1. 13) 0. 051 < 40% 15. 34 14. 10 1. 15 (0. 96, 1. 36) I or II 14. 83 14. 15 1. 08 (0. 97, 1. 21) 0. 19 III or IV 12. 45 13. 54 0. 96 (0. 80, 1. 15) No Device 13. 08 13. 72 0. 99 (0. 89, 1. 09) 0. 002 ICD or Bi. V-ICD 32. 43 16. 37 2. 00 (1. 31, 3. 05) 2 15. 96 10. 02 1. 54 (1. 10, 2. 16) 0. 15 ≥ 3 14. 06 14. 42 1. 02 (0. 92, 1. 12) NYHA Class Device therapy CHADS 2 score


Available now online from European Heart Journal http: //eurheartj. oxfordjournals. org/cgi/content/full/ehr 342

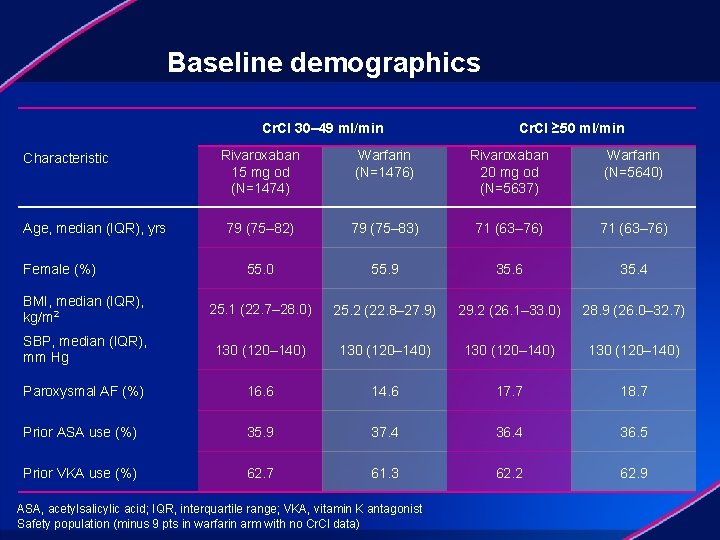
Baseline demographics Cr. Cl 30– 49 ml/min Cr. Cl ≥ 50 ml/min Rivaroxaban 15 mg od (N=1474) Warfarin (N=1476) Rivaroxaban 20 mg od (N=5637) Warfarin (N=5640) 79 (75– 82) 79 (75– 83) 71 (63– 76) 55. 0 55. 9 35. 6 35. 4 BMI, median (IQR), kg/m 2 25. 1 (22. 7– 28. 0) 25. 2 (22. 8– 27. 9) 29. 2 (26. 1– 33. 0) 28. 9 (26. 0– 32. 7) SBP, median (IQR), mm Hg 130 (120– 140) Paroxysmal AF (%) 16. 6 14. 6 17. 7 18. 7 Prior ASA use (%) 35. 9 37. 4 36. 5 Prior VKA use (%) 62. 7 61. 3 62. 2 62. 9 Characteristic Age, median (IQR), yrs Female (%) ASA, acetylsalicylic acid; IQR, interquartile range; VKA, vitamin K antagonist Safety population (minus 9 pts in warfarin arm with no Cr. Cl data)

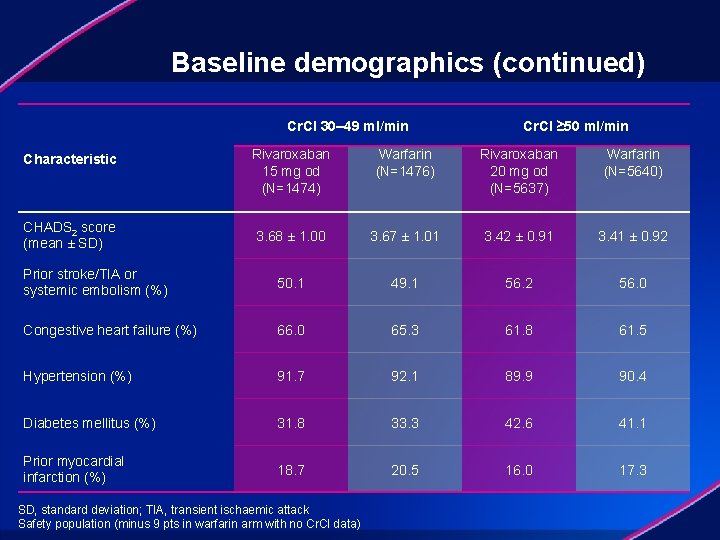
Baseline demographics (continued) Cr. Cl 30– 49 ml/min Cr. Cl ≥ 50 ml/min Rivaroxaban 15 mg od (N=1474) Warfarin (N=1476) Rivaroxaban 20 mg od (N=5637) Warfarin (N=5640) 3. 68 ± 1. 00 3. 67 ± 1. 01 3. 42 ± 0. 91 3. 41 ± 0. 92 Prior stroke/TIA or systemic embolism (%) 50. 1 49. 1 56. 2 56. 0 Congestive heart failure (%) 66. 0 65. 3 61. 8 61. 5 Hypertension (%) 91. 7 92. 1 89. 9 90. 4 Diabetes mellitus (%) 31. 8 33. 3 42. 6 41. 1 Prior myocardial infarction (%) 18. 7 20. 5 16. 0 17. 3 Characteristic CHADS 2 score (mean ± SD) SD, standard deviation; TIA, transient ischaemic attack Safety population (minus 9 pts in warfarin arm with no Cr. Cl data)

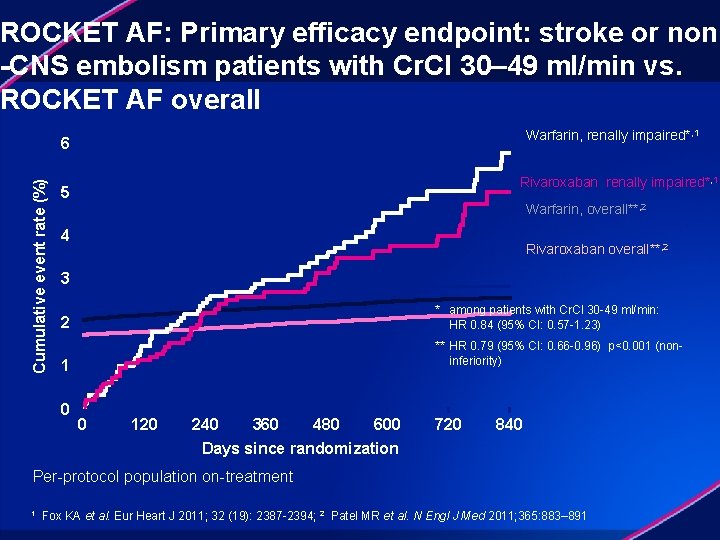
Cumulative event rate (%) ROCKET AF: stroke or non-CNS embolism among patients with Cr. Cl 30– 49 ml/min No. at risk: Rivaroxaban Warfarin Rivaroxaban HR (95% CI): 0. 84 (0. 57, 1. 23) Days since randomization 1, 434 1, 439 1, 226 1, 261 1, 103 1, 140 1, 027 1, 052 806 832 621 656 Event rates are % per year; Based on Protocol Compliant on Treatment Population Fox KA et al. Eur Heart J 2011; 32 (19): 2387 -2394 442 455 272

ROCKET AF: Primary efficacy endpoint: stroke or non -CNS embolism patients with Cr. Cl 30– 49 ml/min vs. ROCKET AF overall Warfarin, renally impaired*, 1 Cumulative event rate (%) 6 Rivaroxaban renally impaired*, 1 5 Warfarin, overall**, 2 4 Rivaroxaban overall**, 2 3 * among patients with Cr. Cl 30 -49 ml/min: HR 0. 84 (95% CI: 0. 57 -1. 23) 2 ** HR 0. 79 (95% CI: 0. 66 -0. 96) p<0. 001 (noninferiority) 1 0 0 120 240 480 600 360 Days since randomization 720 840 Per-protocol population on-treatment 1 Fox KA et al. Eur Heart J 2011; 32 (19): 2387 -2394; 2 Patel MR et al. N Engl J Med 2011; 365: 883– 891

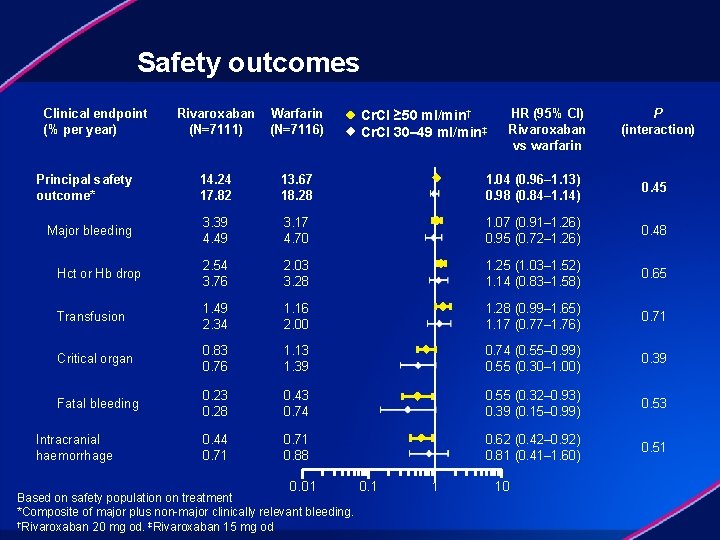
Safety outcomes Clinical endpoint (% per year) Rivaroxaban (N=7111) Warfarin (N=7116) Principal safety outcome* 14. 24 17. 82 13. 67 18. 28 1. 04 (0. 96– 1. 13) 0. 98 (0. 84– 1. 14) 0. 45 Major bleeding 3. 39 4. 49 3. 17 4. 70 1. 07 (0. 91– 1. 26) 0. 95 (0. 72– 1. 26) 0. 48 Hct or Hb drop 2. 54 3. 76 2. 03 3. 28 1. 25 (1. 03– 1. 52) 1. 14 (0. 83– 1. 58) 0. 65 Transfusion 1. 49 2. 34 1. 16 2. 00 1. 28 (0. 99– 1. 65) 1. 17 (0. 77– 1. 76) 0. 71 Critical organ 0. 83 0. 76 1. 13 1. 39 0. 74 (0. 55– 0. 99) 0. 55 (0. 30– 1. 00) 0. 39 Fatal bleeding 0. 23 0. 28 0. 43 0. 74 0. 55 (0. 32– 0. 93) 0. 39 (0. 15– 0. 99) 0. 53 0. 44 0. 71 0. 88 0. 62 (0. 42– 0. 92) 0. 81 (0. 41– 1. 60) 0. 51 Intracranial haemorrhage 0. 01 Based on safety population on treatment *Composite of major plus non-major clinically relevant bleeding. †Rivaroxaban 20 mg od. ‡Rivaroxaban 15 mg od Cr. Cl ≥ 50 ml/min† Cr. Cl 30– 49 ml/min‡ 0. 1 1 HR (95% CI) Rivaroxaban vs warfarin 10 P (interaction)

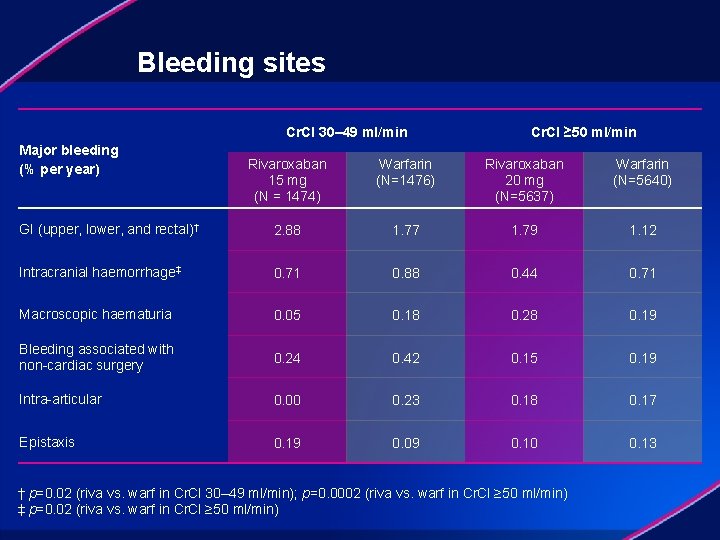
Bleeding sites Cr. Cl 30– 49 ml/min Major bleeding (% per year) Cr. Cl ≥ 50 ml/min Rivaroxaban 15 mg (N = 1474) Warfarin (N=1476) Rivaroxaban 20 mg (N=5637) Warfarin (N=5640) GI (upper, lower, and rectal)† 2. 88 1. 77 1. 79 1. 12 Intracranial haemorrhage‡ 0. 71 0. 88 0. 44 0. 71 Macroscopic haematuria 0. 05 0. 18 0. 28 0. 19 Bleeding associated with non-cardiac surgery 0. 24 0. 42 0. 15 0. 19 Intra-articular 0. 00 0. 23 0. 18 0. 17 Epistaxis 0. 19 0. 09 0. 10 0. 13 † p=0. 02 (riva vs. warf in Cr. Cl 30– 49 ml/min); p=0. 0002 (riva vs. warf in Cr. Cl ≥ 50 ml/min) ‡ p=0. 02 (riva vs. warf in Cr. Cl ≥ 50 ml/min)

ROCKET AF – PREVENZIONE SECONDARIA 28

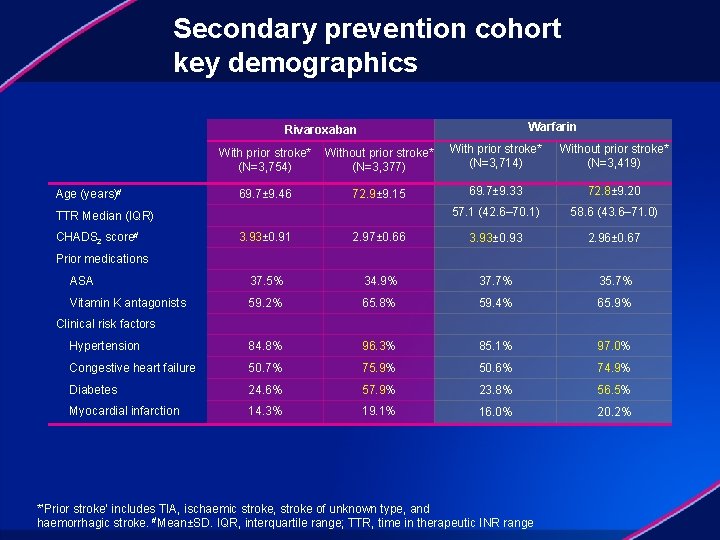
Secondary prevention cohort key demographics Warfarin Rivaroxaban With prior stroke* Without prior stroke* (N=3, 754) (N=3, 377) Age (years)# 69. 7± 9. 46 72. 9± 9. 15 TTR Median (IQR) CHADS 2 score# With prior stroke* (N=3, 714) Without prior stroke* (N=3, 419) 69. 7± 9. 33 72. 8± 9. 20 57. 1 (42. 6– 70. 1) 58. 6 (43. 6– 71. 0) 3. 93± 0. 91 2. 97± 0. 66 3. 93± 0. 93 2. 96± 0. 67 ASA 37. 5% 34. 9% 37. 7% 35. 7% Vitamin K antagonists 59. 2% 65. 8% 59. 4% 65. 9% Hypertension 84. 8% 96. 3% 85. 1% 97. 0% Congestive heart failure 50. 7% 75. 9% 50. 6% 74. 9% Diabetes 24. 6% 57. 9% 23. 8% 56. 5% Myocardial infarction 14. 3% 19. 1% 16. 0% 20. 2% Prior medications Clinical risk factors *‘Prior stroke’ includes TIA, ischaemic stroke, stroke of unknown type, and haemorrhagic stroke. #Mean±SD. IQR, interquartile range; TTR, time in therapeutic INR range

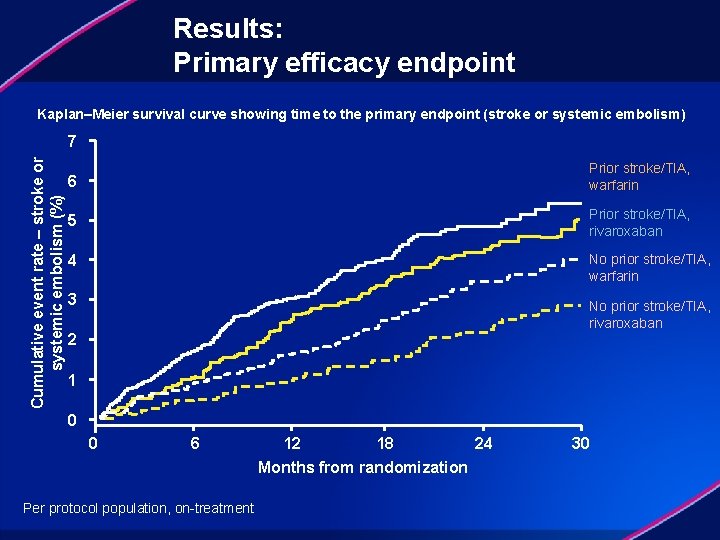
Results: Primary efficacy endpoint Kaplan–Meier survival curve showing time to the primary endpoint (stroke or systemic embolism) Cumulative event rate – stroke or systemic embolism (%) 7 6 Prior stroke/TIA, warfarin 5 Prior stroke/TIA, rivaroxaban 4 No prior stroke/TIA, warfarin 3 No prior stroke/TIA, rivaroxaban 2 1 0 0 6 Per protocol population, on-treatment 12 18 24 Months from randomization 30

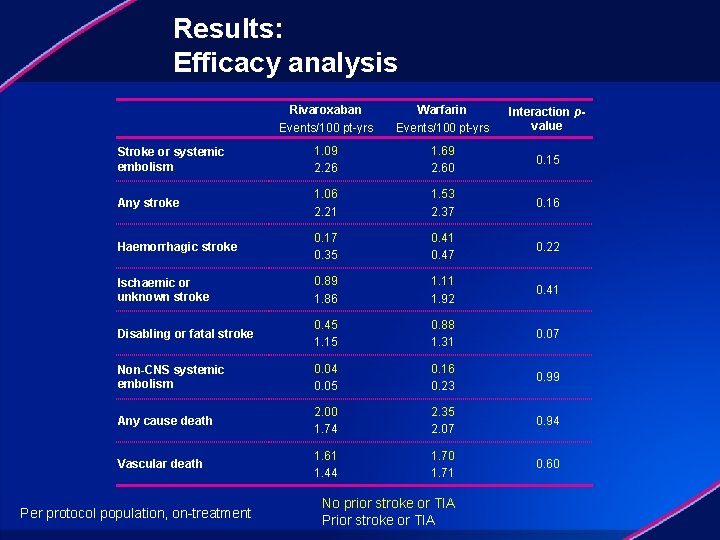
Results: Efficacy analysis Rivaroxaban Events/100 pt-yrs Warfarin Events/100 pt-yrs Interaction pvalue Stroke or systemic embolism 1. 09 2. 26 1. 69 2. 60 0. 15 Any stroke 1. 06 2. 21 1. 53 2. 37 0. 16 Haemorrhagic stroke 0. 17 0. 35 0. 41 0. 47 0. 22 Ischaemic or unknown stroke 0. 89 1. 86 1. 11 1. 92 0. 41 Disabling or fatal stroke 0. 45 1. 15 0. 88 1. 31 0. 07 Non-CNS systemic embolism 0. 04 0. 05 0. 16 0. 23 0. 99 Any cause death 2. 00 1. 74 2. 35 2. 07 0. 94 Vascular death 1. 61 1. 44 1. 70 1. 71 0. 60 Per protocol population, on-treatment No prior stroke or TIA Prior stroke or TIA

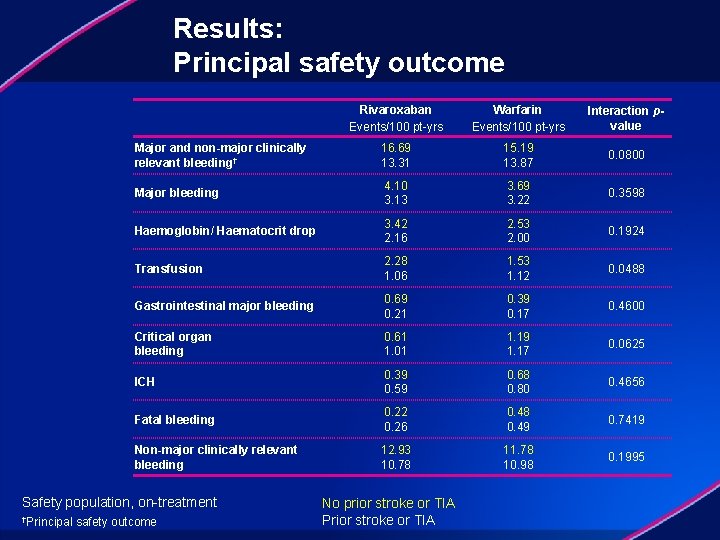
Results: Principal safety outcome Rivaroxaban Events/100 pt-yrs Warfarin Events/100 pt-yrs Interaction pvalue Major and non-major clinically relevant bleeding† 16. 69 13. 31 15. 19 13. 87 0. 0800 Major bleeding 4. 10 3. 13 3. 69 3. 22 0. 3598 Haemoglobin/ Haematocrit drop 3. 42 2. 16 2. 53 2. 00 0. 1924 Transfusion 2. 28 1. 06 1. 53 1. 12 0. 0488 Gastrointestinal major bleeding 0. 69 0. 21 0. 39 0. 17 0. 4600 Critical organ bleeding 0. 61 1. 01 1. 19 1. 17 0. 0625 ICH 0. 39 0. 59 0. 68 0. 80 0. 4656 Fatal bleeding 0. 22 0. 26 0. 48 0. 49 0. 7419 Non-major clinically relevant bleeding 12. 93 10. 78 11. 78 10. 98 0. 1995 Safety population, on-treatment †Principal safety outcome No prior stroke or TIA Prior stroke or TIA

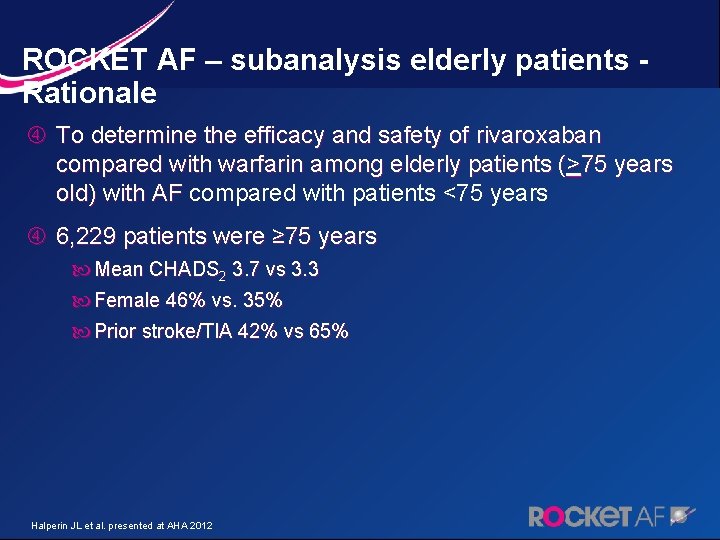
ROCKET AF – subanalysis elderly patients - Rationale To determine the efficacy and safety of rivaroxaban compared with warfarin among elderly patients (>75 years old) with AF compared with patients <75 years old) with AF 6, 229 patients were ≥ 75 years Mean CHADS 2 3. 7 vs 3. 3 Female 46% vs. 35% Prior stroke/TIA 42% vs 65% Halperin JL et al. presented at AHA 2012

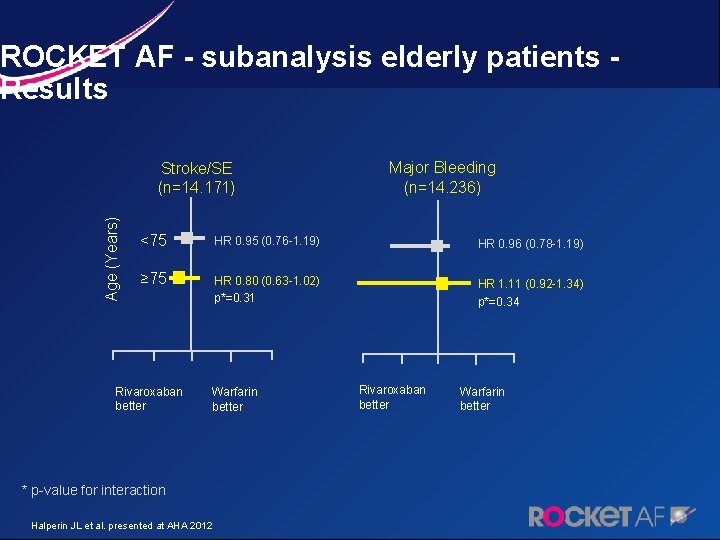
ROCKET AF - subanalysis elderly patients - Results Age (Years) Stroke/SE (n=14. 171) Major Bleeding (n=14. 236) <75 HR 0. 95 (0. 76 -1. 19) HR 0. 96 (0. 78 -1. 19) ≥ 75 HR 0. 80 (0. 63 -1. 02) p*=0. 31 HR 1. 11 (0. 92 -1. 34) p*=0. 34 Rivaroxaban better Warfarin better * p-value for interaction Halperin JL et al. presented at AHA 2012 Rivaroxaban better Warfarin better

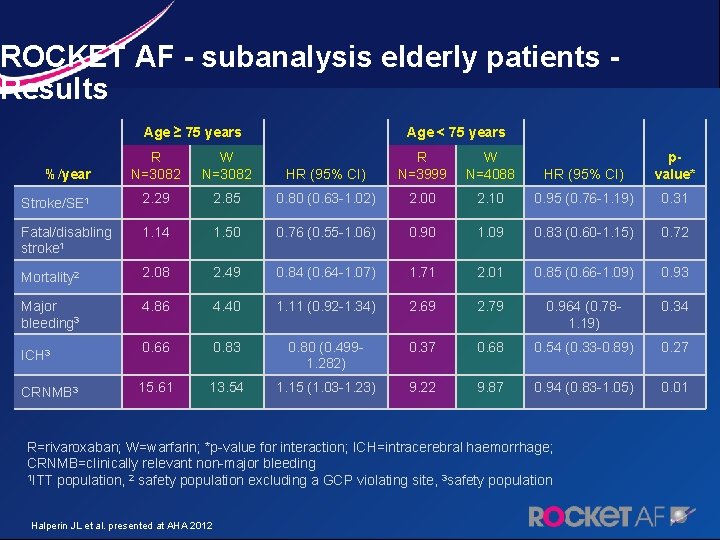
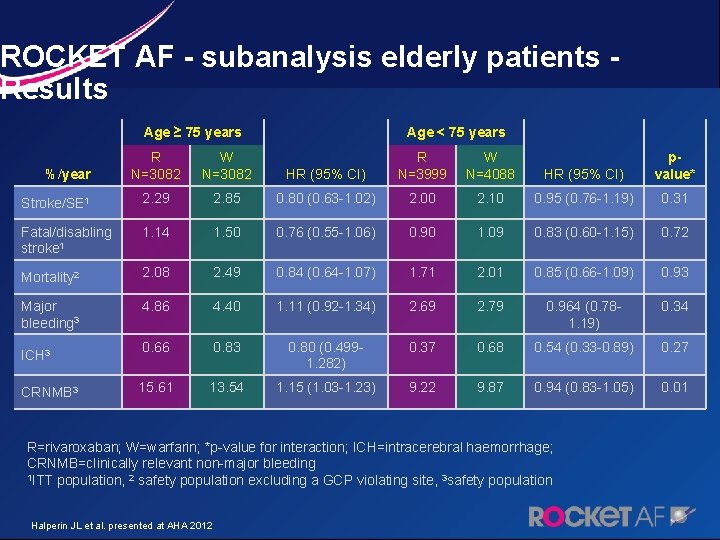
ROCKET AF - subanalysis elderly patients - Results Age ≥ 75 years R N=3082 W N=3082 Stroke/SE 1 2. 29 Fatal/disabling stroke 1 Age < 75 years HR (95% CI) R N=3999 W N=4088 HR (95% CI) pvalue* 2. 85 0. 80 (0. 63 -1. 02) 2. 00 2. 10 0. 95 (0. 76 -1. 19) 0. 31 1. 14 1. 50 0. 76 (0. 55 -1. 06) 0. 90 1. 09 0. 83 (0. 60 -1. 15) 0. 72 Mortality 2 2. 08 2. 49 0. 84 (0. 64 -1. 07) 1. 71 2. 01 0. 85 (0. 66 -1. 09) 0. 93 Major bleeding 3 4. 86 4. 40 1. 11 (0. 92 -1. 34) 2. 69 2. 79 0. 964 (0. 781. 19) 0. 34 0. 66 0. 83 0. 80 (0. 4991. 282) 0. 37 0. 68 0. 54 (0. 33 -0. 89) 0. 27 15. 61 13. 54 1. 15 (1. 03 -1. 23) 9. 22 9. 87 0. 94 (0. 83 -1. 05) 0. 01 %/year ICH 3 CRNMB 3 R=rivaroxaban; W=warfarin; *p-value for interaction; ICH=intracerebral haemorrhage; CRNMB=clinically relevant non-major bleeding 1 ITT population, 2 safety population excluding a GCP violating site, 3 safety population Halperin JL et al. presented at AHA 2012

ROCKET AF - subanalysis elderly patients - Results Age ≥ 75 years R N=3082 W N=3082 Stroke/SE 1 2. 29 Fatal/disabling stroke 1 Age < 75 years HR (95% CI) R N=3999 W N=4088 HR (95% CI) pvalue* 2. 85 0. 80 (0. 63 -1. 02) 2. 00 2. 10 0. 95 (0. 76 -1. 19) 0. 31 1. 14 1. 50 0. 76 (0. 55 -1. 06) 0. 90 1. 09 0. 83 (0. 60 -1. 15) 0. 72 Mortality 2 2. 08 2. 49 0. 84 (0. 64 -1. 07) 1. 71 2. 01 0. 85 (0. 66 -1. 09) 0. 93 Major bleeding 3 4. 86 4. 40 1. 11 (0. 92 -1. 34) 2. 69 2. 79 0. 964 (0. 781. 19) 0. 34 0. 66 0. 83 0. 80 (0. 4991. 282) 0. 37 0. 68 0. 54 (0. 33 -0. 89) 0. 27 15. 61 13. 54 1. 15 (1. 03 -1. 23) 9. 22 9. 87 0. 94 (0. 83 -1. 05) 0. 01 %/year ICH 3 CRNMB 3 R=rivaroxaban; W=warfarin; *p-value for interaction; ICH=intracerebral haemorrhage; CRNMB=clinically relevant non-major bleeding 1 ITT population, 2 safety population excluding a GCP violating site, 3 safety population Halperin JL et al. presented at AHA 2012

 Nuovi antidiabetici orali
Nuovi antidiabetici orali Nuovi antidiabetici orali
Nuovi antidiabetici orali Esempi di documenti orali
Esempi di documenti orali I nuovi prodotti alimentari
I nuovi prodotti alimentari Nuovi percorsi professionali
Nuovi percorsi professionali Per vino nuovo otri nuovi
Per vino nuovo otri nuovi Nuovi istituti tecnici
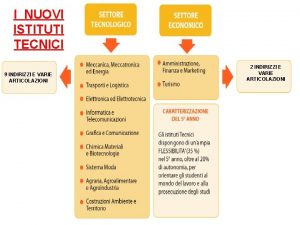
Nuovi istituti tecnici Indirizzi tecnici
Indirizzi tecnici Su pensieri nuovi facciamo versi antichi
Su pensieri nuovi facciamo versi antichi Nuovi prodotti alimentari
Nuovi prodotti alimentari La nascita delle lingue e delle letterature romanze
La nascita delle lingue e delle letterature romanze L'esperienza delle cose moderne e la lezione delle antique
L'esperienza delle cose moderne e la lezione delle antique Regni rinascimentali mappa
Regni rinascimentali mappa The protist dilemma
The protist dilemma Euthyphro plato summary
Euthyphro plato summary Pvq p q
Pvq p q Contoh circulus in probando
Contoh circulus in probando The hot dog buns dilemma answers
The hot dog buns dilemma answers Edd regel
Edd regel Deontologie voorbeelden
Deontologie voorbeelden How sociologists view social problems: the abortion dilemma
How sociologists view social problems: the abortion dilemma Volkswagen scandal ethical issues
Volkswagen scandal ethical issues Haldane's dilemma
Haldane's dilemma Dilemma
Dilemma Amphiboly fallacy
Amphiboly fallacy Ano ang dapat isaalang alang sa pagtatala ng datos
Ano ang dapat isaalang alang sa pagtatala ng datos False dichotomy in the crucible
False dichotomy in the crucible Prisoners dilemma
Prisoners dilemma Perbedaan ethical dilemma dan ethical lapse
Perbedaan ethical dilemma dan ethical lapse Kohlberg dilemma worksheet answers
Kohlberg dilemma worksheet answers Prisoners dilemma
Prisoners dilemma Prisoner's dilemma nash equilibrium
Prisoner's dilemma nash equilibrium The xyz affair dilemma
The xyz affair dilemma Selective repeat dilemma
Selective repeat dilemma Elements of science fiction and myths legends folktales
Elements of science fiction and myths legends folktales Ethisch dilemma accountant voorbeeld
Ethisch dilemma accountant voorbeeld Pooja morar
Pooja morar Good boy/girl orientation
Good boy/girl orientation