How does the cell manufacture these magnificent machines























- Slides: 39

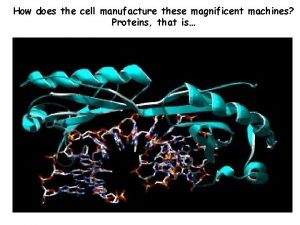
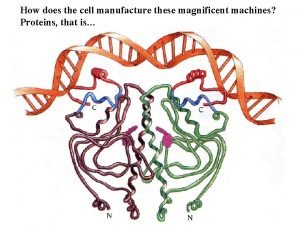
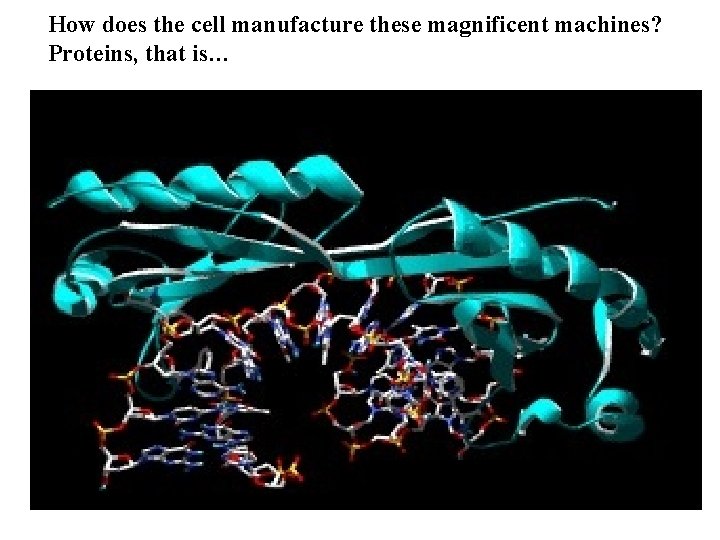
How does the cell manufacture these magnificent machines? Proteins, that is…

Proteins • Long polymers of amino acids, joined by peptide (amide) bonds are called polypeptides • Polypeptides fold into stable threedimensional shapes and are called proteins • Shape determines the function of proteins (active sites are on the surface)

Proteins - classified by functions Enzymes - catalytic activity and function Transport Proteins - bind & carry ligands Storage Proteins - ovalbumin, gluten, casein, ferretin Contractile (Motor): can contract, change shape, elements of cytoskeleton (actin, myosin, tubulin) Structural (Support): collagen of tendons & cartilage, elastin of ligaments (tropoelastin), keratin of hair, feathers, & nails, fibroin of silk & webs Defensive (Protect): antibodies (Ig. G), fibrinogen & thrombin, snake venoms, bacterial toxins Regulatory (Signal): regulate metabolic processes, hormones, transcription factors & enhancers, growth factor proteins Receptors (detect stimuli): light & rhodopsin, membrane receptor proteins and acetylcholine or insulin.


Structure of Proteins the Variety of Protein Structures may be INFINITE. . . average protein has 300 -400 amino acid's & has a MW of 30 k. D to 45 k. D a PROTEIN of 300 amino acids made with 20 different kinds of amino acids can have 20300 different linear arrays of aa's [10390 different proteins] 1 st protein sequenced was Beef Insulin by Fred Sanger - 1958 Nobel Prize winner to date about 100, 000 protein have been sequenced only about 10, 000 structures known [2 K/yr] E. coli make about 3, 000 proteins, humans make about 100, 000 proteins.

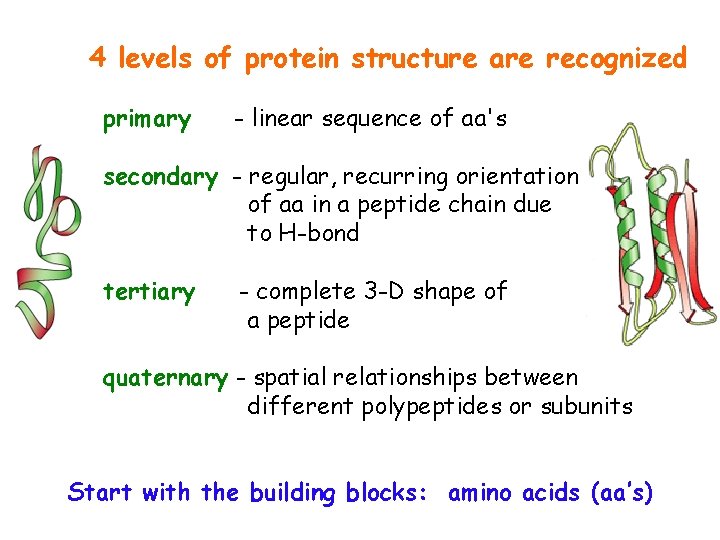
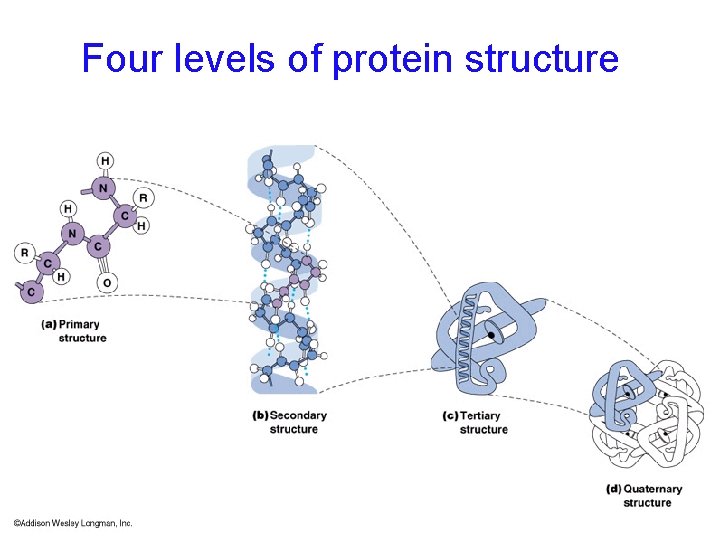
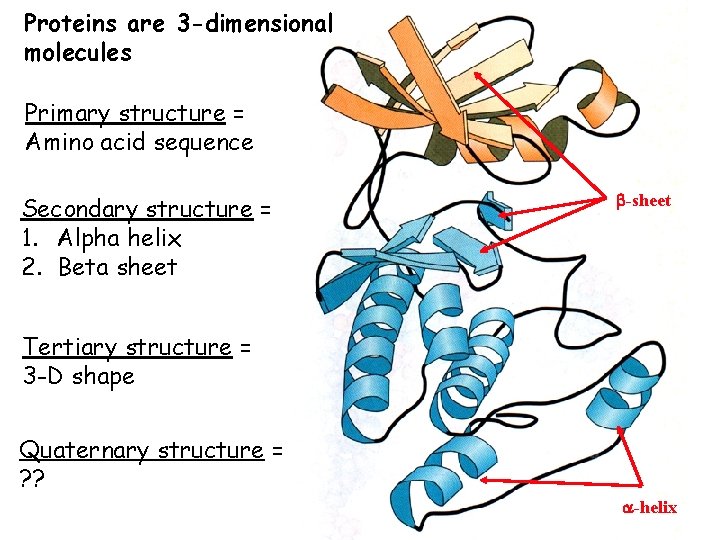
4 levels of protein structure are recognized primary - linear sequence of aa's secondary - regular, recurring orientation of aa in a peptide chain due to H-bond tertiary - complete 3 -D shape of a peptide quaternary - spatial relationships between different polypeptides or subunits Start with the building blocks: amino acids (aa’s)

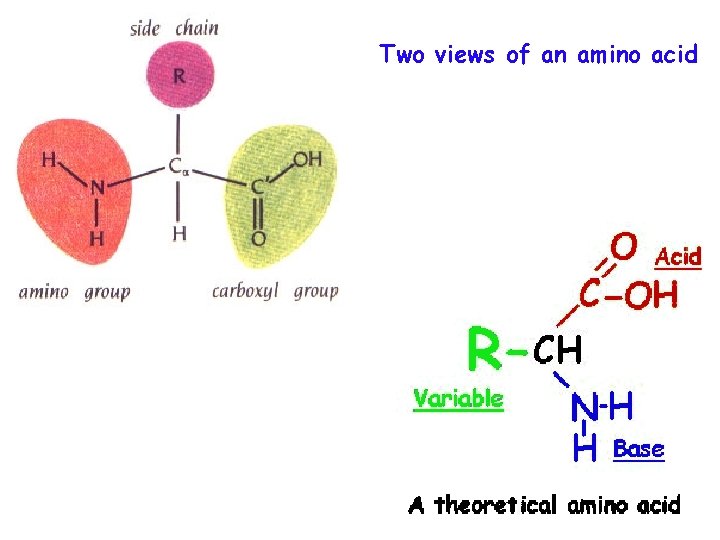
Two views of an amino acid

There are three types of side chains…. • Nonpolar (hydrophobic) • Polar uncharged (hydrophilic) • Polar charged (hydrophilic)





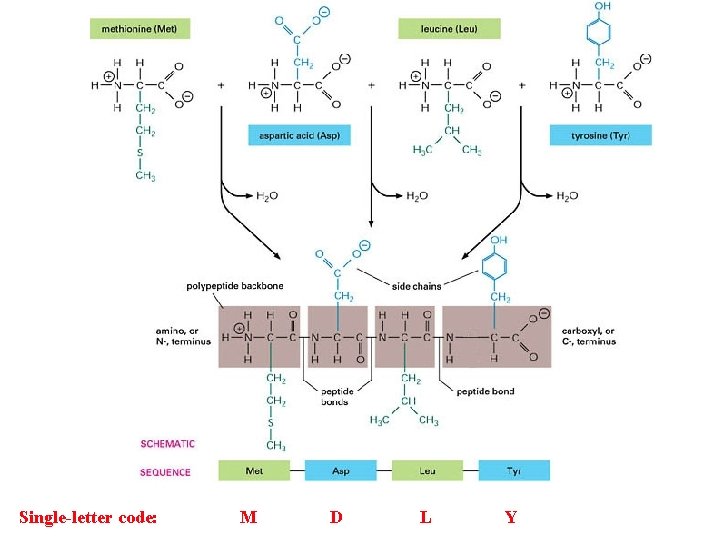
Single-letter code: M D L Y


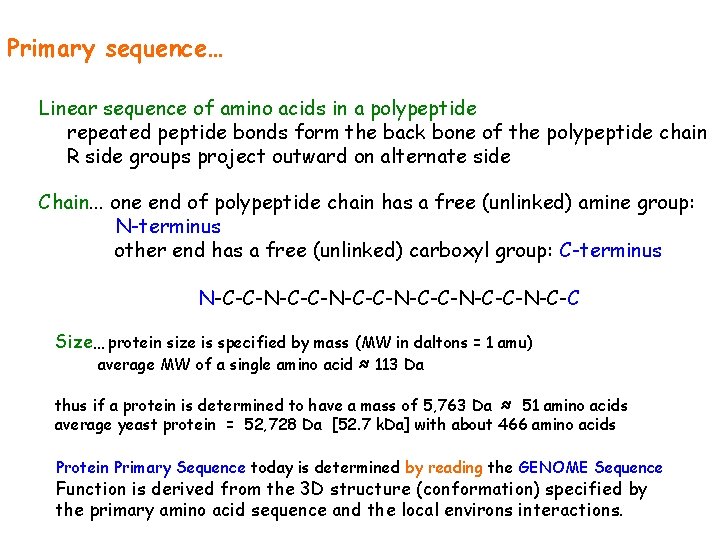
Primary sequence… Linear sequence of amino acids in a polypeptide repeated peptide bonds form the back bone of the polypeptide chain R side groups project outward on alternate side Chain. . . one end of polypeptide chain has a free (unlinked) amine group: N-terminus other end has a free (unlinked) carboxyl group: C-terminus N-C-C-N-C-C-N-C-C-N-C-C Size… protein size is specified by mass (MW in daltons = 1 amu) average MW of a single amino acid ≈ 113 Da thus if a protein is determined to have a mass of 5, 763 Da ≈ 51 amino acids average yeast protein = 52, 728 Da [52. 7 k. Da] with about 466 amino acids Protein Primary Sequence today is determined by reading the GENOME Sequence Function is derived from the 3 D structure (conformation) specified by the primary amino acid sequence and the local environs interactions.


Four levels of protein structure

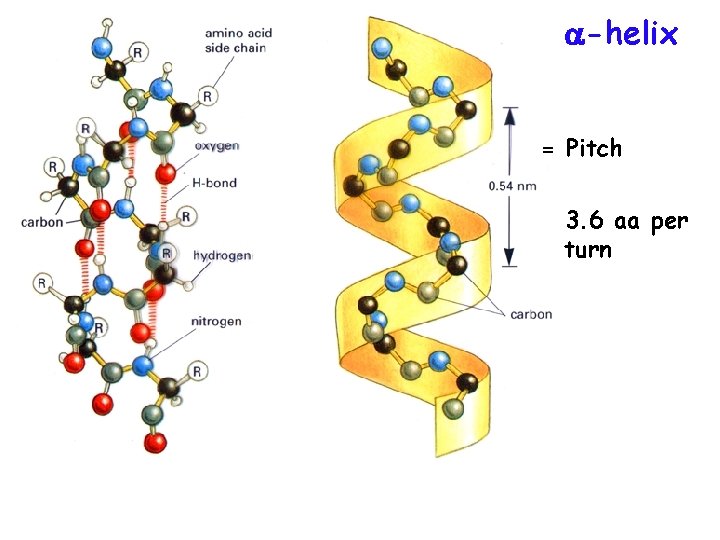
-helix = Pitch 3. 6 aa per turn

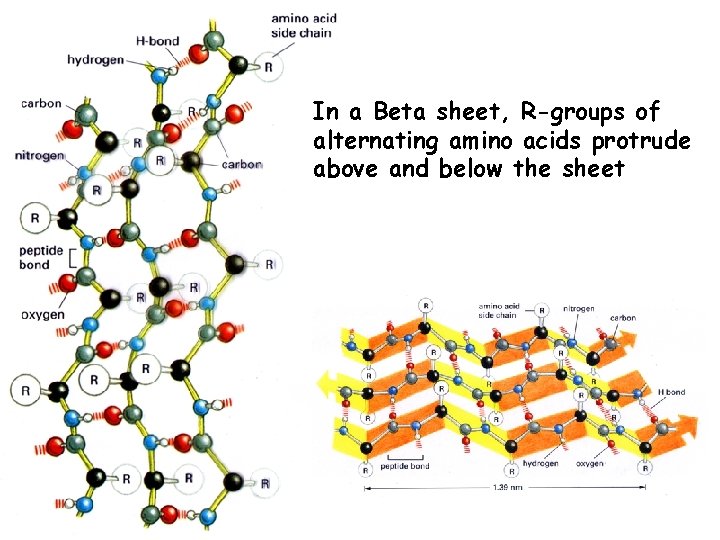
In a Beta sheet, R-groups of alternating amino acids protrude above and below the sheet

Proteins are 3 -dimensional molecules Primary structure = Amino acid sequence Secondary structure = 1. Alpha helix 2. Beta sheet -sheet Tertiary structure = 3 -D shape Quaternary structure = ? ? -helix

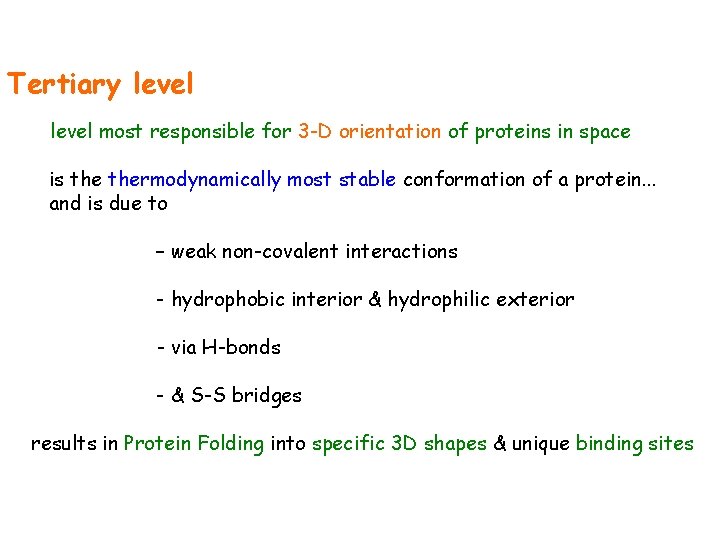
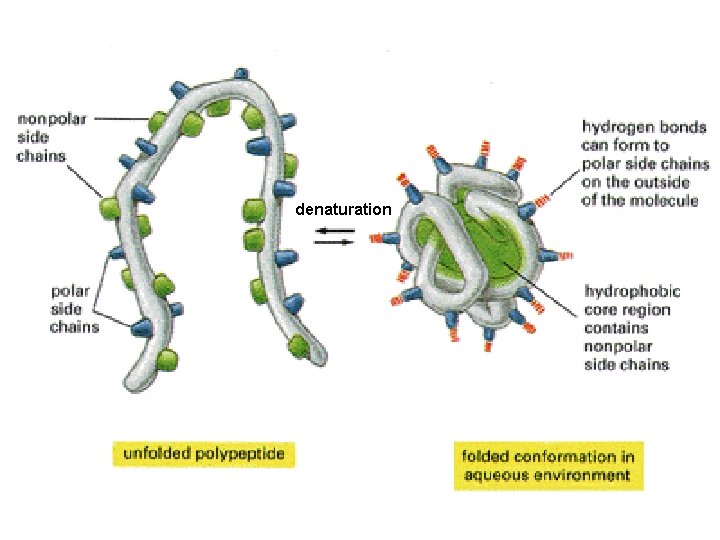
Tertiary level most responsible for 3 -D orientation of proteins in space is thermodynamically most stable conformation of a protein. . . and is due to – weak non-covalent interactions - hydrophobic interior & hydrophilic exterior - via H-bonds - & S-S bridges results in Protein Folding into specific 3 D shapes & unique binding sites

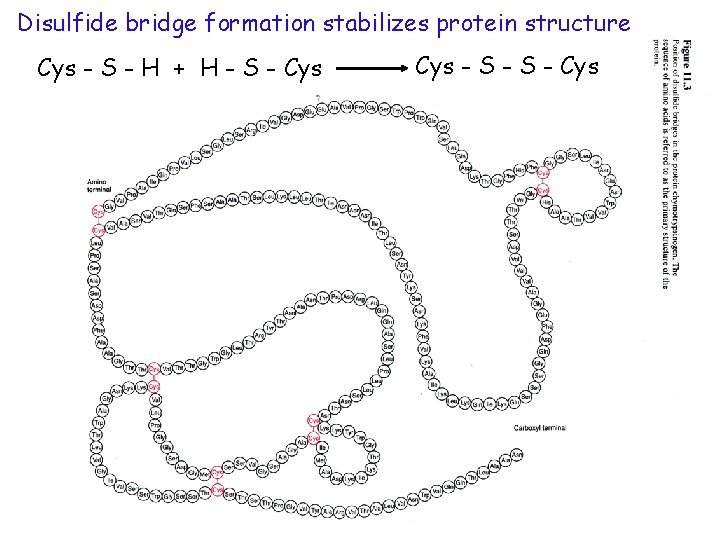
Disulfide bridge formation stabilizes protein structure Cys - S - H + H - S - Cys


denaturation












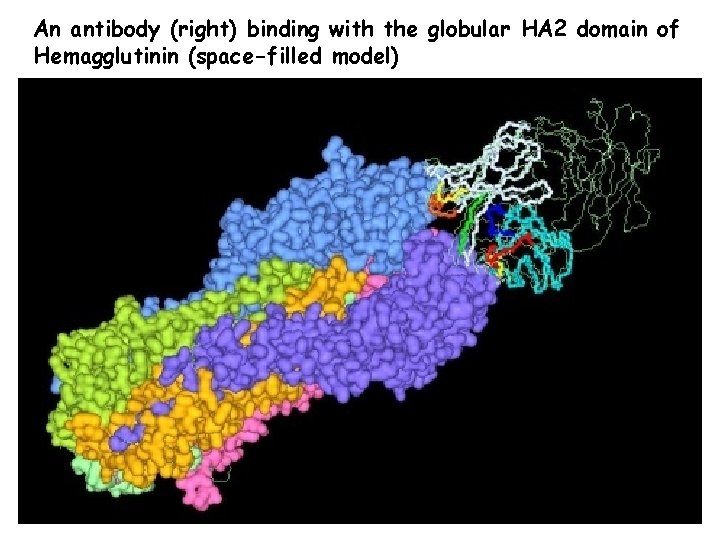
An antibody (right) binding with the globular HA 2 domain of Hemagglutinin (space-filled model)



 How does beowulf prepare for his battle with grendel
How does beowulf prepare for his battle with grendel Whiskery spitter meaning
Whiskery spitter meaning Mehmed the magnificent
Mehmed the magnificent The magnificent bull poem
The magnificent bull poem Suleiman the magnificent expansion
Suleiman the magnificent expansion Hemo the magnificent
Hemo the magnificent Suleiman the magnificent absolutism
Suleiman the magnificent absolutism Magnificent african cake
Magnificent african cake Yemen architecture
Yemen architecture Manufacture vs production
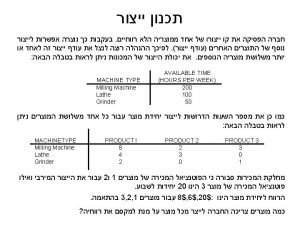
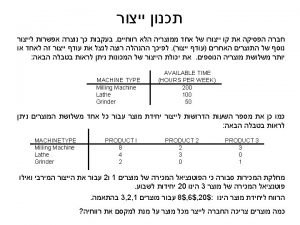
Manufacture vs production Hyster year chart
Hyster year chart Manufacture of portland cement
Manufacture of portland cement Higher design and manufacture
Higher design and manufacture Otto hoffman's method
Otto hoffman's method What is the use of terylene
What is the use of terylene Cambridge technicals level 3 engineering
Cambridge technicals level 3 engineering Chemical manufacture
Chemical manufacture Incremental analysis formula
Incremental analysis formula A firm is planning to manufacture a new product
A firm is planning to manufacture a new product Manufacture
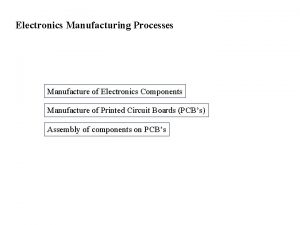
Manufacture Electronics manufacturing process
Electronics manufacturing process Manufacture of cement
Manufacture of cement Foundry machinery manufacture exporter
Foundry machinery manufacture exporter Example of production plan
Example of production plan The rapid manufacture of large numbers of identical objects
The rapid manufacture of large numbers of identical objects Im strong and stiff found only in plants
Im strong and stiff found only in plants Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton là gì
Tư thế worm breton là gì Chúa sống lại
Chúa sống lại Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ 101012 bằng
101012 bằng