Hormones Hot Flashes Highlights in Womens Health Laura




































































- Slides: 81

Hormones, Hot Flashes, & Highlights in Women’s Health Laura Davisson, MD Assistant Professor, Section of General Internal Medicine Clinical Care Co-Director, Center of Excellence in

History of Women’s Health

History of Women’s Health n n n 1941: Diethylstilbestrol (DES) approved by FDA 1953: Study published showing ineffectiveness at preventing miscarriage Until 1971: Continued to be aggressively marketed and routinely prescribed 1971: Identified as cause of vaginal clear cell adenocarcinoma in DES daughters 1971: FDA advised physicians to stop prescribing to pregnant women and national effort begun to find women prescribed DES while pregnant

History of Women’s Health n n n Such tragedies in pregnant women led to fear of using women of childbearing age in trials 1970 s: regulations restricted testing in women of reproductive age Ultimately led to widespread exclusion of women from trials Throughout most of 20 th century: treatments tested solely on men 1990: GAO report brought to light underrepresentation of women in federally funded trials

History of Women’s Health Now… n Several Federal agencies have changed policies to promote inclusion of women in studies n Learning that women affected by some diseases at different rates than men n Learning that women can present differently than men n There is emerging body of gender-specific research to guide practice in women’s

Today’s Goals Review literature regarding several important women’s health topics n Increase awareness of emerging discipline of women’s health n

Outline n Hormones n Hot flashes n Highlights

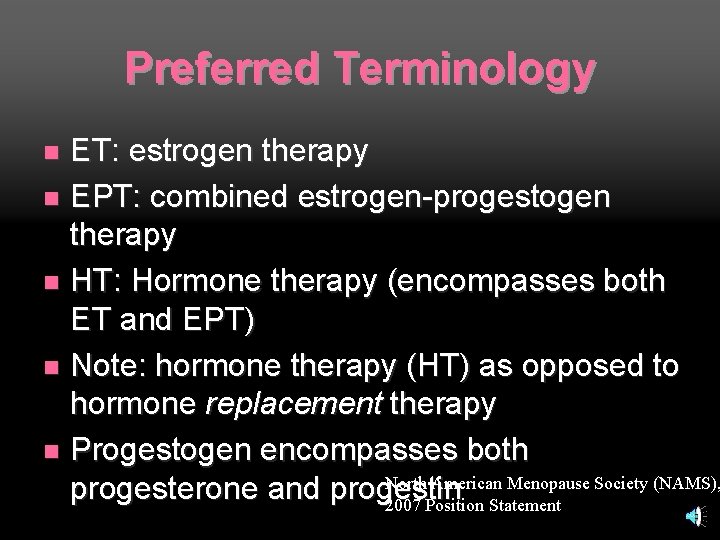
Preferred Terminology ET: estrogen therapy n EPT: combined estrogen-progestogen therapy n HT: Hormone therapy (encompasses both ET and EPT) n Note: hormone therapy (HT) as opposed to hormone replacement therapy n Progestogen encompasses both North American Menopause Society (NAMS), progesterone and progestin 2007 Position Statement n

1990’s Recommendations based on observational studies “Women who have coronary heart disease or who are at increased risk of coronary heart disease are likely to benefit from hormone therapy” American College of Physicians, 1992 “Probable beneficial effect of estrogen on heart disease” American College of Obstetricians and Gynecologists, 1992 “Estrogen replacement therapy does look promising as a long-term protection against heart attack” American Heart Association, 1996

Heart and Estrogen/Progestin Replacement Study (HERS) 1998

HERS Purpose: To evaluate secondary prevention of CHD with hormone therapy (HT) in postmenopausal women with known coronary disease

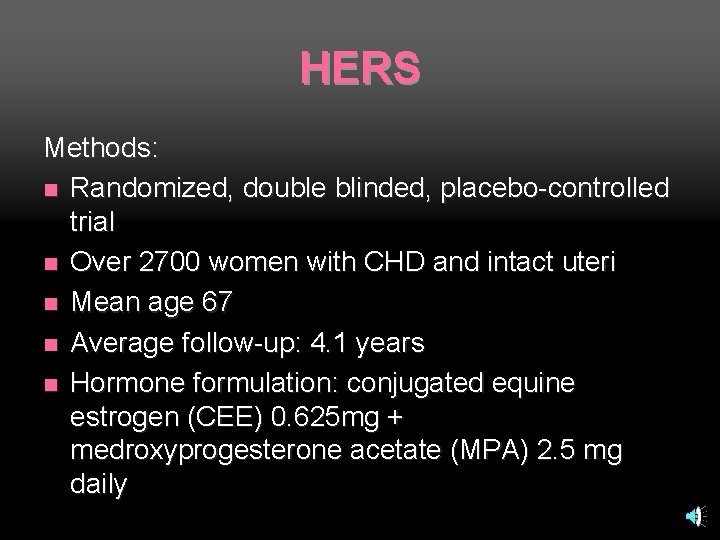
HERS Methods: n Randomized, double blinded, placebo-controlled trial n Over 2700 women with CHD and intact uteri n Mean age 67 n Average follow-up: 4. 1 years n Hormone formulation: conjugated equine estrogen (CEE) 0. 625 mg + medroxyprogesterone acetate (MPA) 2. 5 mg daily

HERS Results: No significant differences in CHD found in treatment vs. placebo groups

HERS II (2002) Subsequent unblinded follow-up of HERS subjects n Looked at long-term outcomes for 2. 7 more years n Confirmed HERS findings that HT did NOT decrease risk of CHD in women with known history of CHD n

HERS I and II Interpretation of HERS trials: HT not effective in secondary prevention of CHD

WHI Women’s Health Initiative

WHI Observational Study n Clinical Trials: n Hormone Therapy n Dietary Modification n Calcium/Vitamin D n

Some of the 100+ WHI papers: Estrogen plus progestin and the risk of coronary heart disease. NEJM, 2003 Effect of estrogen plus progestin on stroke in postmenopausal women. The women’s health initiative: a randomized trial. JAMA, 2003 Estrogen plus progestin and colorectal cancer in postmenopausal women. NEJM, 2004 Estrogen plus progestin and risk of venous thrombosis. JAMA, 2004 Conjugated equine estrogens and coronary heart disease: the women’s health initiative. Arch Intern Med, 2006 Venous thrombosis and conjugated equine estrogen in women without a uterus. Arch Intern Med, 2006

WHI Multi-million dollar, 15 -year project sponsored by NIH, NHLBI n Over 160, 000 women aged 50 -79 n 40 Clinical Centers across US n One of most definitive, far-reaching clinical trials of post-menopausal women's health ever done n

WHI Hormone Therapy Trial Long-term study to evaluate HT’s effects on CHD, osteoporotic fractures, and breast cancer in postmenopausal women n Two arms: n Estrogen plus Progestin (E+P) n Estrogen Alone (E Alone) n

WHI Hormone Therapy Trial: E+P arm

WHI: E+P arm Methods: n Over 16, 000 women with intact uteri n Randomly assigned to E+P vs. placebo for 5 years (planned duration 8. 5 years) n Hormone formulation: CEE 0. 625 mg plus MPA 2. 5 mg daily

WHI: E+P arm Results: n Trial halted early--July 2002 n Overall harm found in treatment group

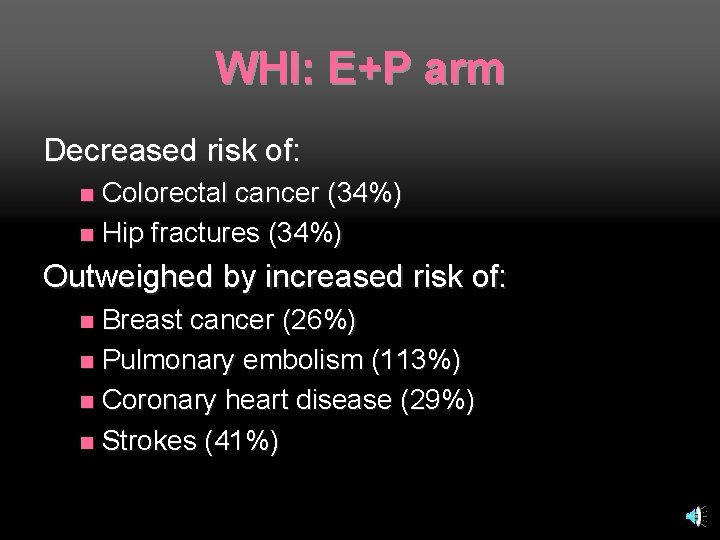
WHI: E+P arm Decreased risk of: Colorectal cancer (34%) n Hip fractures (34%) n Outweighed by increased risk of: Breast cancer (26%) n Pulmonary embolism (113%) n Coronary heart disease (29%) n Strokes (41%) n

WHI: E+P arm Absolute excess risks: n Excess CHD events: 7/10, 000 womanyears n Excess stroke events : 8/10, 000 womanyears n Excess pulmonary emboli: 8/10, 000 woman-years n Excess invasive breast cancer: 8/10, 000 woman-years

WHI: E+P arm Absolute Benefits: n Fewer colorectal cancers: 6/10, 000 woman-years n Fewer hip fractures: 5/10, 000 womanyears

WHI: E+P arm Summary of additional adverse events: 19 per 10, 000 woman-years n Number needed to harm for 5 years E+P use: 101 n

WHI Hormone Therapy Trial: E Alone arm

WHI: E Alone arm Methods: n Over 10, 000 women aged 50 -79 with prior hysterectomy n Randomly assigned to 0. 625 mg CEE vs. placebo n Followed for average 6. 8 years

WHI: E Alone arm Results: n Trial halted one year early--February 2004 n Increased risk of stroke among women in hormone group

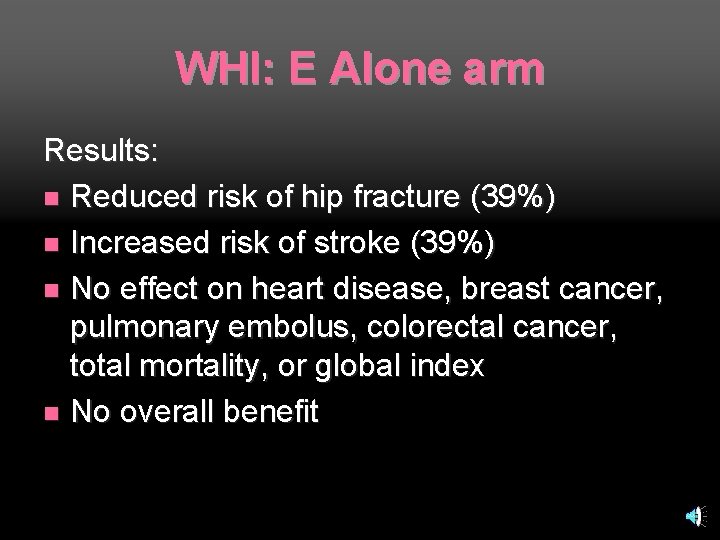
WHI: E Alone arm Results: n Reduced risk of hip fracture (39%) n Increased risk of stroke (39%) n No effect on heart disease, breast cancer, pulmonary embolus, colorectal cancer, total mortality, or global index n No overall benefit

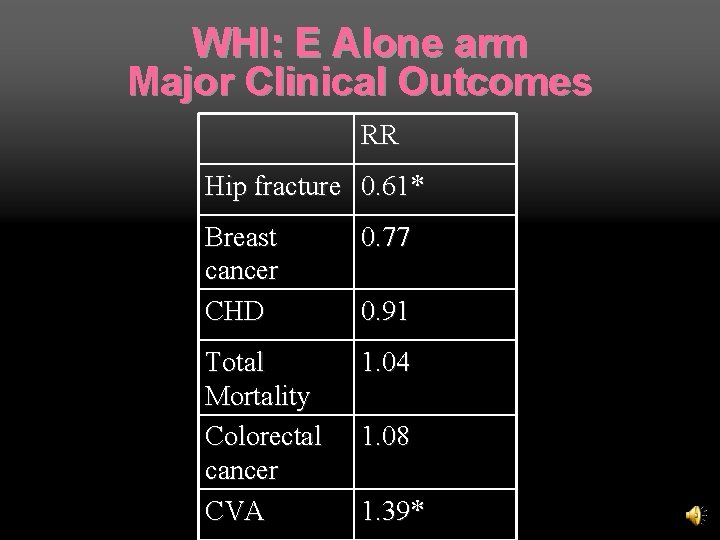
WHI: E Alone arm Major Clinical Outcomes RR Hip fracture 0. 61* Breast cancer CHD 0. 77 Total Mortality Colorectal cancer CVA 1. 04 0. 91 1. 08 1. 39*

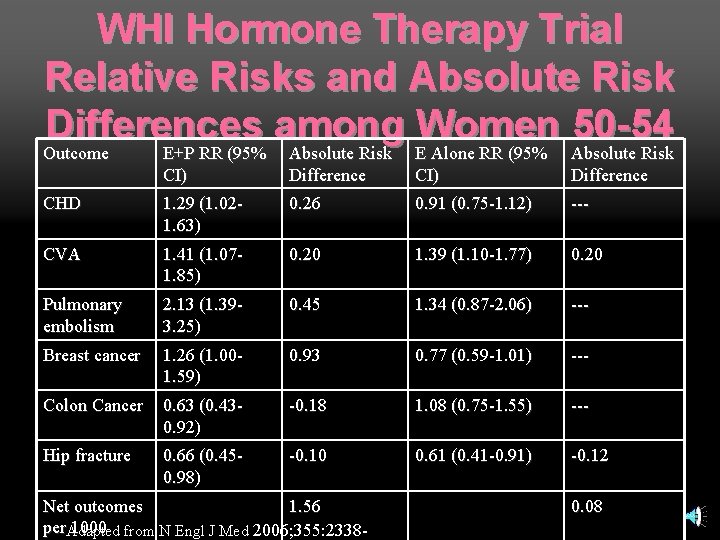
WHI Hormone Therapy Trial Relative Risks and Absolute Risk Differences among Women 50 -54 Outcome E+P RR (95% Absolute Risk E Alone RR (95% Absolute Risk Outcome E+P RR (95% CI) Absolute Risk Difference E Alone RR (95% CI) Absolute Risk Difference CHD 1. 29 (1. 021. 63) 0. 26 0. 91 (0. 75 -1. 12) --- CVA 1. 41 (1. 071. 85) 0. 20 1. 39 (1. 10 -1. 77) 0. 20 Pulmonary embolism 2. 13 (1. 393. 25) 0. 45 1. 34 (0. 87 -2. 06) --- Breast cancer 1. 26 (1. 001. 59) 0. 93 0. 77 (0. 59 -1. 01) --- Colon Cancer 0. 63 (0. 430. 92) -0. 18 1. 08 (0. 75 -1. 55) --- Hip fracture 0. 66 (0. 450. 98) -0. 10 0. 61 (0. 41 -0. 91) -0. 12 Net outcomes 1. 56 per. Adapted 1000 from N Engl J Med 2006; 355: 2338 - 0. 08

WHI Hormone Therapy Trial: Divergent results n Heart disease n n n Pulmonary embolism n n n Increased: E+P Neutral: E Alone Breast cancer n n Increased: E+P Neutral: E Alone n Colorectal cancer n n n Decreased: E+P Neutral: E Alone Global index n n Increased: E+P Neutral: E Alone

WHI Hormone Therapy Trial: Concordant results n Increased: n Decreased: n Fractures n Vasomotor symptoms n Diabetes n Neutral: n HRQOL Strokes n Dementia (>65) n Gallstones n Urinary incontinence n

WHI Hormone Therapy Trial Limitations: n Only tested CEE and MPA hormone regimens n Early termination of trials can lead to bias n Average mid-60’s, not typical 50 yearold women seeking relief from menopausal symptoms n Possible that recently menopausal women with low baseline risk of heart disease may have more favorable balance of benefits and risks

WHI Hormone Therapy Trial Conclusions: n HT should not be recommended for chronic disease prevention in postmenopausal women n Rate of adverse events was higher with E+P than E Alone n Possible that progestins exacerbate risks n Possible that other formulations, routes of administration, or lower doses might be associated with fewer adverse events (little supportive evidence)

Divergence of clinical trials from observational studies n n Highlights importance of randomized controlled clinical trials Confounding may explain lower heart disease rates in hormone users in observational studies because they were: n n n Leaner Less likely to smoke More physically active More likely to see doctors regularly More highly educated Another possible explanation for discrepancy: timing of initiation of HT

Timing of initiation of HT Emerging data suggests disparities may be related to timing of initiation of HT in relation to proximity of menopause n On average, women in WHI and HERS initiated HT more than a decade after menopause n Insufficient numbers of younger, symptomatic, newly postmenopausal women to determine whether similar patterns apply to them n

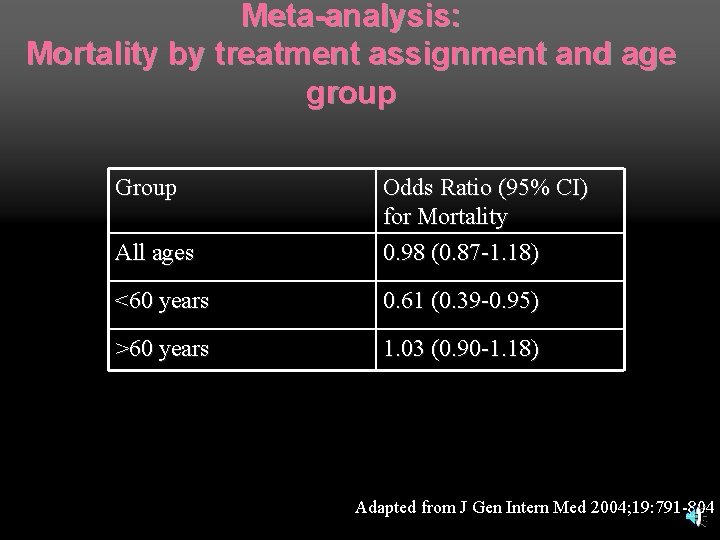
Meta-analysis: Mortality by treatment assignment and age group Group All ages Odds Ratio (95% CI) for Mortality 0. 98 (0. 87 -1. 18) <60 years 0. 61 (0. 39 -0. 95) >60 years 1. 03 (0. 90 -1. 18) Adapted from J Gen Intern Med 2004; 19: 791 -804

Rossouw, JE, et al. JAMA. Apr 4, 2007; 297: 1465 -1477

Timing of initiation of HT Purpose: To explore whether the effects of HT on risk of cardiovascular disease vary by age or years since menopause began JAMA. Apr, 2007; 297: 1465 -1477

Timing of initiation of HT Methods: n Secondary analysis of WHI HT trial results n Combined data from the two trial arms to improve statistical power to be able to examine trends across categories of age and years since menopause n Main outcome: CHD n Other outcomes: mortality and a global index JAMA. Apr, 2007; 297: 1465 -1477

Timing of initiation of HT Results: n Trend for reduced CHD risk in women closer to menopause but no subgroup met statistical significance n Nonsignificant tendency for total mortality to be reduced among younger women (50 -59) n Risk of stroke did not vary by age or time since menopause JAMA. Apr, 2007; 297: 1465 -1477

Timing of initiation of HT Conclusions: n Offers reassurance that HT a reasonable option for short-term treatment of menopausal symptoms n Raises the question of whethere may be a protective effect against CHD in younger, recently postmenopausal women JAMA. Apr, 2007; 297: 1465 -1477

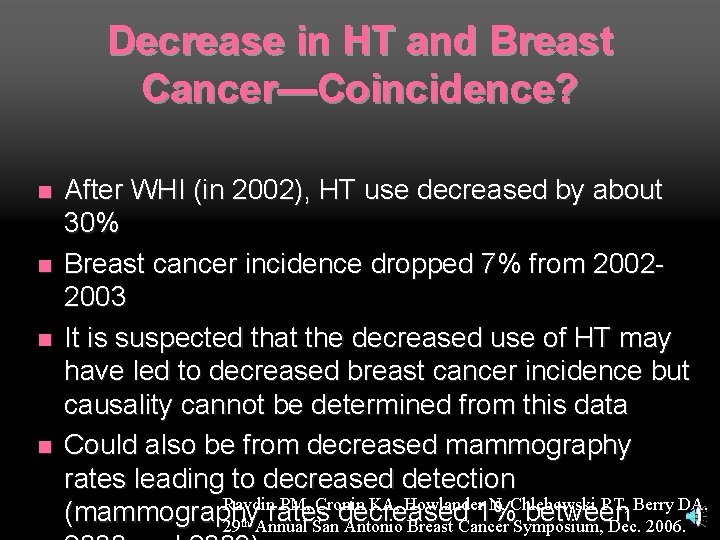
Decrease in HT and Breast Cancer—Coincidence? n n After WHI (in 2002), HT use decreased by about 30% Breast cancer incidence dropped 7% from 20022003 It is suspected that the decreased use of HT may have led to decreased breast cancer incidence but causality cannot be determined from this data Could also be from decreased mammography rates leading to decreased detection Ravdin PM, Cronin KA, Howlander N, Chlebowski RT, Berry DA. (mammography rates decreased 1% between 29 th Annual San Antonio Breast Cancer Symposium, Dec. 2006.

Hormone Therapy (HT): Current Guidelines

HT: current guidelines Indications by current labeling: n Treatment of moderate-severe vasomotor symptoms n Treatment of moderate-severe urogenital symptoms n Prevention of osteoporosis (not treatment) in women at significant risk

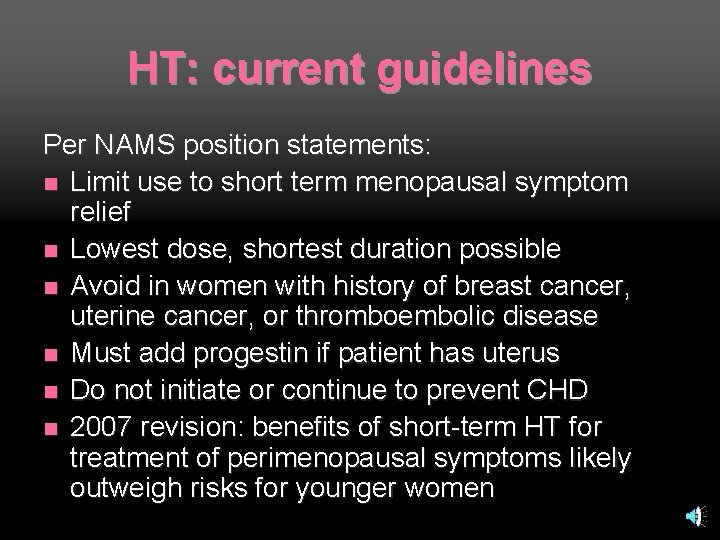
HT: current guidelines Per NAMS position statements: n Limit use to short term menopausal symptom relief n Lowest dose, shortest duration possible n Avoid in women with history of breast cancer, uterine cancer, or thromboembolic disease n Must add progestin if patient has uterus n Do not initiate or continue to prevent CHD n 2007 revision: benefits of short-term HT for treatment of perimenopausal symptoms likely outweigh risks for younger women

Outline n Hormones n Hot flashes n Highlights

Definition of natural menopause Retrospectively defined: no menstrual period for 12 months n Average age: 51 -52 years n

Perimenopause Around ages 42 -52 years n Irregular cycles: shorter or longer; heavier or lighter; missed completely n Vasomotor symptoms: hot flashes, night sweats n Urogenital symptoms: dryness, itching, dyspareunia, incontinence, increased bladder infections n FSH rises (often normal range during perimenopause) n

Perimenopause Many vasomotor symptoms will improve within several months n Most will resolve within 4 -5 years n Substantial minority (10 -15%) continue to have troublesome symptoms for years n

Treatment of vasomotor symptoms Data to guide therapy is lacking n Studies complicated by high rate of placebo effect (around 25%) n Trials have been small and brief, providing little information about long-term efficacy and risks n

Treatment of vasomotor symptoms: Estrogen

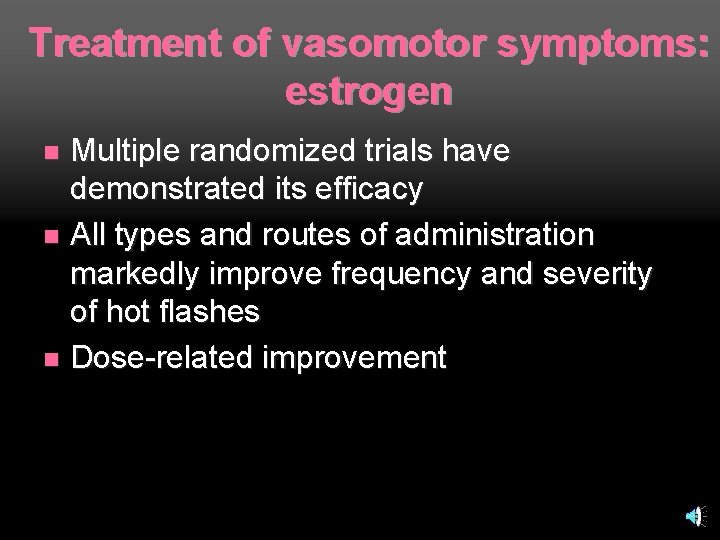
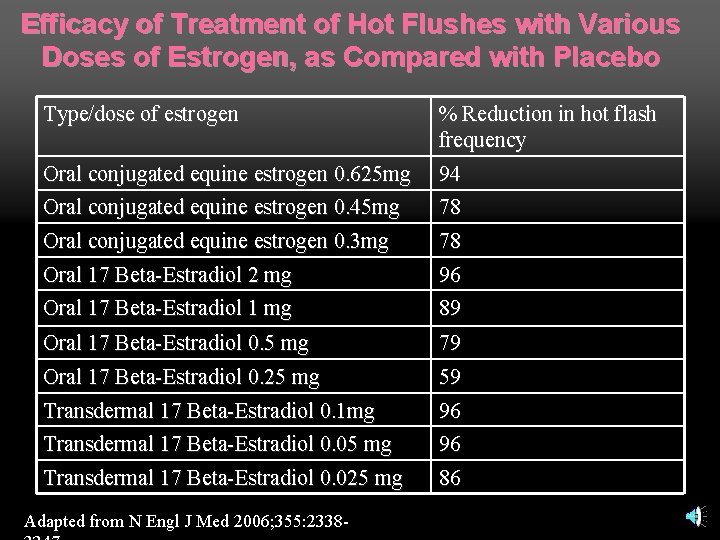
Treatment of vasomotor symptoms: estrogen Multiple randomized trials have demonstrated its efficacy n All types and routes of administration markedly improve frequency and severity of hot flashes n Dose-related improvement n

Efficacy of Treatment of Hot Flushes with Various Doses of Estrogen, as Compared with Placebo Type/dose of estrogen Oral conjugated equine estrogen 0. 625 mg Oral conjugated equine estrogen 0. 45 mg Oral conjugated equine estrogen 0. 3 mg Oral 17 Beta-Estradiol 2 mg % Reduction in hot flash frequency 94 78 78 Oral 17 Beta-Estradiol 1 mg 96 89 Oral 17 Beta-Estradiol 0. 5 mg Oral 17 Beta-Estradiol 0. 25 mg 79 59 Transdermal 17 Beta-Estradiol 0. 1 mg Transdermal 17 Beta-Estradiol 0. 05 mg 96 96 Transdermal 17 Beta-Estradiol 0. 025 mg 86 Adapted from N Engl J Med 2006; 355: 2338 -

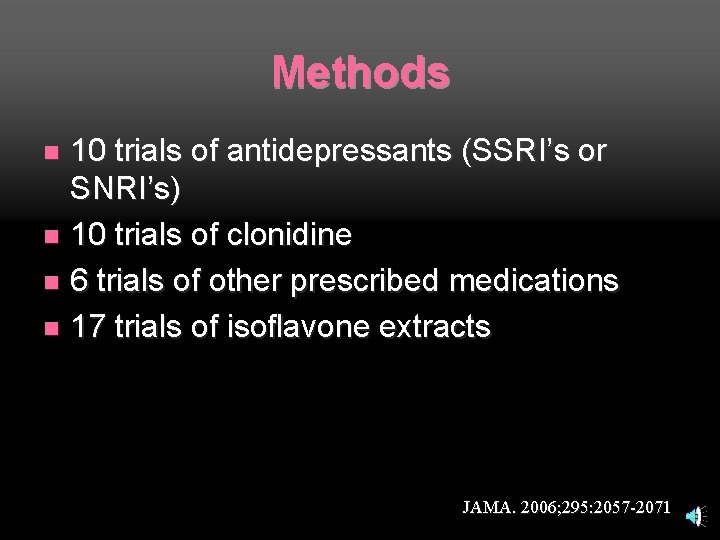
Nelson, HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: Systematic review and metaanalysis. JAMA. 2006; 295: 2057 -2071

Methods 10 trials of antidepressants (SSRI’s or SNRI’s) n 10 trials of clonidine n 6 trials of other prescribed medications n 17 trials of isoflavone extracts n JAMA. 2006; 295: 2057 -2071

Results and Conclusions Isoflavone extracts: no definite evidence of benefit n SSRI’s, SNRI’s, clonidine, gabapentin: some evidence of efficacy but less than estrogen n Few trials of non-hormonal therapies have been published n Most have methodological deficiencies n Generalizability limited JAMA. 2006; 295: 2057 -2071 n

Evidence of Efficacy of Non-hormonal Prescription Drugs for Treatment of Hot Flashes from Randomized, Controlled Clinical Trials Treatment MPA Megestrol Gabapentin Evidence of Benefit Yes Yes Clonidine Methyldopa Citalopram Fluoxetine Paroxetine Sertraline Mixed No No Mixed Yes No Venlafaxine Mixed Adapted from N Engl J Med 2006; 355: 2338 -2347

Nedrow A, Miller J, Walker M, Nygren P, Huffman LH, Nelson HD. Complementary and alternative therapies for the management of menopause-related symptoms: A systematic evidence review. Arch Intern Med. 2006; 166: 1453 -1465

Complementary and Alternative Therapies Systematic review included randomized controlled trials and meta-analyses n Phytoestrogens: mixed results n Black cohosh: mixed results n Mind-body, energy, manipulative, bodybased therapies and whole medical systems: little benefit n Data insufficient to support effectiveness of any therapy in this review n Arch Intern Med. 2006; 166: 1453 -1465

Treatment of vasomotor symptoms: black cohosh 2006: longest and largest placebocontrolled, double-blind trial to date of black cohosh n Showed no improvement in vasomotor symptoms n In combination with other studies provides strong evidence that black cohosh Ann Intern Med. 2006; 145: 869 -879. ineffective n

Treatment of vasomotor symptoms No convincing evidence of efficacy for treatment of vasomotor symptoms for: n n n n n Evening primrose oil Ginseng Wild yam cream Yoga Chinese herbs Dong quai Kava Red clover extract Vitamin E ANY complementary and alternative therapy

“Bioidentical” hormone replacement

“Bioidentical” hormone replacement n n n Term “bioidentical” not defined, has no scientific meaning These products made by compounding pharmacies Individualized dosage determined by salivary hormone levels Promoted by patient testimonials, often celebrities, as safer and more effective than conventional therapy Not produced according to federal Good Manufacturing Practice, not approved by FDA No evidence of effectiveness or safety

Practical/behavioral measures Paced respirations n Dress in layers n Lower ambient temperature n Avoid turtlenecks, down comforters, alcohol, spicy foods, bright lights n

Summary of Treatment Options for Vasomotor Symptoms n n Practical/behavioral measures—first line for mild symptoms HT for moderate to severe symptoms—most effective Topical treatment if urogenital symptoms only Non-hormonal drugs can be tried if want to avoid estrogens (off-label): n n n Antidepressants including venlafaxine, fluoxetine, paroxetine Gabapentin Clonidine

Treatment of vasomotor symptoms —if HT used: Follow NAMS guidelines (avoid use in patients with contraindications, use low doses/short durations) n Reasonable to try discontinuing every 6 -12 months since vasomotor symptoms are usually temporary n Possible that women will have recurrence of symptoms after stopping n If symptoms recur, try gradually tapering dose or number of days per week used (no guidelines) n

Outline n Hormones n Hot flashes n Highlights

Low-Fat Dietary Pattern and Risk of Colorectal Cancer Low-Fat Dietary Pattern and Risk of Invasive Breast Cancer The Women’s Health Initiative Randomized Controlled Dietary Modification Trial JAMA, February 8, 2006; 295

WHI Dietary Modification Trial Background: n Observational data suggests association of dietary fat intake with breast cancer, cardiovascular disease, and colorectal cancer n Purpose: to evaluate effectiveness of lowfat diet

WHI Dietary Modification Trial Methods: n Over 48, 000 women, aged 50 -79 n Subjects randomized to low-fat/high fruit, vegetable, grain diet vs. usual eating pattern n Followed for mean of 8. 1 years

WHI Dietary Modification Trial Results--no significant reduced risk of any outcome: n Breast cancer n Colorectal cancer n Stroke n CHD

WHI Dietary Modification Trial Limitations: n Did not specify types fats--potential for benefit of diet lower in saturated and trans fat n Few met target of 20% calories from fat--may have been underpowered to detect difference n Positive trend toward decreased coronary heart disease, breast cancer, and colon polyps as trial progressed—did not reach statistical significance n Health implications of diet may take years to be fully realized--benefits may show up in future follow-up

Women’s Intervention Nutrition Study (WINS) n n n Randomized, prospective, multicenter clinical trial Tested dietary intervention for reducing recurrence of breast cancer Intervention: decrease fat intake to 15% of calories (30% at baseline) Over 2000 women with resected, early-stage breast cancer receiving conventional cancer management Analysis performed after median follow-up of 60 months J Natl Cancer Inst. 2006 Dec 20; 98(24): 1767 -76

Women’s Intervention Nutrition Study (WINS) Results: n Intervention group achieved fat intake of 20% of calories (29% in control group) n Intervention group weighed 6 pounds less than control group (same at baseline) n 24% risk reduction for relapse in intervention group n Number needed to treat: 38 n Subgroup analysis suggested most benefit in hormone receptor negative cancers (not J Natl Cancer Inst. 2006 Dec 20; 98(24): 1767 -76 statistically significant)

Women’s Intervention Nutrition Study (WINS) Limitations: n Effect could have been from weight loss rather than the dietary fat on its own n Generalizability of benefit of low fat diet is limited: only studied recurrence of breast cancer in women with previous breast cancer history J Natl Cancer Inst. 2006 Dec 20; 98(24): 1767 -76

Interpretation of these trials: Should women bother eating a low-fat diet? n n n Studies need to be interpreted with caution because conducting clinical trials of lifestyle changes is difficult WINS study suggests that reduced dietary fat intake with influence on weight may benefit breast cancer patients WHI Dietary Modification trial did not show benefit, but experts are not recommending change in current recommendations Longer follow-up of these trials will answer more questions about its benefit Low-fat diet still recommended for overall health

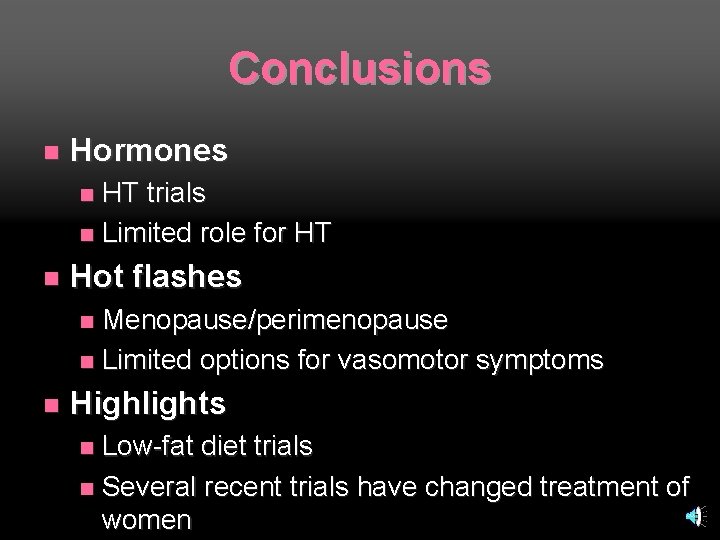
Conclusions n Hormones HT trials n Limited role for HT n n Hot flashes Menopause/perimenopause n Limited options for vasomotor symptoms n n Highlights Low-fat diet trials n Several recent trials have changed treatment of women n
 Laura conocía bien a elián. elián conocía bien a laura.
Laura conocía bien a elián. elián conocía bien a laura. Laura lilly hot
Laura lilly hot Needs of adolescence
Needs of adolescence Womens rights
Womens rights Late night womens hour
Late night womens hour Aylesbury womens aid
Aylesbury womens aid Womens right
Womens right Difference between mens and womens soccer
Difference between mens and womens soccer Womens college kumbakonam
Womens college kumbakonam Womens college kumbakonam
Womens college kumbakonam Womens right
Womens right Womens shelter edmonton
Womens shelter edmonton Womens rights
Womens rights Womans anatomy
Womans anatomy Womens history month door
Womens history month door Womens community shelters
Womens community shelters Ballybeen womens centre
Ballybeen womens centre Scarborough womens centre
Scarborough womens centre Mens lacrosse helmet
Mens lacrosse helmet Bristol womens voice
Bristol womens voice Key highlights icons
Key highlights icons Proposal highlights
Proposal highlights Highlights memorandum
Highlights memorandum Investment highlights
Investment highlights Highlights from the book of isaiah
Highlights from the book of isaiah Highlights from the book of isaiah
Highlights from the book of isaiah Work immersion expected behavior
Work immersion expected behavior The passage highlights……
The passage highlights…… Principles of art
Principles of art White hot vs red hot temperature
White hot vs red hot temperature Advantage of hot working process
Advantage of hot working process Perbedaan hot lava dan hot lava volcano
Perbedaan hot lava dan hot lava volcano Hot nor hot
Hot nor hot Role of steroid hormone
Role of steroid hormone Hypotalanus
Hypotalanus Function of steroids
Function of steroids Functions of ethylene
Functions of ethylene Hormones secreted by adenohypophysis
Hormones secreted by adenohypophysis Hipofisis gland
Hipofisis gland Amine hormone
Amine hormone Lipid soluble hormones examples
Lipid soluble hormones examples Releasing inhibiting hormones
Releasing inhibiting hormones Lipid soluble hormones examples
Lipid soluble hormones examples Lipid soluble hormones examples
Lipid soluble hormones examples Gonadal hormones
Gonadal hormones Vasopresine
Vasopresine Bioflix activity homeostasis hormones and homeostasis
Bioflix activity homeostasis hormones and homeostasis What is a tropic hormone
What is a tropic hormone Amino acid-based hormones
Amino acid-based hormones Examples of amine hormones
Examples of amine hormones Amino acid-based hormones
Amino acid-based hormones What is a tropic hormone
What is a tropic hormone Nontropic hormones
Nontropic hormones Four classes of hormones
Four classes of hormones Tropic hormones hypothalamus
Tropic hormones hypothalamus Hormones
Hormones Tropic casade
Tropic casade Hormones during pregnancy
Hormones during pregnancy What are the lipid soluble hormones
What are the lipid soluble hormones Autocrine hormones
Autocrine hormones Hormones after sex
Hormones after sex Hypophysis
Hypophysis Hypothalamic hormones
Hypothalamic hormones Incentive theory ap psychology
Incentive theory ap psychology Male and female hormones
Male and female hormones Hypothalamus and pituitary gland connection
Hypothalamus and pituitary gland connection Biochemistry of hormones
Biochemistry of hormones Qsxx
Qsxx Gastrointestinal hormones
Gastrointestinal hormones What is thyrodism
What is thyrodism Hypothalamus hormones
Hypothalamus hormones Vasocongestion definition
Vasocongestion definition Example of hormonal stimulus
Example of hormonal stimulus Male and female hormones
Male and female hormones Posterior pituitary hormones
Posterior pituitary hormones Hormones
Hormones Galactokinetic hormone
Galactokinetic hormone Bioflix activity homeostasis hormones and homeostasis
Bioflix activity homeostasis hormones and homeostasis Hormones released by
Hormones released by Respond to
Respond to Plant hormones and responses
Plant hormones and responses Hypothyroidism dwarfism
Hypothyroidism dwarfism