Hematologic Disease in Pregnancy Raymond Powrie MD FRCPC















- Slides: 45

Hematologic Disease in Pregnancy Raymond Powrie, MD, FRCP(C) FACP Professor of Medicine and Obstetrics and Gynecology Alpert Medical School at Brown University Interim Chief of Medicine Women and Infants Hospital of Rhode Island Chief Medical Quality Officer Care New England Healthcare System

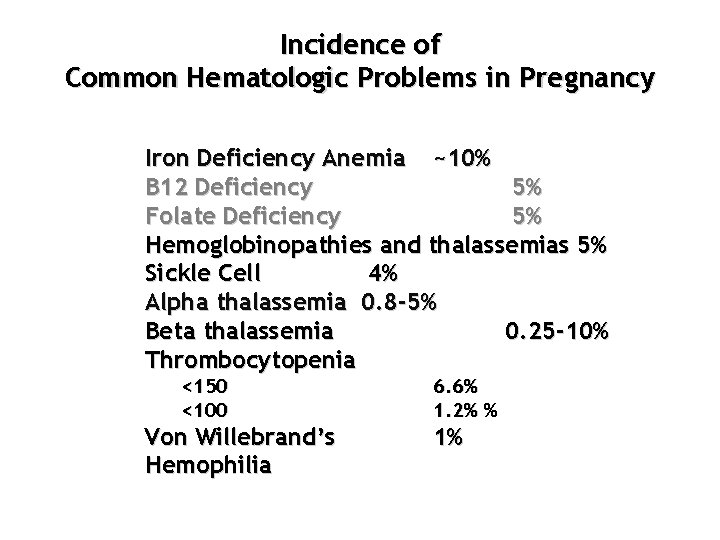
Incidence of Common Hematologic Problems in Pregnancy Iron Deficiency Anemia ~10% B 12 Deficiency 5% Folate Deficiency 5% Hemoglobinopathies and thalassemias 5% Sickle Cell 4% Alpha thalassemia 0. 8 -5% Beta thalassemia 0. 25 -10% Thrombocytopenia <150 <100 Von Willebrand’s Hemophilia 6. 6% 1. 2% % 1%

Anemia

Anemia • Defined <10. 5 g/d. L • Hgb < 6 g/d. L has clear fetal consequences

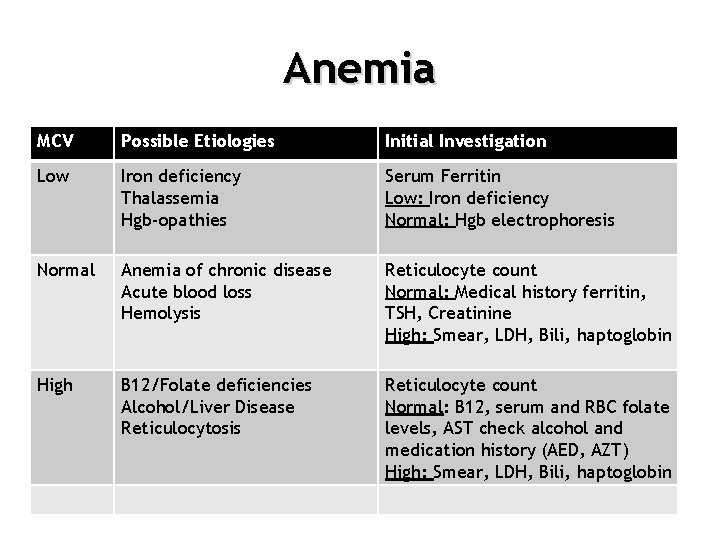
Anemia MCV Possible Etiologies Initial Investigation Low Iron deficiency Thalassemia Hgb-opathies Serum Ferritin Low: Iron deficiency Normal: Hgb electrophoresis Normal Anemia of chronic disease Acute blood loss Hemolysis Reticulocyte count Normal: Medical history ferritin, TSH, Creatinine High: Smear, LDH, Bili, haptoglobin High B 12/Folate deficiencies Alcohol/Liver Disease Reticulocytosis Reticulocyte count Normal: B 12, serum and RBC folate levels, AST check alcohol and medication history (AED, AZT) High: Smear, LDH, Bili, haptoglobin

Iron Deficiency Anemia • Evaluate for GI blood loss • Ferrous sulfate 325 mg two to three times a day – Supplement with vitamin C or red meat to increase absorption – Do not take with calcium, tea, coffee or wine • Expect a 0. 8 g/d. L/week with iron supplementation after a few weeks • Rarely a need for parenteral iron

Thalassemia Hemoglobinopathies • 5% of the world’s population • Mediterranean, Africa, Indian Subcontinent, SE Asia, the Pacific


• Thalassemias • Hemoglobinapathies • Hemoglobin chains NOT made so there is anemia • Hemoglobin chains made with flaws so there is anemia

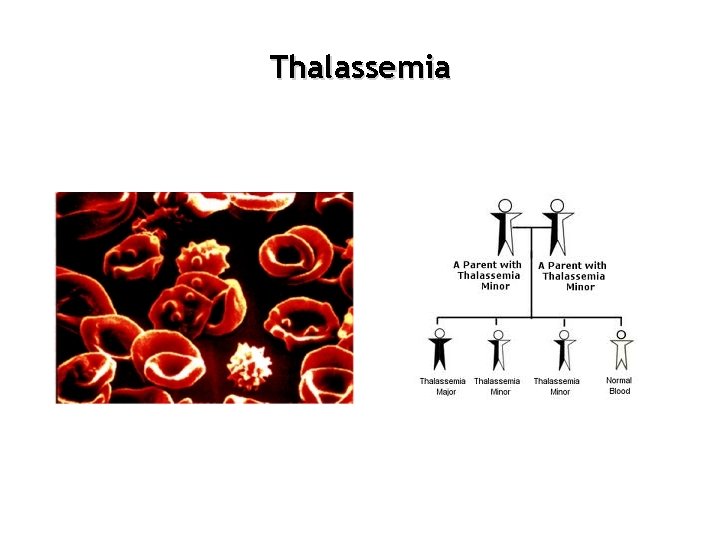
Thalassemia

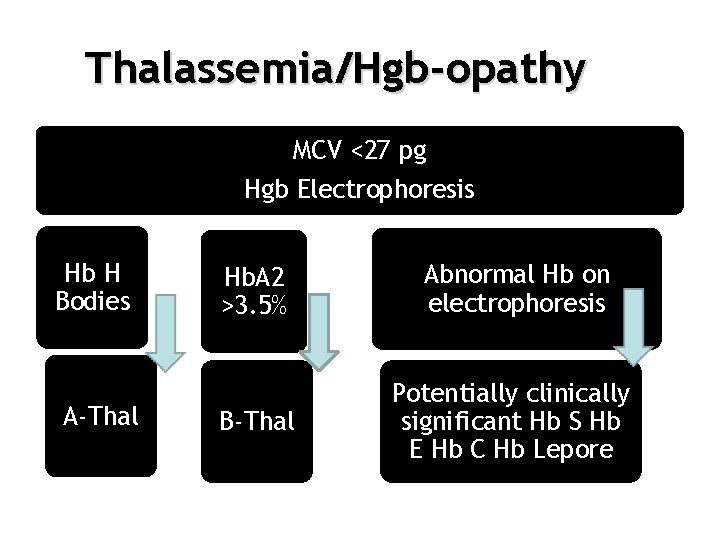
Thalassemia/Hgb-opathy MCV <27 pg Hgb Electrophoresis Hb H Bodies A-Thal Hb. A 2 >3. 5% Abnormal Hb on electrophoresis B-Thal Potentially clinically significant Hb S Hb E Hb C Hb Lepore

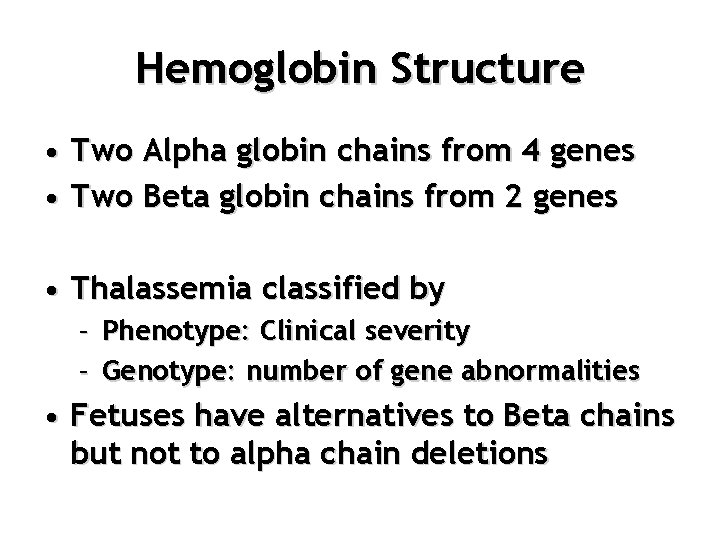
Hemoglobin Structure • Two Alpha globin chains from 4 genes • Two Beta globin chains from 2 genes • Thalassemia classified by – Phenotype: Clinical severity – Genotype: number of gene abnormalities • Fetuses have alternatives to Beta chains but not to alpha chain deletions

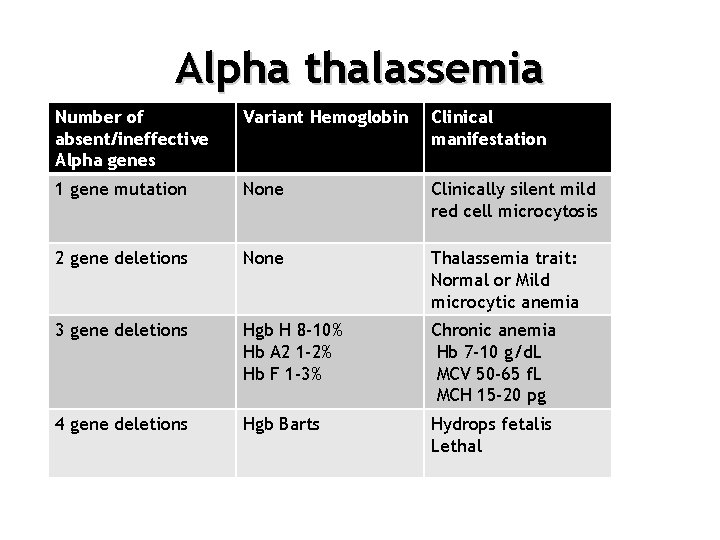
Alpha thalassemia Number of absent/ineffective Alpha genes Variant Hemoglobin Clinical manifestation 1 gene mutation None Clinically silent mild red cell microcytosis 2 gene deletions None Thalassemia trait: Normal or Mild microcytic anemia 3 gene deletions Hgb H 8 -10% Hb A 2 1 -2% Hb F 1 -3% Chronic anemia Hb 7 -10 g/d. L MCV 50 -65 f. L MCH 15 -20 pg 4 gene deletions Hgb Barts Hydrops fetalis Lethal

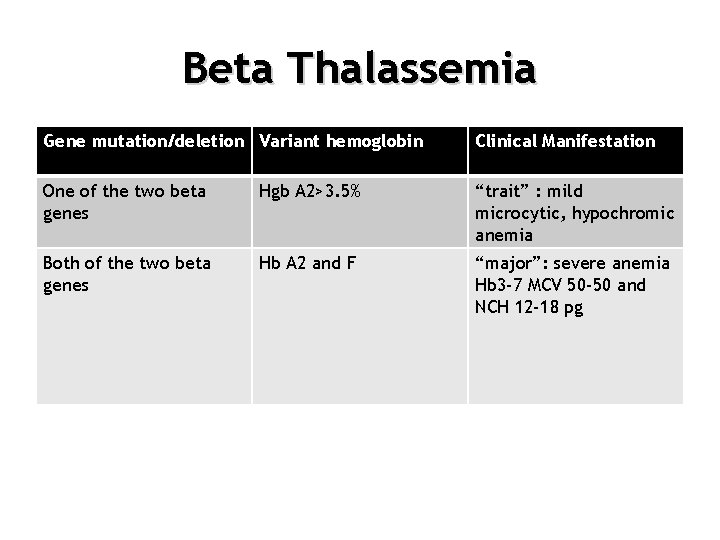
Beta Thalassemia Gene mutation/deletion Variant hemoglobin Clinical Manifestation One of the two beta genes Hgb A 2>3. 5% “trait” : mild microcytic, hypochromic anemia Both of the two beta genes Hb A 2 and F “major”: severe anemia Hb 3 -7 MCV 50 -50 and NCH 12 -18 pg

Sickle Cell Disease

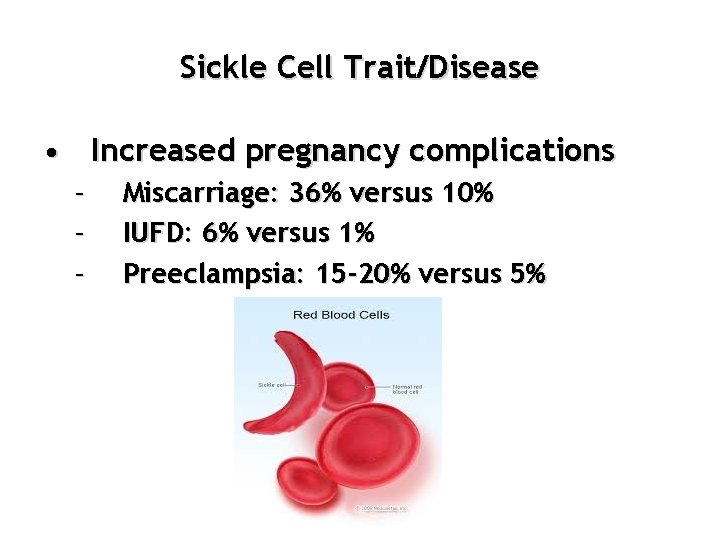
Sickle Cell Trait/Disease • Increased pregnancy complications – – – Miscarriage: 36% versus 10% IUFD: 6% versus 1% Preeclampsia: 15 -20% versus 5%

Sickle Cell Disease • Prevention of Crises – Continuous good hydration – Folate 1 mg daily – No role for prophylactic blood transfusions

Sickle Disease • Treatment of Crises – Aggressive pain control with narcotics – Hydration – Oxygen – Continued folate – Careful search for and treatment of any infection – Transfusion to achieve at least 50% normal hemoglobin if severe Chest crises have a significant mortality associated with them and should be taken very seriously

Combinations • Hb E 20 -60% of Southeast Asians carry this gene • Hb C 3% of Africans • Hb S 12% of Africans • Hb Lepore • Significant when combined with thalassemia traits or found in homozygous states

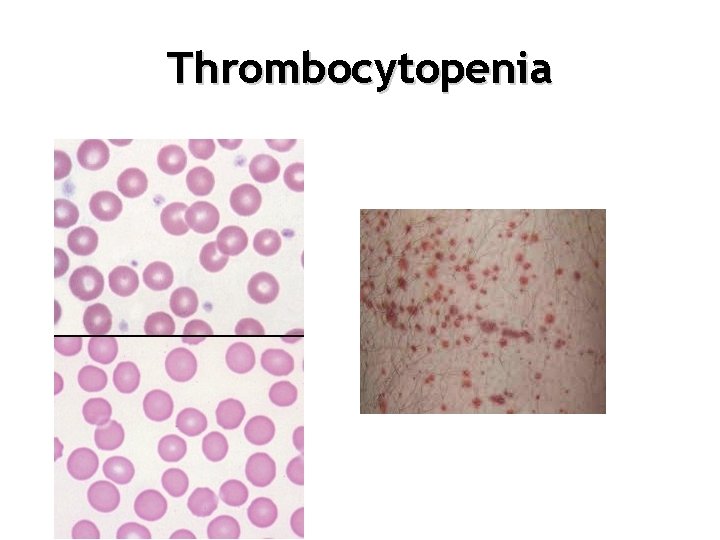
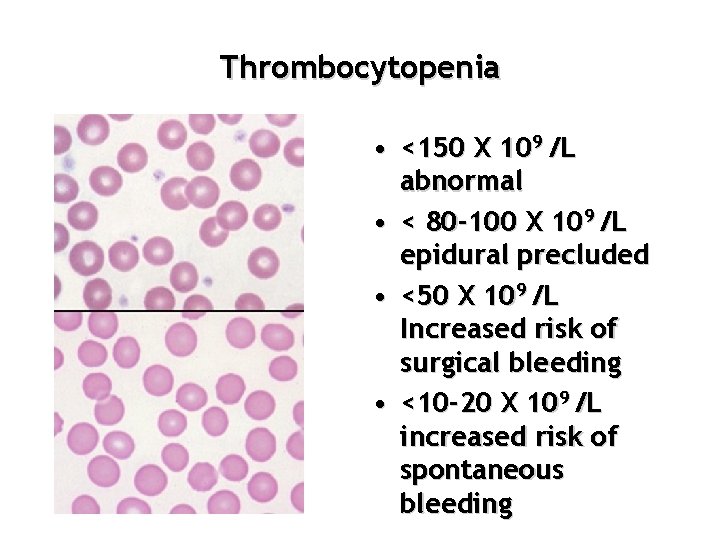
Thrombocytopenia

Thrombocytopenia • <150 X 109 /L abnormal • < 80 -100 X 109 /L epidural precluded • <50 X 109 /L Increased risk of surgical bleeding • <10 -20 X 109 /L increased risk of spontaneous bleeding

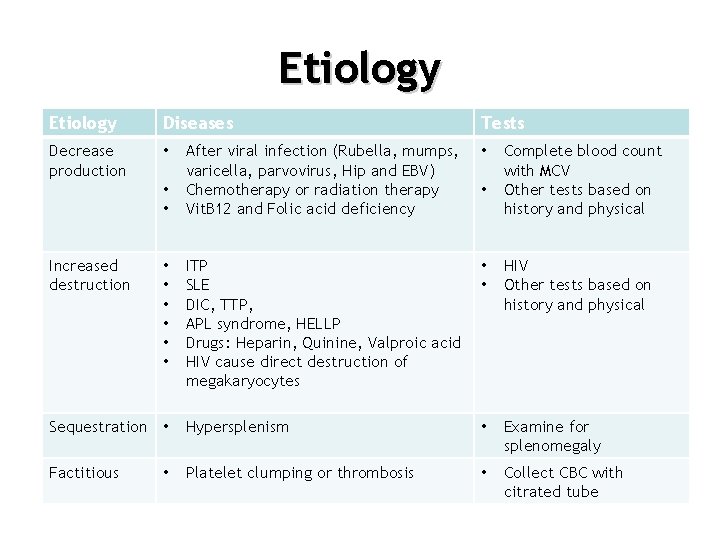
Etiology Diseases Decrease production • Tests After viral infection (Rubella, mumps, varicella, parvovirus, Hip and EBV) Chemotherapy or radiation therapy Vit. B 12 and Folic acid deficiency • ITP SLE DIC, TTP, APL syndrome, HELLP Drugs: Heparin, Quinine, Valproic acid HIV cause direct destruction of megakaryocytes • • HIV Other tests based on history and physical Sequestration • Hypersplenism • Examine for splenomegaly Factitious Platelet clumping or thrombosis • Collect CBC with citrated tube • • Increased destruction • • Complete blood count with MCV Other tests based on history and physical

Gestational Thrombocytopenia • The frequency in the largest series of consecutive women admitted for delivery is 5% (Burrows RF, Kelton JG, N Engl J med 1993; 329: 1263) • Defined by the following five criteria: – Mild and asymptomatic thrombocytopenia, More than 70. 000 – No past history of thrombocytopenia (except previous pregnancy) – Occurrence during late gestation – No association with fetal thrombocytopenia – Spontaneous resolution after delivery

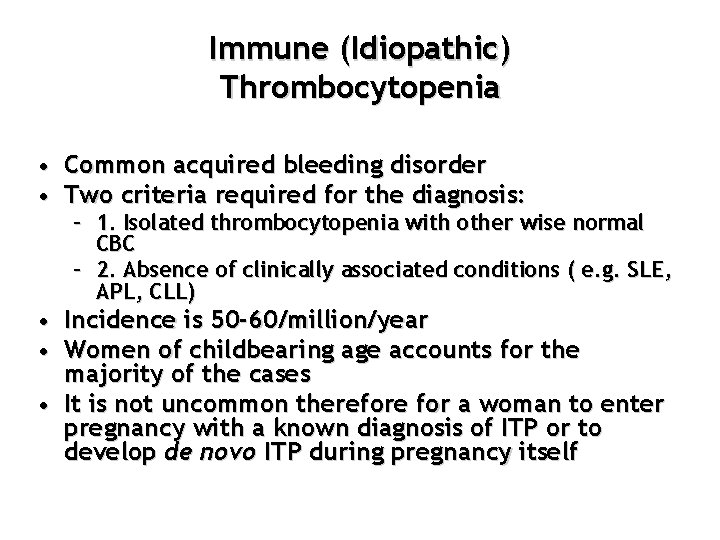
Immune (Idiopathic) Thrombocytopenia • Common acquired bleeding disorder • Two criteria required for the diagnosis: – 1. Isolated thrombocytopenia with other wise normal CBC – 2. Absence of clinically associated conditions ( e. g. SLE, APL, CLL) • Incidence is 50 -60/million/year • Women of childbearing age accounts for the majority of the cases • It is not uncommon therefore for a woman to enter pregnancy with a known diagnosis of ITP or to develop de novo ITP during pregnancy itself

Clinical Manifestations • Marked variability in the clinical presentation • Onset usually insidious but can be acute and abrupt • Usually asymptomatic • Bleeding usually muco-cutaneous – – Petichiae, purpura, and easy bruising (Expected)) Epistaxis, gingival bleeding , and menorrhagia Common) GI bleeding and gross hematuria( Rare) Intracranial hemorrhage ( Fatal, Very rare)

Diagnosis • Diagnostic approach to ITP in a is the same as in nonpregnant patients. – No “gold standard” test to diagnose ITP. – Diagnosis is reached after exclusion of other causes using clinical history and exam, CBC and peripheral blood smear. – Additional lab tests to consider include testing for autoimmune disorders, and exclusion of HIV Antiplatelet – Antibody testing is not recommended by American Society of Hematology

Effect Of Pregnancy On ITP • ITP accounts for 3 -4% of the cases of thrombocytopenia detected in pregnancy • Pregnancy does not per se alter the natural course of ITP or increase the risk of relapse in women with previously diagnosed ITP

Effect Of ITP On Pregnancy • Thrombocytopenia in the infant can occur due to passive diffusion of autoimmune antibodies across the placenta • In one review of 10 studies of 600 pregnancies in 469 women with known ITP reported neonatal thrombocytopenia: – < 150. 000 in 28% – < 50. 000 in 11% – ICH in 1. 2% • Maternal platelet count at delivery is not predictive of neonatal platelet • The risk of spontaneous bleeding in pregnant women is low

Treatment of ITP • The goal for treatment of ITP is to provide a safe platelet count to prevent major bleeding, rather than returning the platelet count to normal • Treatment is considered if the platelet count is <30 -50, 000. • Some will treat for platelet counts <80, 000 to allow regional anesthesia

Treatment in pregnancy Prednisone is First line • 1 mg/kg/day for 7 -10 days and then gradually taper to keep platelet above the desired threshold • The lowest possible dose should be aimed for < 10 mg daily, most likely throughout the pregnancy • Can increase the risk of GDM, HTN, maternal infection and preterm delivery but no fetal effect

Treatment (Continued) IVIG (gamma globulin) • May be considered first line in third trimester – 1 g/kg ( pregnancy wt) daily for 2 days • • Provides effective ( 80% )but temporary improvement in platelet count (usually last for 2 -4 weeks) Indicated in women with moderate or severe bleeding symptoms or whose platelet < 10, 000 Can be repeated but expensive and time consuming to administer No effect on fetal platelet count

Treatment (Continued) • Azathioprine • Is a steroid-sparing agent • used in pregnancy in conjunction with corticosteroids in women with refractory ITP Splenectomy • Laparoscopic splenectomy has been safely carried out mainly in second trimester

Treatment (Continued) • High doses Methylpredisone 1 gm IV • Dexamethasone 40 g daily for 4 days – can cross the placenta with fetal effect • Rituximab Monoclonal antibody therapy – used with caution in pregnancy as it crosses the placenta and can cause temporary suppression of fetal B lymphocytes and unclear long term effect on development of fetal immune system • Platelet transfusion – for severe, symptomatic thrombocytopenia

Delivery • Vaginal delivery preferable but mode of delivery should be determined by obstetric indications • Neuroaxial anesthesia: – Although the precise platelet count needed to safely perform neuroaxial analgesia is unknown, a platelet count variously given as >50. 000 or > 80. 000 is considered safe for epidural/spinal anesthesia/analgesia if coagulation is otherwise normal.

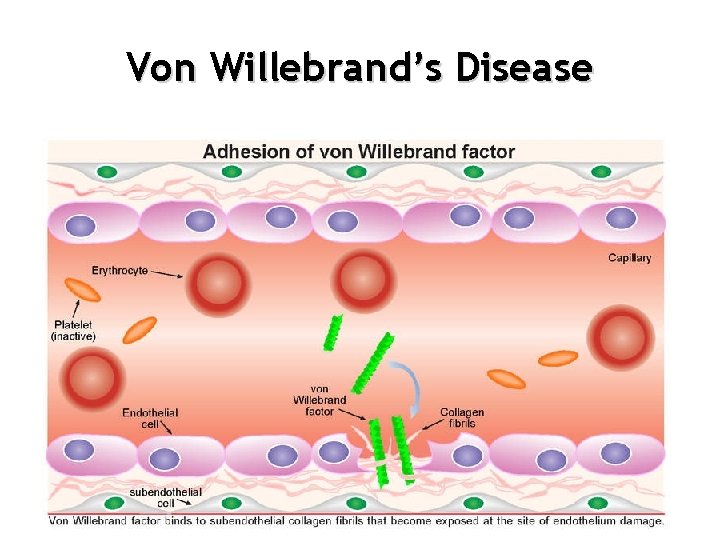
von Willebrand’s Disease VWD • Most common inherited bleeding disorder: 1% of population • Quantitative or qualitative deficiency in von Willebrand factor (VWF) • VWF has two roles – Helps platelets stick to each other, to damaged tissue and to fibrinogen – Carrier protein for FACTOR VIII that keeps FACTOR VIII from being broken down prematurely

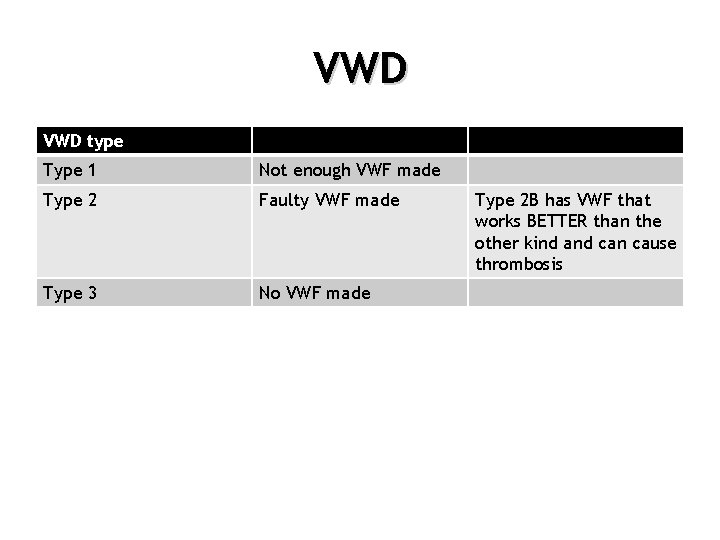
VWD type Type 1 Not enough VWF made Type 2 Faulty VWF made Type 3 No VWF made Type 2 B has VWF that works BETTER than the other kind and can cause thrombosis

Bleeding Disorders Lab • • History Easy bruising especially without injury Bleeding following dental work or surgery Gingival bleeding Menorrhagia • • CBC a. PTT, INR PFA-100 Bleeding time

Von Willebrand’s Disease

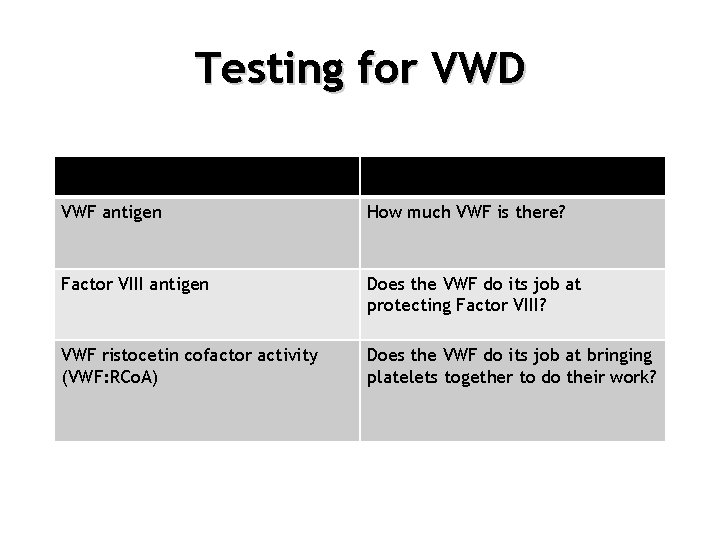
Testing for VWD VWF antigen How much VWF is there? Factor VIII antigen Does the VWF do its job at protecting Factor VIII? VWF ristocetin cofactor activity (VWF: RCo. A) Does the VWF do its job at bringing platelets together to do their work?

Pregnancy • VWF and factor VIII levels go up in pregnancy • Patients generally fine if Ristocetin cofactor activity levels >50 IU/d. L • Patients with type 2 VWD may have worsened thrombocytopenia in pregnancy

Treatment in Pregnancy Review prior tests and confirm diagnosis VWD testing at booking and at 34 weeks • If >50 IU/L: – no treatment • If <50 IU/L – If Type 1 or 2 A • PRN DDAVP 0. 3 mcg/kg IV/SQ postpartum for vaginal delivery • Preventive DDAVP 0. 3 mcg/kg IV/SQ prior to CS – If Type 2 B 2 N 2 M or type 3… give intermediate purity FVIII concentrate 20 -40 IU/kg

Treatment in Pregnancy • Inherited illness but generally high levels of FVIII and VWF in newborn

Hemophilia

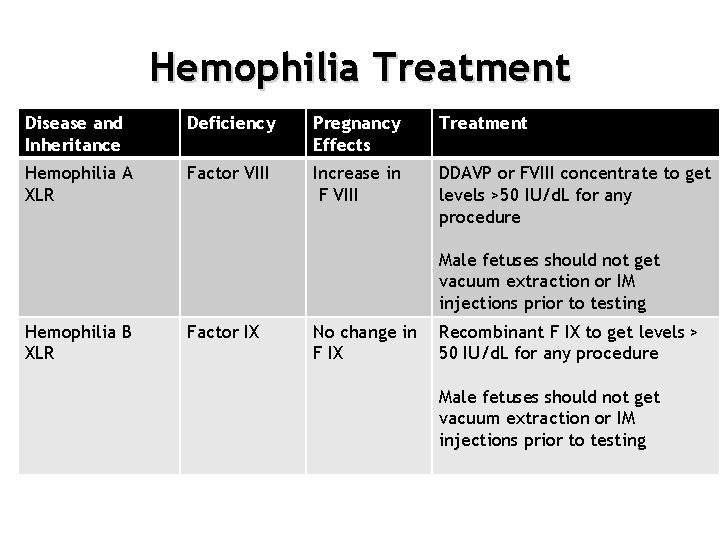
Hemophilia Treatment Disease and Inheritance Deficiency Pregnancy Effects Treatment Hemophilia A XLR Factor VIII Increase in F VIII DDAVP or FVIII concentrate to get levels >50 IU/d. L for any procedure • Hemophilia A Male fetuses should not get vacuum extraction or IM injections prior to testing Hemophilia B XLR Factor IX No change in F IX Recombinant F IX to get levels > 50 IU/d. L for any procedure Male fetuses should not get vacuum extraction or IM injections prior to testing

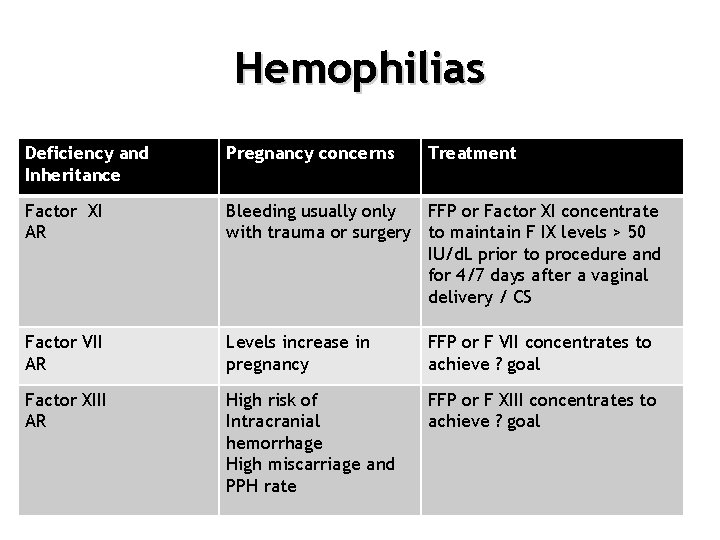
Hemophilias Deficiency and Inheritance Pregnancy concerns Treatment Factor XI AR Bleeding usually only FFP or Factor XI concentrate with trauma or surgery to maintain F IX levels > 50 IU/d. L prior to procedure and for 4/7 days after a vaginal delivery / CS Factor VII AR Levels increase in pregnancy FFP or F VII concentrates to achieve ? goal Factor XIII AR High risk of Intracranial hemorrhage High miscarriage and PPH rate FFP or F XIII concentrates to achieve ? goal
 Raymond powrie
Raymond powrie Neoplasia
Neoplasia Md frcpc definition
Md frcpc definition Molar pregnancy
Molar pregnancy Hematologic system assessment
Hematologic system assessment Endocrine and hematologic emergencies
Endocrine and hematologic emergencies Assessment of hematologic system
Assessment of hematologic system Hypochromic red cells
Hypochromic red cells Bharathi viswanathan
Bharathi viswanathan Raymond moffatt
Raymond moffatt Raymond's run summary
Raymond's run summary Singhals heuristic algorithm
Singhals heuristic algorithm Freudian slip meaning
Freudian slip meaning Raymond williams analysis of culture
Raymond williams analysis of culture Raymond willians
Raymond willians How does scout react to francis taunts
How does scout react to francis taunts Civics 360
Civics 360 Raymond shen
Raymond shen Dato raymond liew
Dato raymond liew Three levels of management
Three levels of management Joseph pasteur
Joseph pasteur Raymond chandler cathedral
Raymond chandler cathedral Raymond najjar
Raymond najjar Raymond flood
Raymond flood Dolphus raymond quotes
Dolphus raymond quotes Ray kroc
Ray kroc Cattell intelligence
Cattell intelligence Raymond jiang
Raymond jiang Teleast internet prices
Teleast internet prices Frank raymond leavis
Frank raymond leavis Yann mikaeloff
Yann mikaeloff St raymond elementary school uniform
St raymond elementary school uniform Raymond inmon wikipedia
Raymond inmon wikipedia Raymond carlsen
Raymond carlsen Raymond james stuart
Raymond james stuart Tragedy and tradition by raymond williams
Tragedy and tradition by raymond williams Raymond ackerman academy
Raymond ackerman academy Raymond loevy
Raymond loevy Raymond chandler 1939
Raymond chandler 1939 Raymond cox qc
Raymond cox qc Chad vasc score
Chad vasc score Raymond hettinger wikipedia
Raymond hettinger wikipedia Cs391l
Cs391l Definition of ethics
Definition of ethics Deconstructing the popular
Deconstructing the popular Donald d. chamberlin and raymond f. boyce
Donald d. chamberlin and raymond f. boyce