Fractional Distillation of Crude Oil Do Now Write






- Slides: 14

Fractional Distillation of Crude Oil Do Now: Write down 5 things that you know about fractional distillation from GCSE.



What do we already know about crude oil? How can crude oil be made useful?

IDEAS

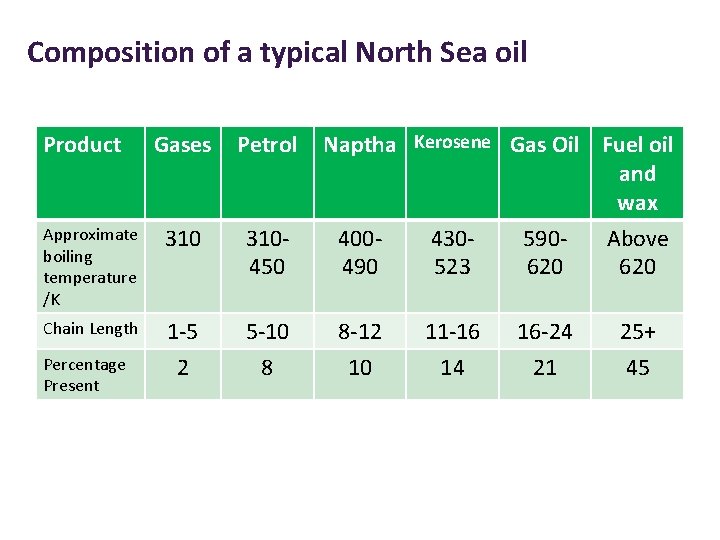
Composition of a typical North Sea oil Product Gases Petrol Approximate boiling temperature /K 310450 Chain Length 1 -5 2 5 -10 8 Percentage Present Naptha Kerosene Gas Oil Fuel oil and wax 400430590 Above 490 523 620 8 -12 10 11 -16 14 16 -24 21 25+ 45

Fractional Distillation • To convert crude oil into useful products we have to separate the mixture. • We do this by heating it and collecting the fractions that boil over different ranges of temperatures. • Each fraction is a mixture of hydrocarbons of similar chain length and therefore similar properties. • The process is called fractional distillation and is done in a fractionating tower. Can you list the STEPS?

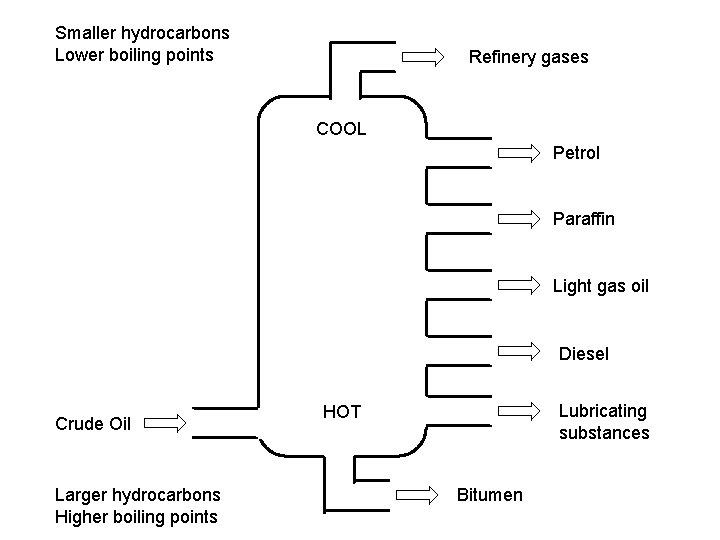
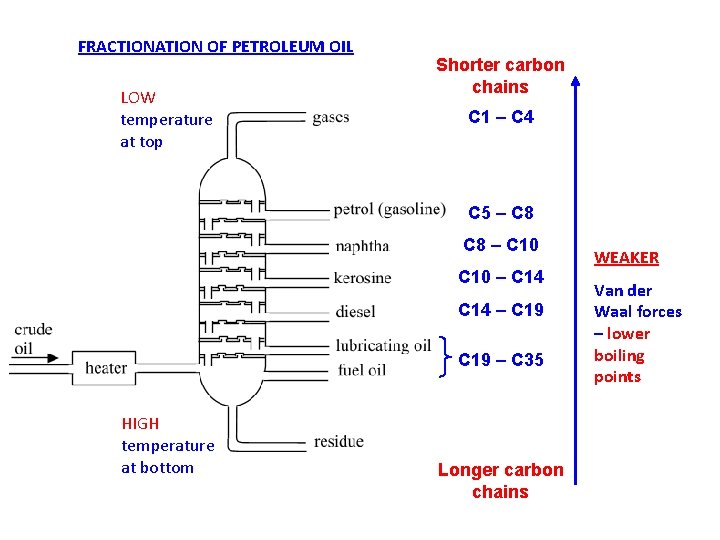
Steps 1. 2. 3. 4. 5. 6. The crude oil is first heated in a furnace A mixture of liquid and vapour passes into a tower that is cooler at the top than at the bottom The vapours pass up the tower via a series of trays containing bubble caps until they arrive at a tray that is sufficiently cool (at a lower temperature than their boiling point). Then they condense to liquid. The mixture of liquids that condenses on each tray is piped off. The shorter chain hydrocarbons condense in the trays nearer to the top of the tower, where it is cooler, because they have lower boiling points. The thick residue that collects at the base of the tower is called tar or bitumen. It can be used for road surfacing but, as supply often exceeds demand, this fraction is often further processed to give more valuable products.


Smaller hydrocarbons Lower boiling points Refinery gases COOL Petrol Paraffin Light gas oil Diesel Crude Oil Larger hydrocarbons Higher boiling points Lubricating substances HOT Bitumen

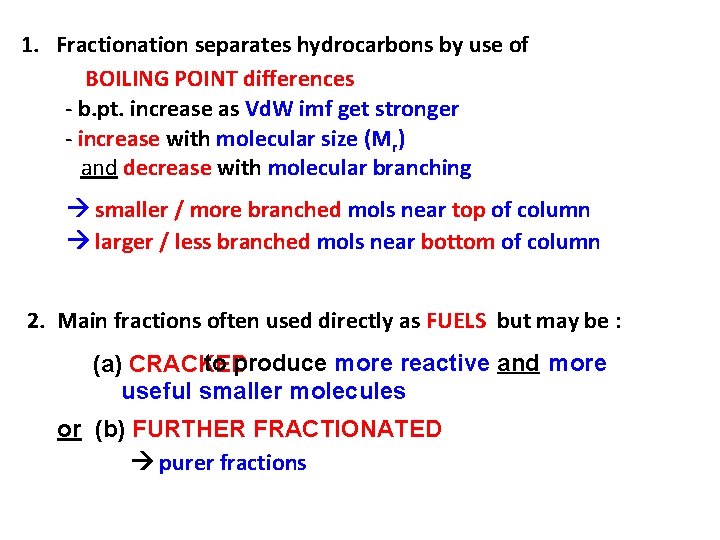
1. Fractionation separates hydrocarbons by use of BOILING POINT differences - b. pt. increase as Vd. W imf get stronger - increase with molecular size (Mr) and decrease with molecular branching smaller / more branched mols near top of column larger / less branched mols near bottom of column 2. Main fractions often used directly as FUELS but may be : to produce more reactive and more (a) CRACKED useful smaller molecules or (b) FURTHER FRACTIONATED purer fractions

FRACTIONATION OF PETROLEUM OIL LOW temperature at top Shorter carbon chains C 1 – C 4 C 5 – C 8 – C 10 – C 14 – C 19 – C 35 HIGH temperature at bottom Longer carbon chains WEAKER Van der Waal forces – lower boiling points

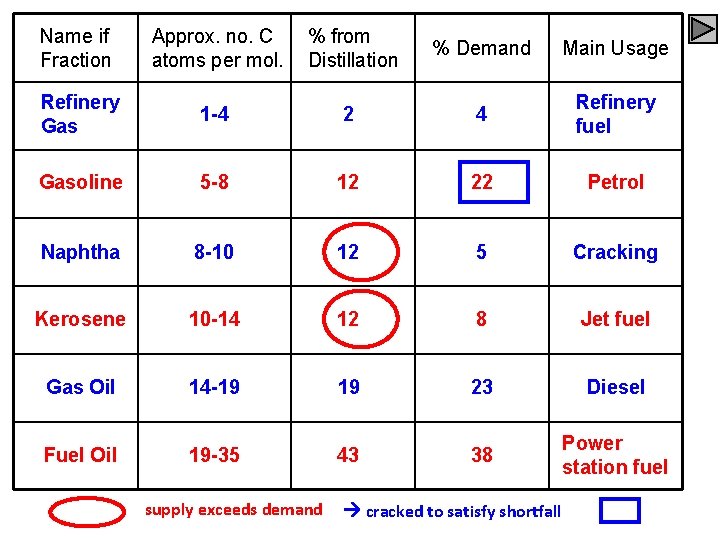
Name if Fraction Approx. no. C atoms per mol. % from Distillation % Demand Main Usage Refinery Gas 1 -4 2 4 Refinery fuel Gasoline 5 -8 12 22 Petrol Naphtha 8 -10 12 5 Cracking Kerosene 10 -14 12 8 Jet fuel Gas Oil 14 -19 19 23 Diesel Fuel Oil 19 -35 43 38 Power station fuel supply exceeds demand cracked to satisfy shortfall

Breaking Bonds • What type of bonds are broken during fractional distillation? • Fractional distillation is a physical process so no covalent bonds within the molecules are broken. • It is the van der Waals forces between the molecules that are broken during vaporisation and reform on condensing.
 Refining of petroleum
Refining of petroleum Soft
Soft Distillation introduction
Distillation introduction Fractional distillation vs destructive distillation
Fractional distillation vs destructive distillation Discussion discussion
Discussion discussion Intensive properties examples
Intensive properties examples Cracking organic chemistry
Cracking organic chemistry Images of fractional distillation
Images of fractional distillation Knockhardy gcse
Knockhardy gcse Fractional distillation
Fractional distillation Industrial application of distillation
Industrial application of distillation How is crude oil formed
How is crude oil formed Canadian oil and gas trusts
Canadian oil and gas trusts Crude oil contains
Crude oil contains Crude oil
Crude oil