Odd one out Look at the following images














- Slides: 21

Odd one out – Look at the following images. Take a line – which is the odd one out and why?

Lesson 2 – Crude Oil and Distillation

Fractional Distillation (C 1. 4) Objectives To state what crude oil is (E) To describe why crude oil must be refined (C) To describe the process of Fractional Distillation (C)

Connect Crude Oil is a mixture of hydrocarbon compounds. THINK-PAIR-SHARE How might we separate this mixture?

Video

Connect the Learning Solve the Jigsaw Stick to A 3 Sheet Peer Assess. You don’t have the same jigsaw as your partner. Draw as many facts as possible and write around. Draw up as many ideas as possible.

Now Listen Just and Listen make notes What’s the topic?

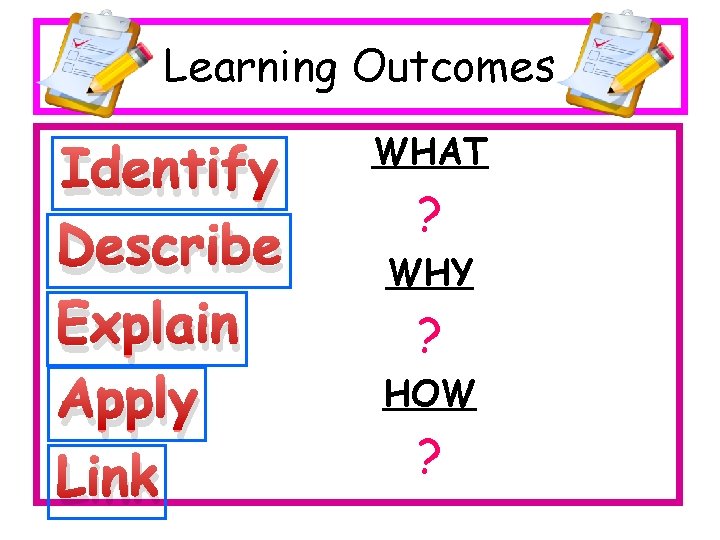
Learning Outcomes Identify Describe Explain Apply Link WHAT ? WHY ? HOW ?

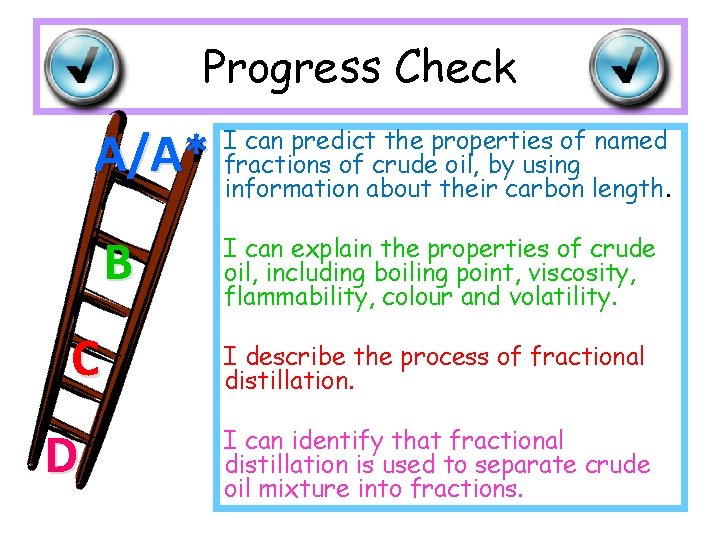
Progress Check A/A* B C D I can predict the properties of named fractions of crude oil, by using information about their carbon length. I can explain the properties of crude oil, including boiling point, viscosity, flammability, colour and volatility. I describe the process of fractional distillation. I can identify that fractional distillation is used to separate crude oil mixture into fractions.

Fractional Distillation http: //www. youtube. com/watch? v=26 AN 1 Lfb. UPc Now watch the teacher demo of distillation and add to your jigsaw sheet from the Connect.

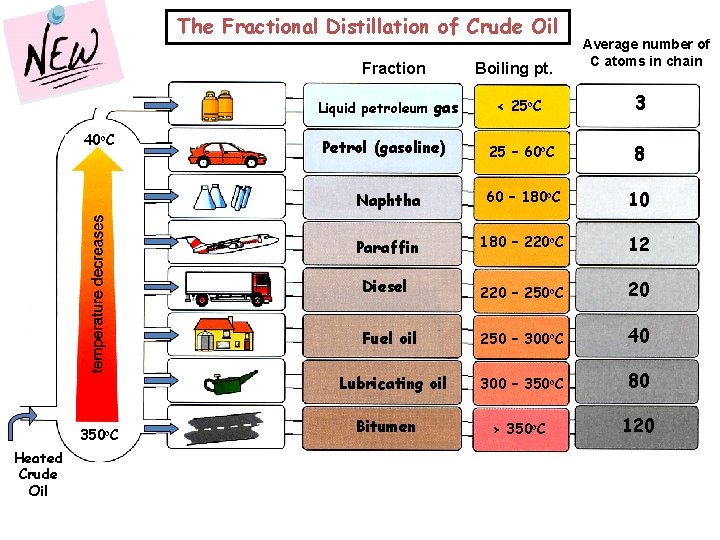
The Fractional Distillation of Crude Oil Fraction < 25 o. C 3 25 – 60 o. C 8 Naphtha 60 – 180 o. C 10 Paraffin 180 – 220 o. C 12 Diesel 220 – 250 o. C 20 Fuel oil 250 – 300 o. C 40 Lubricating oil 300 – 350 o. C 80 > 350 o. C 120 Liquid petroleum gas 40 o. C 350 o. C Heated Crude Oil Boiling pt. Average number of C atoms in chain Petrol (gasoline) Bitumen

Demonstrate your Learning Create a flow chart to describe the process of fractional distillation. Step 1 Step 2 Step 3 You may ask for a help sheet, however you need to present your case first.

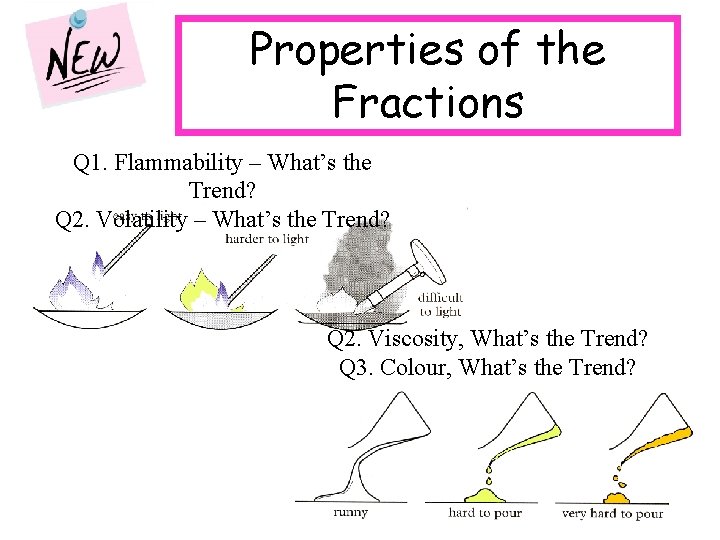
Properties of the Fractions Q 1. Flammability – What’s the Trend? Q 2. Volatility – What’s the Trend? Q 2. Viscosity, What’s the Trend? Q 3. Colour, What’s the Trend?

Search for Meaning Complete the summary sheet on crude oil. ENSURE you clearly explain using key words the properties of the fractions. You have TEN MINUTES.

Review & Reflect Complete the Review and Reflect Exit Ticket

INFO SHEETS

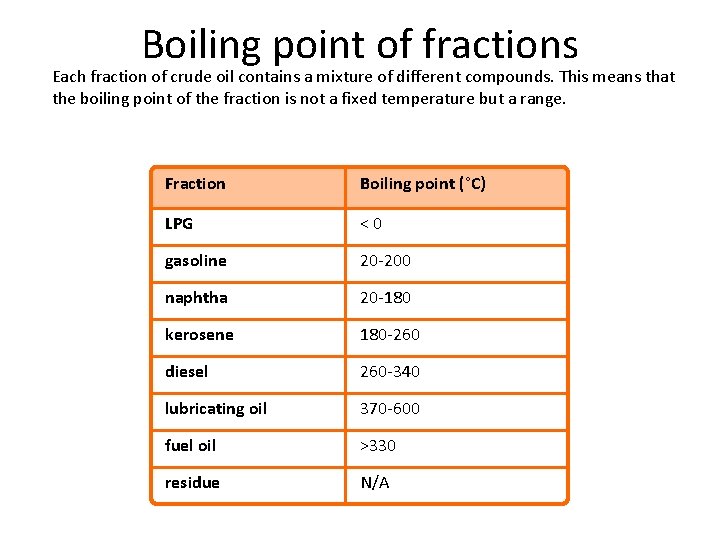
Boiling point of fractions Each fraction of crude oil contains a mixture of different compounds. This means that the boiling point of the fraction is not a fixed temperature but a range. Fraction Boiling point (°C) LPG <0 gasoline 20 -200 naphtha 20 -180 kerosene 180 -260 diesel 260 -340 lubricating oil 370 -600 fuel oil >330 residue N/A

Volatility and flammability Fractions that have a low boiling point evaporate easily. The easier a fraction evaporates, the more volatile it is. When fractions burn, they react with oxygen in the air. The more volatile a fraction is, the easier it mixes with air. This means the fraction ignites and burns easily. Fractions that ignite and burn easily are flammable. Generally, the smaller the molecules in a fraction, the more volatile and flammable the fraction.

What is viscosity? Some fractions of crude oil are thin and runny. Other fractions are thick and sticky. The runniness of a liquid is called viscosity. For example, the residue from fractional distillation has a very high viscosity (it is viscous) and cannot be easily poured. Gasoline has a low viscosity and pours easily. What is the relationship between the length of a hydrocarbon chain and the viscosity of a fraction? The longer the hydrocarbon chains in a fraction, the more viscous the fraction will be.

Molecule size and viscosity Why are fractions with large hydrocarbon molecules more viscous than fractions with small hydrocarbon molecules? The longer chains of large hydrocarbon molecules are easily entangled. Smaller molecules have shorter chains and are less likely to become entangled.

Colour of fractions The colour of a fraction depends on the size of the molecules it contains. As the molecules get smaller, the colour of the fraction becomes lighter, from dark brown to light brown, orange/yellow and transparent. decrease in size of molecules
 Look down to the left
Look down to the left Henri fayol parents
Henri fayol parents Metaphor in one thing one direction
Metaphor in one thing one direction Fraction odd one out
Fraction odd one out Plot setting stage language odd one out
Plot setting stage language odd one out Words
Words Solving equivalent fractions
Solving equivalent fractions Odd one out
Odd one out Food chain of rabbit
Food chain of rabbit Odd one out
Odd one out Odd one out
Odd one out Odd one out
Odd one out Find out the odd words brother sister mother grandson
Find out the odd words brother sister mother grandson Odd one out
Odd one out Odd one out
Odd one out Dessin fractions
Dessin fractions Odd degree odd function
Odd degree odd function Look at the following images
Look at the following images Fractional distillation viscosity
Fractional distillation viscosity What is this picture
What is this picture Find 2 odd miis out
Find 2 odd miis out D4t:h4p::l5r:? a)p4n b)q5m c)p5n d)q4n
D4t:h4p::l5r:? a)p4n b)q5m c)p5n d)q4n