Distillation 1 CONTENTS Flash Distillation Differential Distillation Continuous
















































- Slides: 89

Distillation 1

CONTENTS Flash Distillation Differential Distillation Continuous Distillation with Reflux Simple Steam Distillation Plate Efficiencies

9 -1 Introduction 1 Distillation • It is a unit operation of separation of liquid mixtures into their several components by partial vaporizing and partial condensing, and the most widely used method of achieving this end one of the major operations in chemical and petroleum industries, and the key operation of the oil refinery. The unit operation makes use of difference in volatility of individual components in a mixture. Throughout the chemical industry the demands for pure products, coupled with a relentless pursuit of greater efficiency, has necessitated continued research into the techniques of distillation. 3

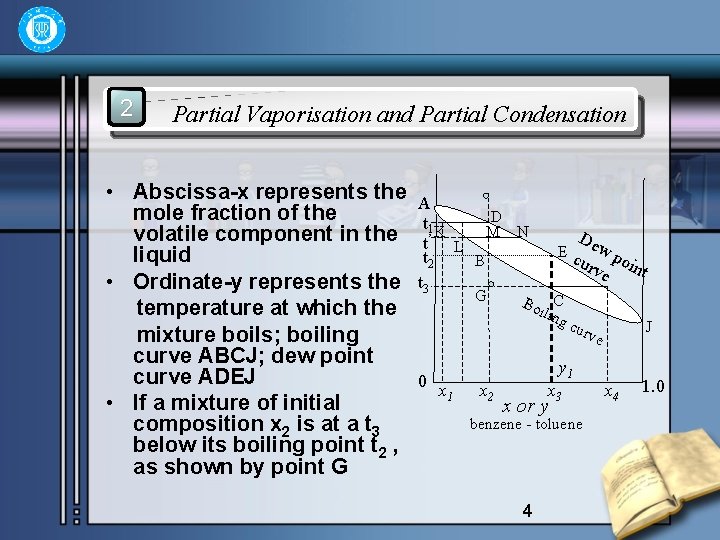
2 Partial Vaporisation and Partial Condensation • Abscissa-x represents the mole fraction of the volatile component in the liquid • Ordinate-y represents the temperature at which the mixture boils; boiling curve ABCJ; dew point curve ADEJ • If a mixture of initial composition x 2 is at a t 3 below its boiling point t 2 , as shown by point G A D t 1 K M N t’ L t 2 B t 3 G De Ec wp urv oin t e Bo C ilin gc J urv e 0 x 1 x 2 y 1 x 3 x or y benzene - toluene 4 x 4 1. 0

• On the diagram, then on heating at constant pressure the following changes will occur: • a) When T reaches T 2 , the liquid will boil, as shown by point B, and some vapor of composition y 2 , shown by point E, is formed. • b) On further heating, the composition of the liquid will change because of the loss of the more volatile component to the vapor and the boiling point will therefore rise to some t’. At this temperature the liquid will have a composition represented by L, and the vapor a composition represented by N. The mass ratio of liquid remained to the vapor formed is (lever rule) 5

• c) On further heating to t 1, all liquid is fully vapourised to give vapor D, of same composition y 1 as the original liquid. • It is seen that partial vaporisation of the liquid gives a vapor richer in the more volatile composition than the liquid. If the vapor initially formed, as for instance at point E, is at once removed by conden- sation, then a liquid of x 3 is obtained, represented by C. The step BEC may be regarded as representing an ideal stage, since the liquid passes from x 2 to x 3, which represents a greater enrichment in the more volatile component than can be obtained by any other single stage of vaporisation. 6

• Starting with superheated vapor represented by H, on cooling to D condensation will commence, and the first drop of liquid will have a composition of K. Further cooling to temperature t’ will give liquid L and vapor N. Thus partial condensation brings about enrichment of the vapor in the more volatile component in the same manner as partial vaporisation. The industrial distillation column is. in essence, a series of units in which these two processes of partial vaporisation and condensation are effected simultaneously. 7

3 Raoult’s and Henry’s Laws • 1. By Dalton’s law of partial pressures, the total pressure is equal to the summation of the partial pressure, that is • Then, since in an ideal gas or vapor the partial pre –ssure is proportional to the mole fraction of the constituent: • For an ideal mixture, the partial pressure is related to the concentration in the liquid phase by Raoult’s law which can be written 8

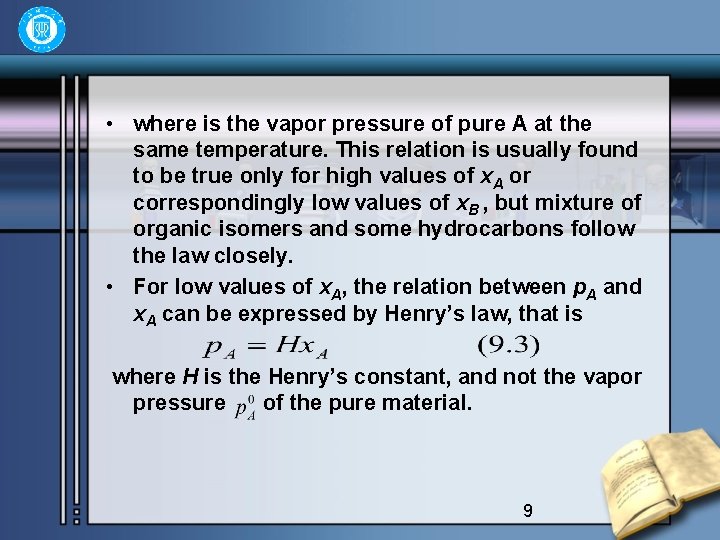
• where is the vapor pressure of pure A at the same temperature. This relation is usually found to be true only for high values of x. A or correspondingly low values of x. B , but mixture of organic isomers and some hydrocarbons follow the law closely. • For low values of x. A, the relation between p. A and x. A can be expressed by Henry’s law, that is where H is the Henry’s constant, and not the vapor pressure of the pure material. 9

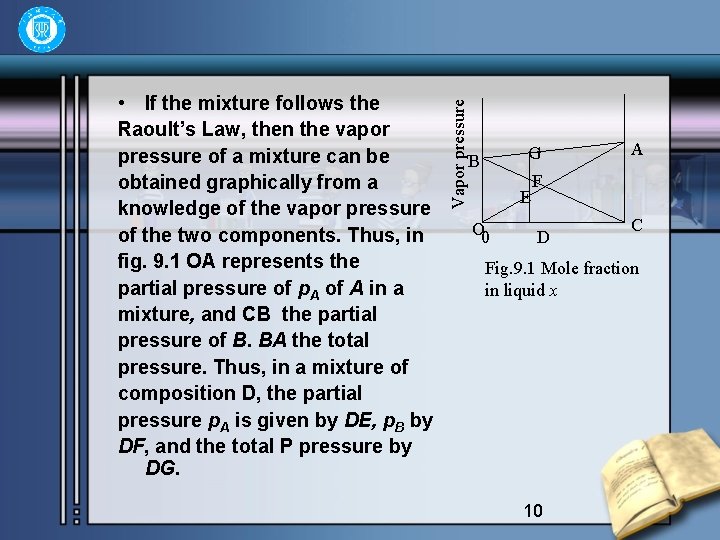
Vapor pressure • If the mixture follows the Raoult’s Law, then the vapor pressure of a mixture can be obtained graphically from a knowledge of the vapor pressure of the two components. Thus, in fig. 9. 1 OA represents the partial pressure of p. A of A in a mixture, and CB the partial pressure of B. BA the total pressure. Thus, in a mixture of composition D, the partial pressure p. A is given by DE, p. B by DF, and the total P pressure by DG. G B E O 0 A F D C Fig. 9. 1 Mole fraction in liquid x 10

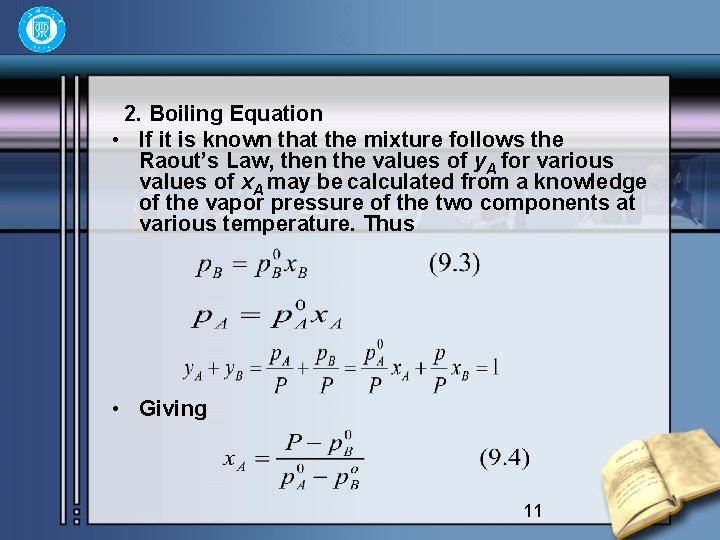
2. Boiling Equation • If it is known that the mixture follows the Raout’s Law, then the values of y. A for various values of x. A may be calculated from a knowledge of the vapor pressure of the two components at various temperature. Thus • Giving 11

3 Relative Volatility • We have known that the distillation unit operation makes use of difference in volatility of individual components in a mixture to separate the liquid mixture into several components. How is the volatility of a component measured? 1. volatility • The volatility is defined as the ratio of the partial pressure to the mol fraction in the liquid, that is 12

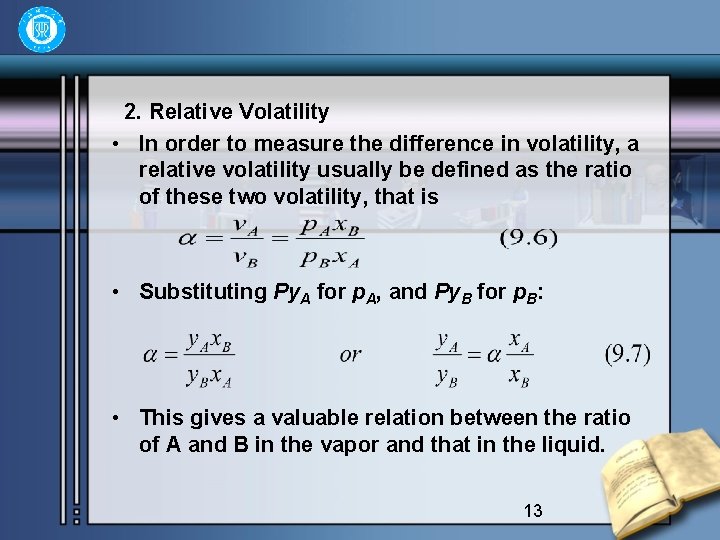
2. Relative Volatility • In order to measure the difference in volatility, a relative volatility usually be defined as the ratio of these two volatility, that is • Substituting Py. A for p. A, and Py. B for p. B: • This gives a valuable relation between the ratio of A and B in the vapor and that in the liquid. 13

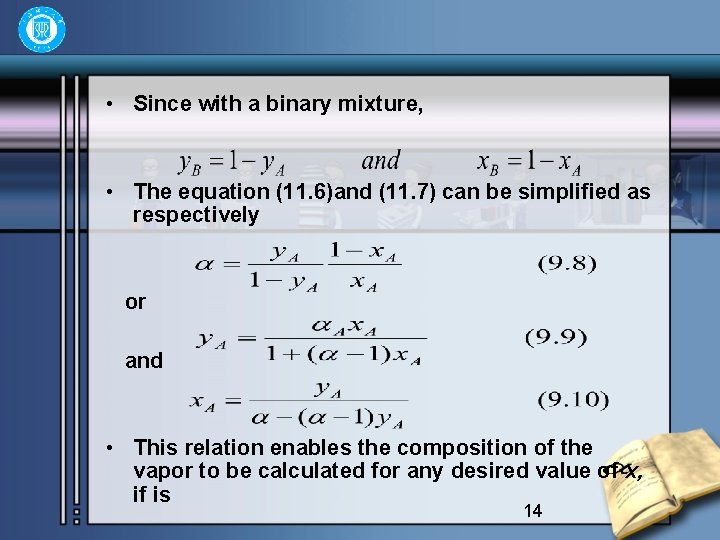
• Since with a binary mixture, • The equation (11. 6)and (11. 7) can be simplified as respectively or and • This relation enables the composition of the vapor to be calculated for any desired value of x, if is 14

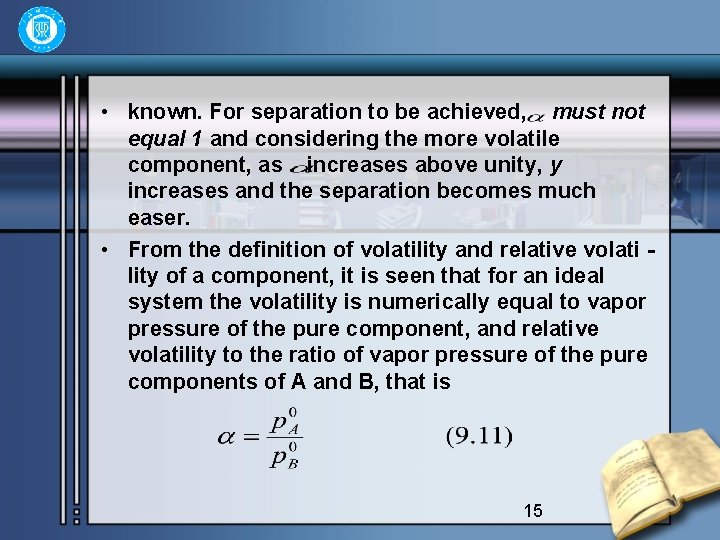
• known. For separation to be achieved, must not equal 1 and considering the more volatile component, as increases above unity, y increases and the separation becomes much easer. • From the definition of volatility and relative volati lity of a component, it is seen that for an ideal system the volatility is numerically equal to vapor pressure of the pure component, and relative volatility to the ratio of vapor pressure of the pure components of A and B, that is 15

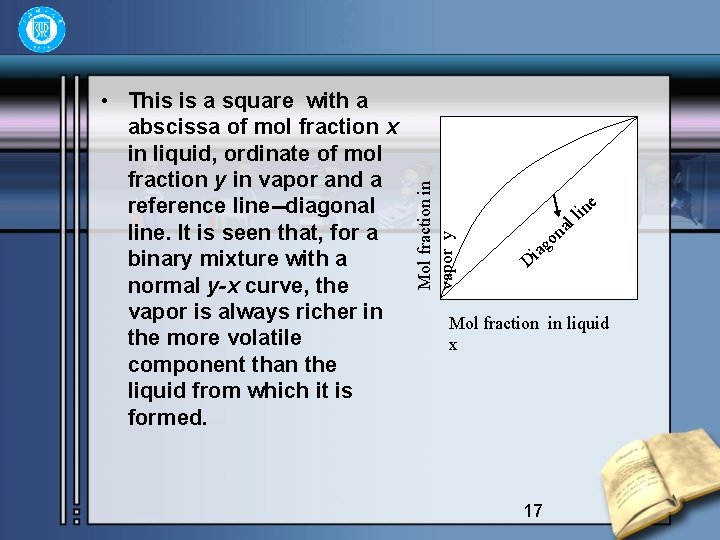
4 Methods of two-Component Mixture Distillation • For distillation purposes it is more convenient to plot y against x at a constant pressure, since the majority of industrial distillation take place at substantially constant pressure. This is shown as following 16

Mol fraction in vapor y • This is a square with a abscissa of mol fraction x in liquid, ordinate of mol fraction y in vapor and a reference line--diagonal line. It is seen that, for a binary mixture with a normal y-x curve, the vapor is always richer in the more volatile component than the liquid from which it is formed. l a on e lin ag i D Mol fraction in liquid x 17

• There are three main methods used in distillation practice which all rely on this basic fact; they are: • 1)differential distillation. (addition) • 2)flash or equilibrium distillation • 3)rectification • Of these, rectification is much more important, and differs from the other two methods in that part of the vapor is condensed and return as liquid to the still, whereas, in the other methods, all the vapor is either removed as much, or is condensed as product. 18

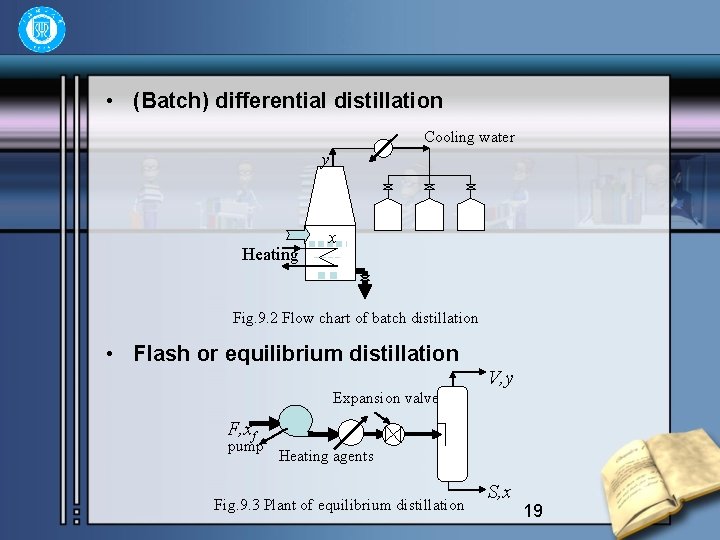
• (Batch) differential distillation Cooling water y Heating x Fig. 9. 2 Flow chart of batch distillation • Flash or equilibrium distillation V, y Expansion valve F, xf pump Heating agents Fig. 9. 3 Plant of equilibrium distillation S, x 19

9 -2 Flash or equilibrium distillation • This method, frequently carried as a continuous process, consist of vaporizing a definite fraction of the liquid feed in such a way that the evolved vapor is in equilibrium with the residual liquid. • The feed is usually pumped through a fired heater and enters the still through a valve where the pressure is reduced. The still is essentially a separator in which the liquid and vapor produced by the reduction in pressure. 20

• Let the concentraction of the feed be x. F ; in mole fraction of the more volatile component. Let F be the molal rate of the feed, V be the molal rate of vapor withdrawn continuously, and L be the mole rate of the liquid. Let y. D and x. B be the concentraction of the vapor and liquid, respectively. • By a material balance for the more volatile component gives: • Let f=V/F be the mole fraction of the feed that is vaporized and withdrawn continuously as vapor. 21

• Combining above two Eqs. gives : (9. 13 ) • or 22

9 -3 Differential Distillation 1. Feature of Differential Distillation • In this process the liquid is boiled slowly and the vapors are withdrawn as rapidly as they form to a condenser, where the condensed vapor is collected. Since the vapor is richer in the more volatile component than the liquid, it follows that the liquid remaining becomes steady weaker in this component, with the result that the component of product progressively alters. Thus, whilst the vapor formed over a short period is in equilibrium with the liquid, the total vapor formed is not in equilibrium with the residual liquid. 23

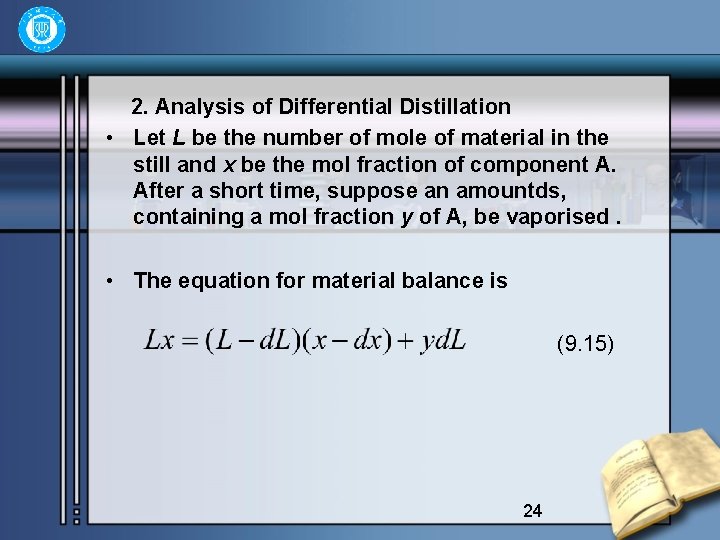
2. Analysis of Differential Distillation • Let L be the number of mole of material in the still and x be the mol fraction of component A. After a short time, suppose an amountds, containing a mol fraction y of A, be vaporised. • The equation for material balance is (9. 15) 24

• Neglecting the term dxd. L and rearranging • Integrating gives • The integral on the right side needs the information on equilibrium relation between y and x, and can be solved graphically or numerically. The average composition of total material distilled, yav , can be obtained by material balance: 25

9. 4 Simple Steam Distillation 1. The Concepts • Where the material to be distilled has high boiling point, and particularly where decomposition might be occur if direct distillation were employed, the process of steam distillation can be used. This method is often used to separate a high-boiling component from small amounts of nonvolatile impurities. • If a layer of liquid water(A) and an immiscible high-boiling component (B) are boiled at 1 atm, then, by the phase rule, for three phases and two components, F=2 -3 -2=1 degree of freedom 26

• When the sum of separate vapor pressures equals the total pressure, the mixture boils and • Then the vapor composition is • Note that by steam distillation, as long as liquid water is present, the high-boiling component B vaporizes at a temperature well below its normal boiling point without using a vacuum. The vapor of water (A) and high-boiling component (B) are usually condensed in a condenser and the resulting two immiscible liquid phase seperated. 27

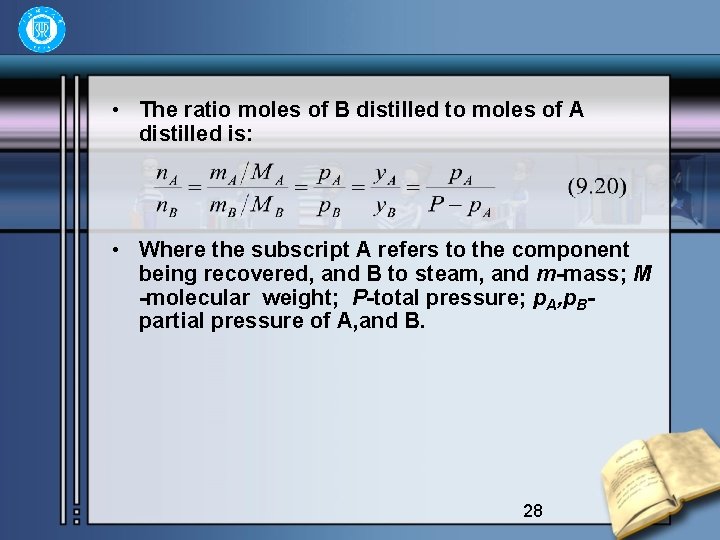
• The ratio moles of B distilled to moles of A distilled is: • Where the subscript A refers to the component being recovered, and B to steam, and m-mass; M -molecular weight; P-total pressure; p. A, p. Bpartial pressure of A, and B. 28

9 -5 Continuous distillation with reflux • For large-scale production, continuous distillation, is often used to separate components of comparable volatility, which requires the use of distillation with reflux. 29

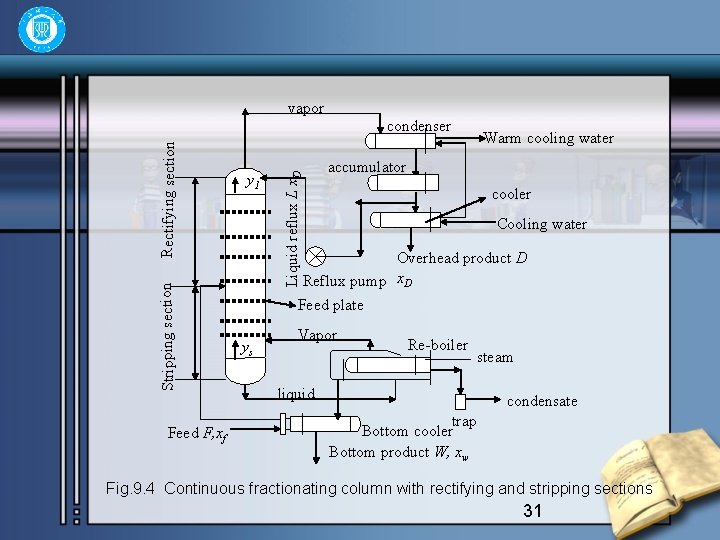
1 The Continuously Fractionating Process • The operation of a typical fractionating column may be followed by reference to Fig. 9. 4. The column consists of a cylindrical structure divided into sections by a series of perforated trays which permit the upward flow of vapor. The liquid reflux flows across each tray, over weir, down a downcomer to the tray below. The vapor rising from the top tray pass to a condenser and then via an accumulator or reflux drum and a reflux divider where part is withdrawn as the overhead product D, and the remainder is returned to the top tray as reflux R. 30

Feed F, xf y 1 Liquid reflux L x. D Stripping section Rectifying section vapor condenser Warm cooling water accumulator cooler Cooling water Overhead product D Reflux pump x. D Feed plate ys Vapor Re-boiler liquid steam condensate trap Bottom cooler Bottom product W, xw Fig. 9. 4 Continuous fractionating column with rectifying and stripping sections 31

• The liquid in the base of the column is frequently heated, either by condensing stream or by a hot oil stream, and the vapor rise through the perorations to the bottom tray. • This operation of partial condensation of the rising vapor and partial vaporisation of the reflux liquid is repeated on each tray. Vapor of composition of y 1 from the top tray is condensed to give the top product D and the reflux R, both of the same the composition y 1. The feed stream is introduced on some intermediate tray where the liquid has approximately the same composition as the feed. The part of the column above the feed point is known as the rectifying section; the lower portion is known as the stripping section. 32

• In the arrangement discussed above, the feed is introduced continuously to the column and two products streams are obtained, one at the top much richer than the feed in the MVC, and the second from the base of the column weaker in the MVC. This is operation of Continuous fractionating. For the separation of small quantities of mixtures, a batch still may be used (commonly in the fine organic chemical industry), which will be discussed in more detail later. 33

2 Action on an ideal plate • On an ideal tray, by definition, the liquid and the vapor leaving the tray are brought into equilibrium. Consider a single tray in an ideal cascade, such as tray n in fig. 9. 5. 34

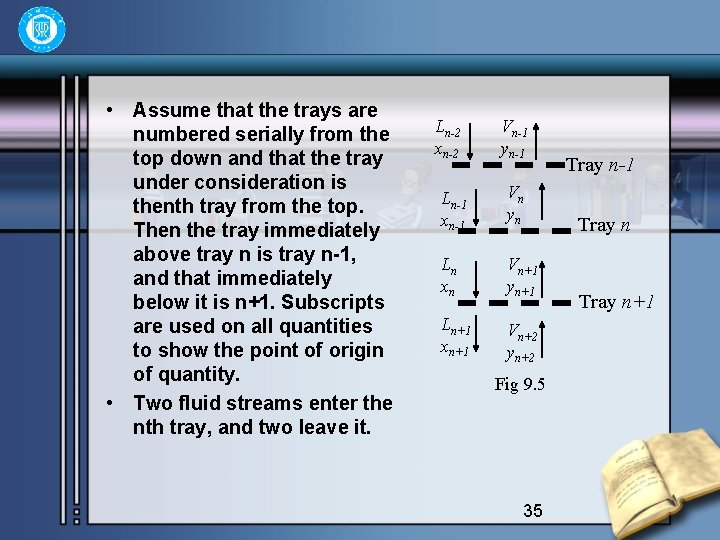
• Assume that the trays are numbered serially from the top down and that the tray under consideration is thenth tray from the top. Then the tray immediately above tray n is tray n-1, and that immediately below it is n+1. Subscripts are used on all quantities to show the point of origin of quantity. • Two fluid streams enter the nth tray, and two leave it. Ln-2 xn-2 Vn-1 yn-1 Ln-1 xn-1 Vn yn Ln xn Vn+1 yn+1 Ln+1 xn+1 Vn+2 yn+2 Fig 9. 5 35 Tray n-1 Tray n+1

A stream of liquid Ln-1 mol/h from tray n-1 and a stream of vapor Vn+1 mol/h from tray (n+1) are brought into intimate contact. A stream of vapor Vn mol/h rises to tray n-1, and a stream of liquid Ln mol/h descends to tray n+1, and the concentrations entering and leaving the nth tray are as follows: • Vapor leaving tray yn; liquid leaving tray xn; • Vapor entering tray yn-1; liquid entering tray xn-1. • By the definition of ideal tray there is equilibrium relationship between xn and yn. That is 36

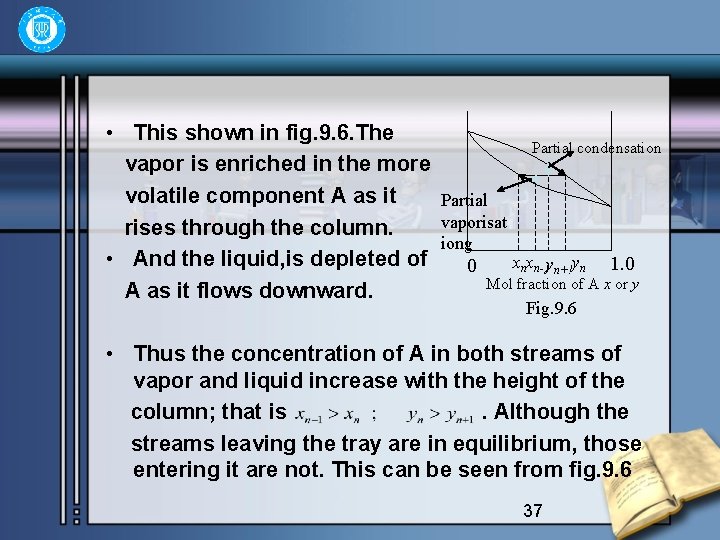
• This shown in fig. 9. 6. The vapor is enriched in the more volatile component A as it rises through the column. • And the liquid, is depleted of A as it flows downward. Partial condensation Partial vaporisat iong 0 xnxn-1 yn+!yn 1. 0 Mol fraction of A x or y Fig. 9. 6 • Thus the concentration of A in both streams of vapor and liquid increase with the height of the column; that is. Although the streams leaving the tray are in equilibrium, those entering it are not. This can be seen from fig. 9. 6 37

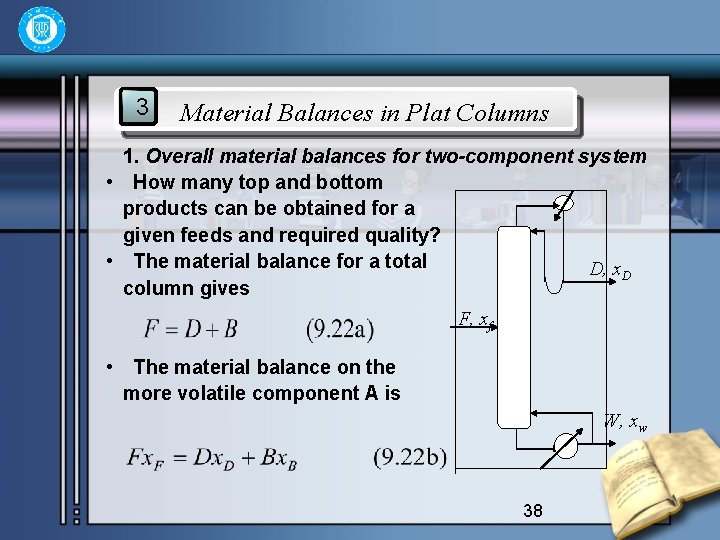
3 Material Balances in Plat Columns 1. Overall material balances for two-component system • How many top and bottom products can be obtained for a given feeds and required quality? • The material balance for a total D, x. D column gives F, xf • The material balance on the more volatile component A is W, xw 38

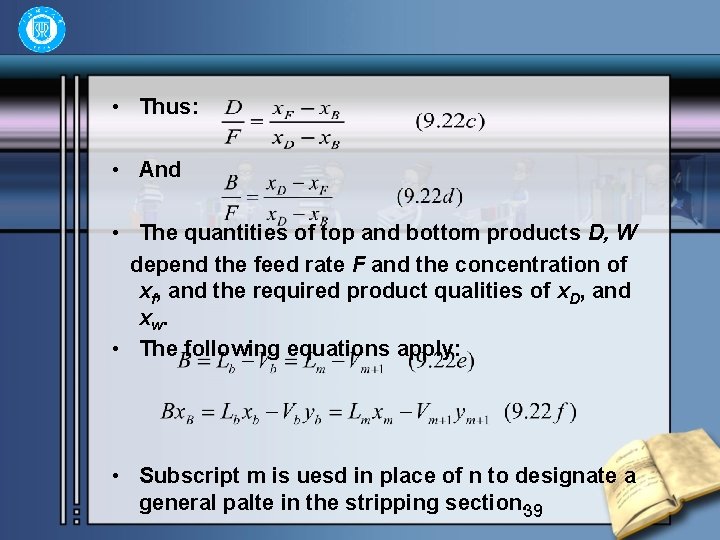
• Thus: • And • The quantities of top and bottom products D, W depend the feed rate F and the concentration of xf, and the required product qualities of x. D, and xw. • The following equations apply: • Subscript m is uesd in place of n to designate a general palte in the stripping section. 39

2. Operating lines • The relationship of concentrations of the vapor and liquid leaving an ideal plate abides by the equilibrium curve. What rule should the relation between concentrations of the vapor leaving an ideal plate and of the liquid entering it obey? • Since a stream of feed is introduced at feed plate, the continuity of material flow in the rectifying section is different from that in the stripping section. • Thus the situations of rectifying section and stripping section must be considered separately. 40

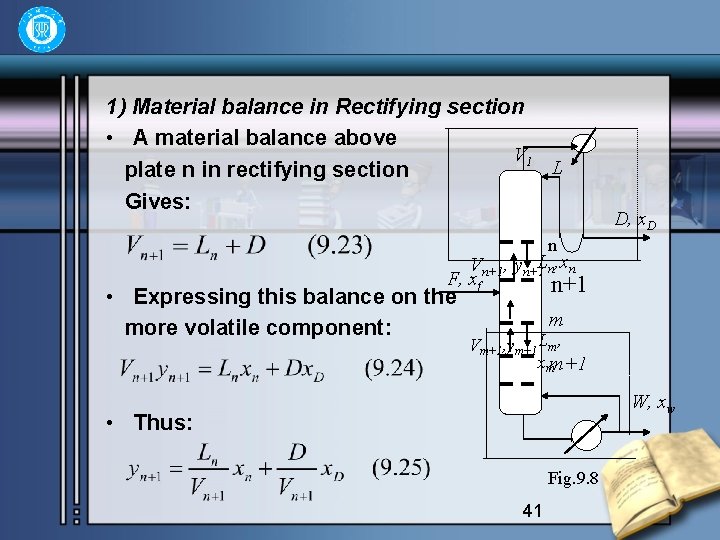
1) Material balance in Rectifying section • A material balance above V 1 L plate n in rectifying section Gives: D, x. D n Vn+1, yn+1 Ln, xn F, xf n+1 • Expressing this balance on the more volatile component: m Vm+1, ym+1 Lm, xmm+1 W, xw • Thus: Fig. 9. 8 41

• Eliminating Vn+1 by Eq. (9. 23), giving: The Eq. (9. 26) is called the equation of the operating line in the rectifying section. 42

2) Material balance in stripping section • Similarly, taking a material balance for the total stream and for the more volatile component from the top to above mth plate and • Thus: 43

• In a different form of Eq. (9. 27 a): • The equation gives the relation between the compositions of the vapor rising to a plate and the liquid on the plate in the stripping section. It is called the equation of operating line for the stripping section of a column. 44

4 Number of plates 1. constant molar overflow • If the molar heats of vaporisation are approximately constant, the flows of liquid and vapor in each part of the column will not vary from tray to tray unless material enters or is withdrawn from the section • This is the concept of constant molar overflow. 45

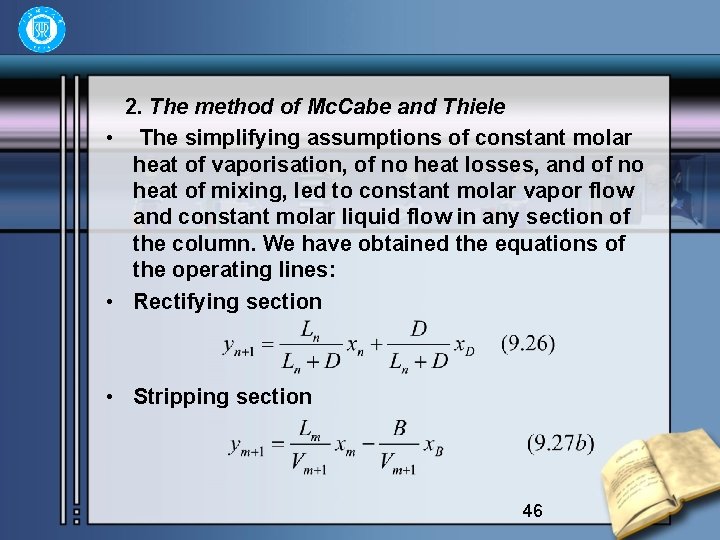
2. The method of Mc. Cabe and Thiele • The simplifying assumptions of constant molar heat of vaporisation, of no heat losses, and of no heat of mixing, led to constant molar vapor flow and constant molar liquid flow in any section of the column. We have obtained the equations of the operating lines: • Rectifying section • Stripping section 46

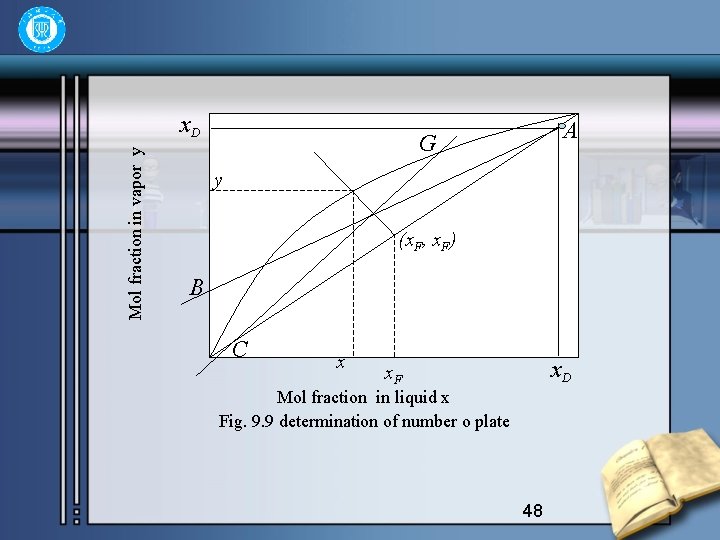
• They are all straight lines. The operating line for rectifying section is a straight line of a slope , and of a intercept. If xn=x. D in equation (9. 26), then • and it must pass through a definite point A(x. D, x. D) on the equilibrium diagram x-y. Further, if xn+1=0, then yn+1= , this represents the another definite point B(0, ). The two definite points make the operating line easily drawn out AB, as shown in fig. 9. 9 47

Mol fraction in vapor y x. D A G y (x. F, x. F) B C x x. D x. F Mol fraction in liquid x Fig. 9. 9 determination of number o plate 48

• Similarly, the equation of operating line for stripping section is also a straight line of slop , and if xm=x. B , then • and this represents the straight line has to pass th -rough the point C(x. B, , x. B). The operating line for stripping section is easy to be drawn out CG with use of the slop and the point C(x. B, , x. B) as shown in fig. 9. 10. 49

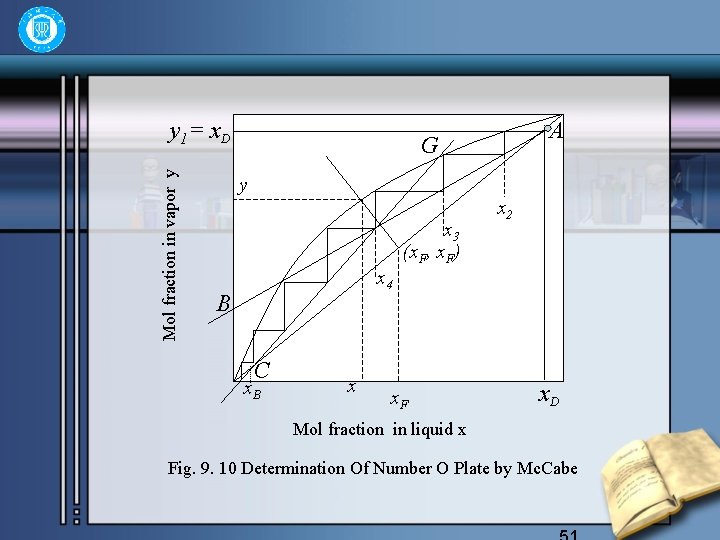
• When the two operating lines have been drawn out, the number of theoretical plates required can be determined by drawing steps between the equ -ilibrium curve and the operating line starting from point A. The method is called Mc. Cabe. Thiele’s. 50

Mol fraction in vapor y y 1= x. D A G y x 3 (x. F, x. F) x 2 x 4 B C x. B x x. F x. D Mol fraction in liquid x Fig. 9. 10 Determination Of Number O Plate by Mc. Cabe

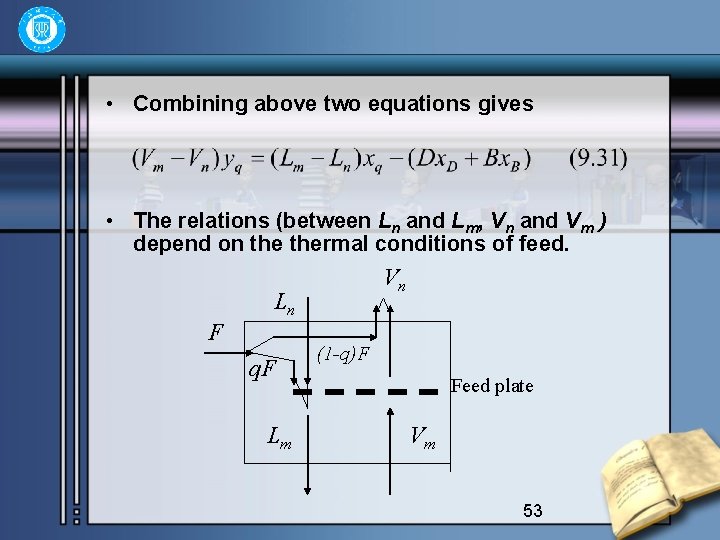
3. Operating Lines(Feed Line) • The equation for this line of intersection can be derived as following: • If the two operating lines intersect at a point with coordinates(x. D, y. D), then 52

• Combining above two equations gives • The relations (between Ln and Lm, Vn and Vm ) depend on thermal conditions of feed. Vn Ln F q. F Lm (1 -q)F Feed plate Vm 53

• Let the feed be partially vapor, and the moles of liquid per unit feed be q, the feed rate be F mol/h. • Thus: the moles of saturated liquid in the feed are q. F, the moles of saturated vapor are (1 -q)F. when the stream of (1 -q)F moles of vapor of feed comes into the column, it flows upward and is made up the vapor stream Vn in rectifying section with the Vm. That is Vn=Vm+(1 -q)F (9. 32) 54

• And the stream of q. F of liquid of feed comes into feed plate, it flow down and adds to the liquid stream Ln making up the liquid stream Lm in stripp- ing section, that is 55

• The means of q is: • Thus, q has the following numerical limits for the various thermal conditions of the feed: • 1) cold feed, q>1 • 2) saturated liquid, q=1 • 3) saturated vapor, q=0 • 4) feed partially vapor, 1>q>0 • 5) feed superheated vapor, q<0 56

• From Eq. (9. 22 b), the last two terms in Eq. (9. 31) can be replaced by Fx. F. Thus, substituting Eq. (9. 32) and (9. 33) into Eq. (9. 31) gives: • And arranging it gives: • The equation is commonly known as the equation of the q-line which represents a straight line, and on which all intersections of the operating lines must fall. 57

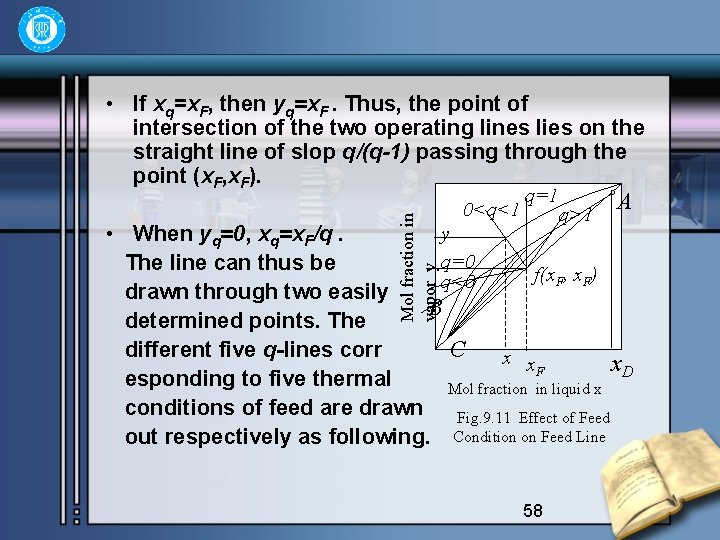
Mol fraction in vapor y • If xq=x. F, then yq=x. F. Thus, the point of intersection of the two operating lines lies on the straight line of slop q/(q-1) passing through the point (x. F, x. F). 0<q<1 q=1 q>1 A y • When yq=0, xq=x. F/q. q=0 The line can thus be f(x. F, x. F) q<0 drawn through two easily B determined points. The different five q-lines corr C x x x. D F esponding to five thermal Mol fraction in liquid x conditions of feed are drawn Fig. 9. 11 Effect of Feed out respectively as following. Condition on Feed Line 58

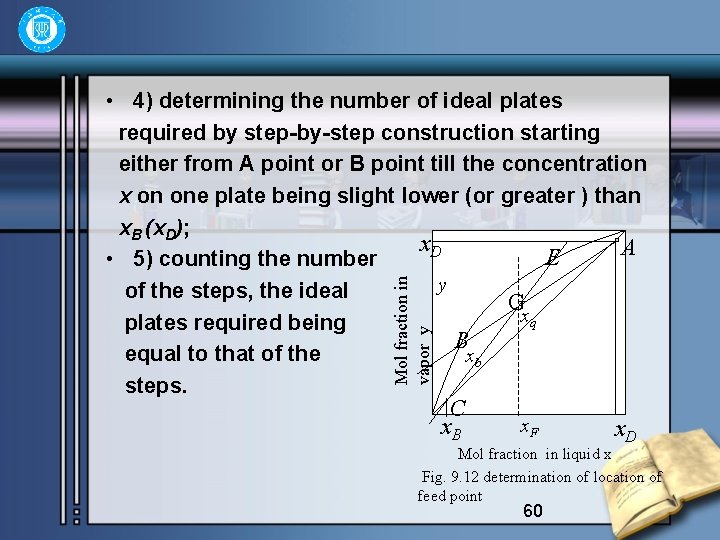
• Summarizing the steps of determination of ideal plates required by Mc. Cabe-thiele method for a given condition of feed, requirement of separation and reflux L gives • 1) plotting the equilibrium curve on x-y diagram based on the data of equilibrium; • 2)determining the four definite points of A(x. D, x. D), f(x. F, x. F), C(x. B, x. B) and B(0, Dx. D/Vn )on the diagonal line or ordinate of the x-y diagram; • 3) drawing the operating line of rectifying section through the points of A and B; drawing the q-line through the two points of f(x. F, x. F) and slope q/(q 1); drawing the operating lines of stripping section; 59

Mol fraction in vapor y • 4) determining the number of ideal plates required by step-by-step construction starting either from A point or B point till the concentration x on one plate being slight lower (or greater ) than x. B (x. D); x. D A E • 5) counting the number y of the steps, the ideal Gx q plates required being B equal to that of the xb steps. C x. B x. F x. D Mol fraction in liquid x Fig. 9. 12 determination of location of feed point 60

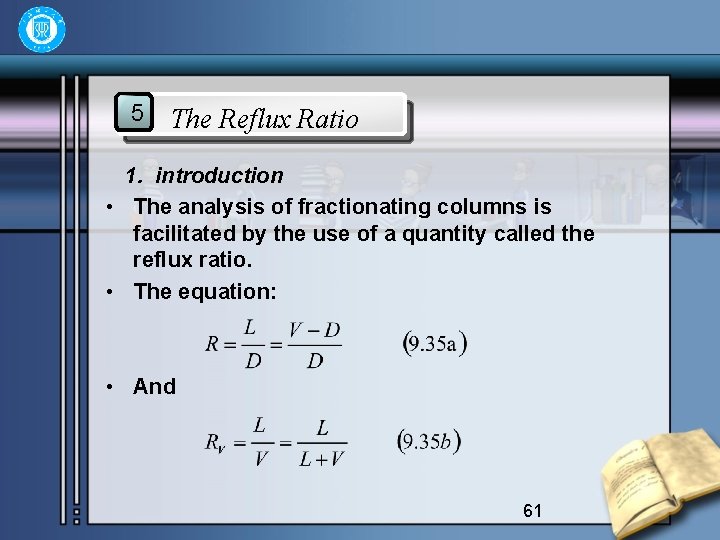
5 The Reflux Ratio 1. introduction • The analysis of fractionating columns is facilitated by the use of a quantity called the reflux ratio. • The equation: • And 61

• Thus, introducing R in equation(9. 26): • Any change in R will therefore modify the slope of the operating line. If R is known, the operating line of rectifying section is most easily drawn by joining point A(xd, xd) to B(0, xd/(R+1)). This method avoid the calculation of the actual flow rates Ln, Vv, when only the number of plates to be estimated. 62

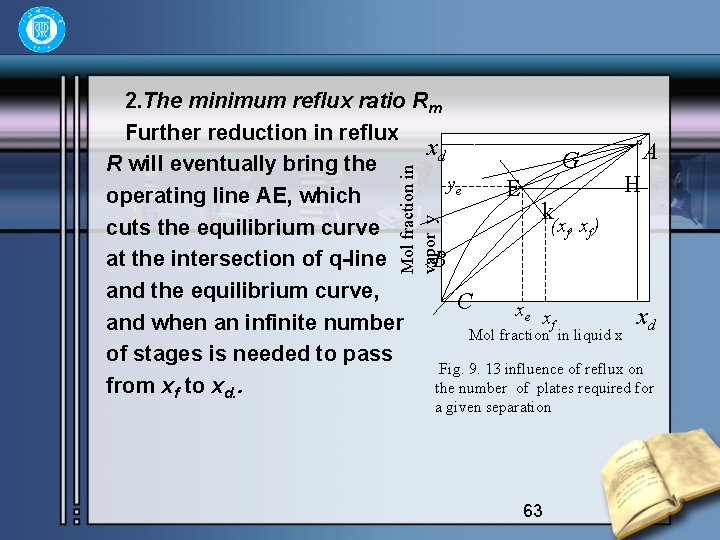
Mol fraction in vapor y 2. The minimum reflux ratio Rm Further reduction in reflux xd A G R will eventually bring the ye H E operating line AE, which k (xf, xf) cuts the equilibrium curve at the intersection of q-line B and the equilibrium curve, C xe x xd f and when an infinite number Mol fraction in liquid x of stages is needed to pass Fig. 9. 13 influence of reflux on from xf to xd. . the number of plates required for a given separation 63

• This arises from the fact that under these conditions the steps become very close together at liquid composition near xf , and no enrichment occurs from the feed plate to the plate above. These conditions are known as minimum reflux, and the reflux ratio is denoted by Rm. Any small increase in R beyond Rm will give a workable system, though a large number of plates will be required. It is important to note that any line such as AG, which is equivalent to a smaller value of R than Rm, represents an impossible condition, since it is impossible to pass beyond point G towards xf. 64

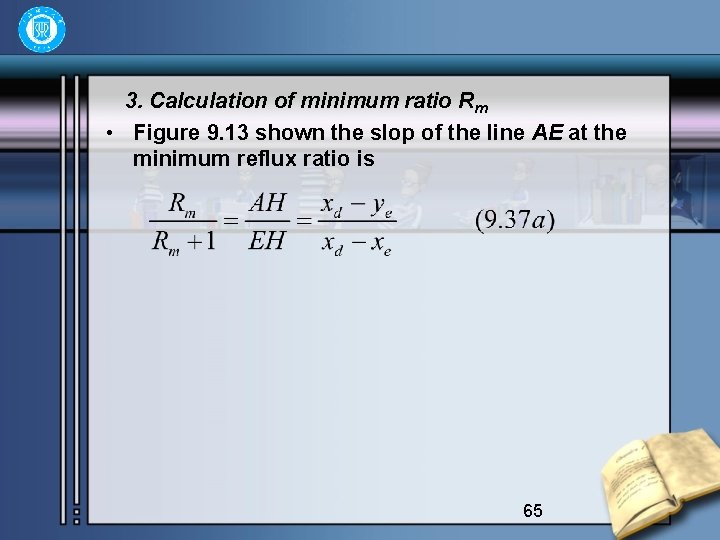
3. Calculation of minimum ratio Rm • Figure 9. 13 shown the slop of the line AE at the minimum reflux ratio is 65

• Arranging the equation obtain • This is an universal formula for calculating the minimum reflux Rm. • 1) For feed at boiling point, the q-line is vertical xe=xf, ye=yf is in equilibrium with xe. 66

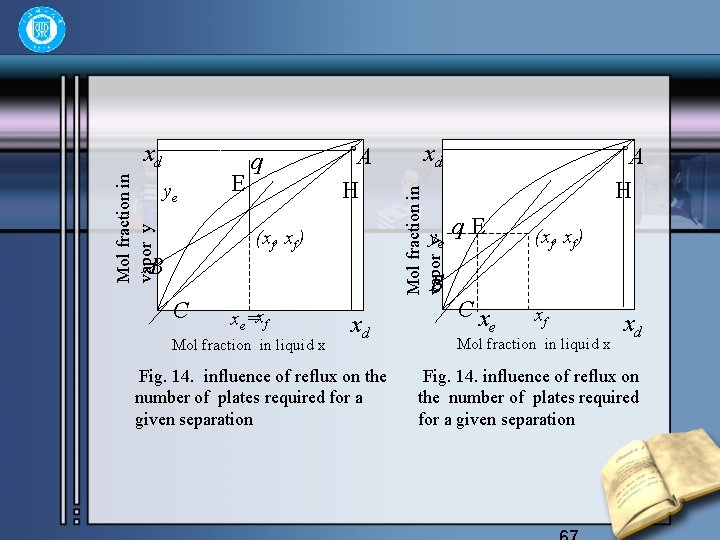
ye E q A H xd Mol fraction in vapor y xd ye (xf, xf) B B C xe=xf Mol fraction in liquid x xd Fig. 14. influence of reflux on the number of plates required for a given separation A H q. E Cx (xf, xf) xf e Mol fraction in liquid x xd Fig. 14. influence of reflux on the number of plates required for a given separation

• 2)For feed saturated vapor, the q-line is horizontal, so ye=xf , xe is in equilibrium with yf, and 68

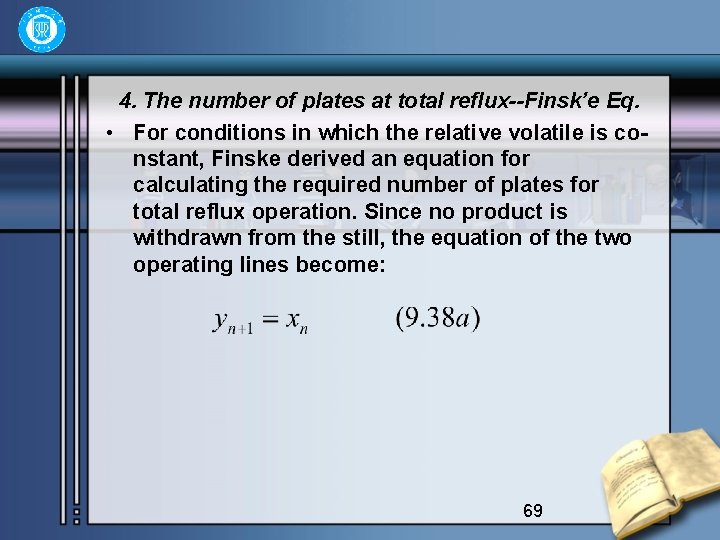
4. The number of plates at total reflux--Finsk’e Eq. • For conditions in which the relative volatile is constant, Finske derived an equation for calculating the required number of plates for total reflux operation. Since no product is withdrawn from the still, the equation of the two operating lines become: 69

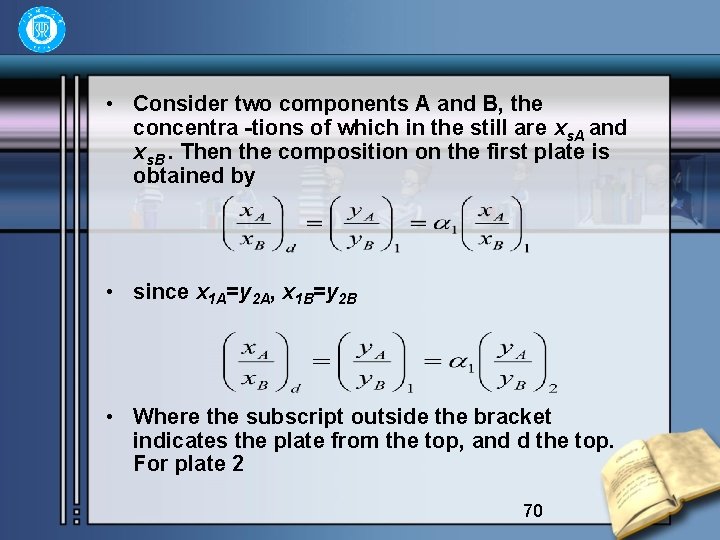
• Consider two components A and B, the concentra -tions of which in the still are xs. A and xs. B. Then the composition on the first plate is obtained by • since x 1 A=y 2 A, x 1 B=y 2 B • Where the subscript outside the bracket indicates the plate from the top, and d the top. For plate 2 70

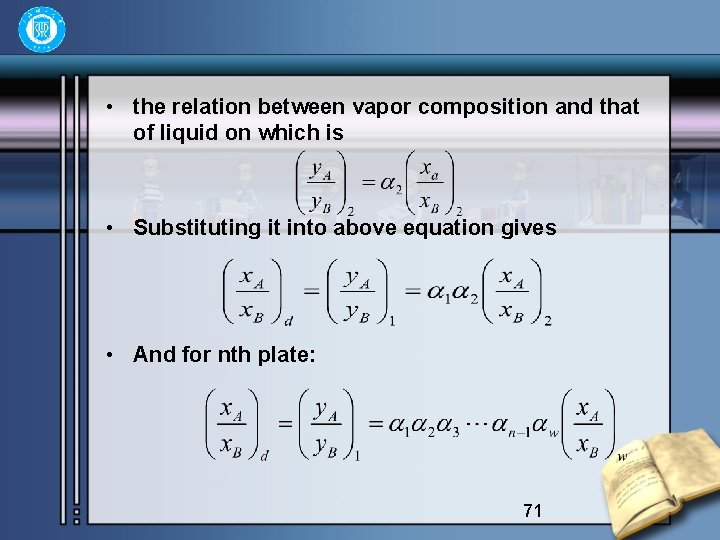
• the relation between vapor composition and that of liquid on which is • Substituting it into above equation gives • And for nth plate: 71

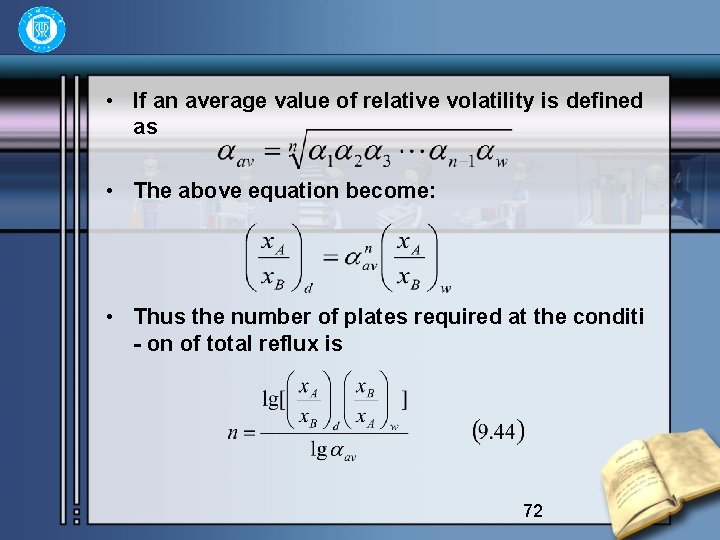
• If an average value of relative volatility is defined as • The above equation become: • Thus the number of plates required at the conditi - on of total reflux is 72

• and n is the required number of theoretical plates in the column. The equation is called Finske’s Eq. 73

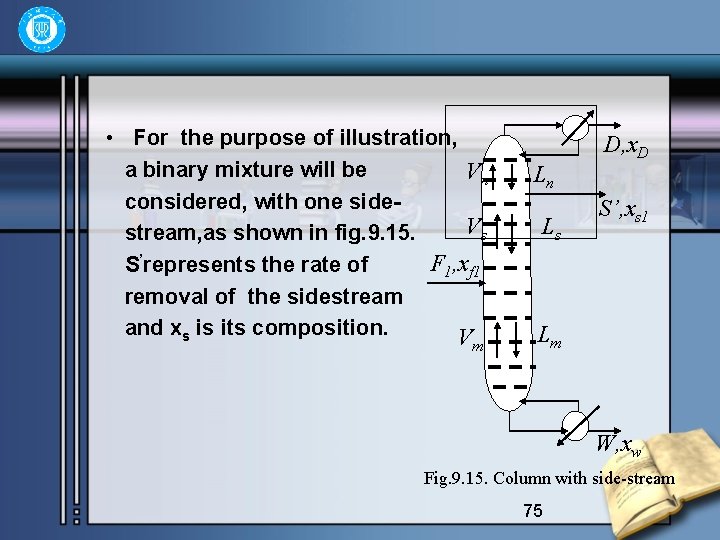
5. Multiple feeds and sidestream • In general, a side-stream is defined as any product stream other than the overhead product and residue (Sl’in fig. 9. 15). Likewise, F 1 and F 2 constitute separate feed stream to the column. Side-streams are most often removed with muti-component systems, but they can be used with binary system. 74

• For the purpose of illustration, a binary mixture will be Vn considered, with one side. Vs stream, as shown in fig. 9. 15. F 1, xf 1 S’represents the rate of removal of the sidestream and xs is its composition. V m Ln Ls D, x. D S’, xs 1 Lm W, xw Fig. 9. 15. Column with side-stream 75

• Multiple feeds and side-streams will alter the con -tinuity of fluid flowing, so the number of sections of a column is not as the same as that of a column which has only one feed stream, one top product stream and one bottom product stream. Usually, the number of the operating lines is equal to the summation of the numbers of feed streams and side-streams and top product stream and bottom product stream minus unity. For fig. 9. 15, the number of operating lines=1+1+2 -1=3. • For that part of the column above the sidestream the operating line is still given by 76

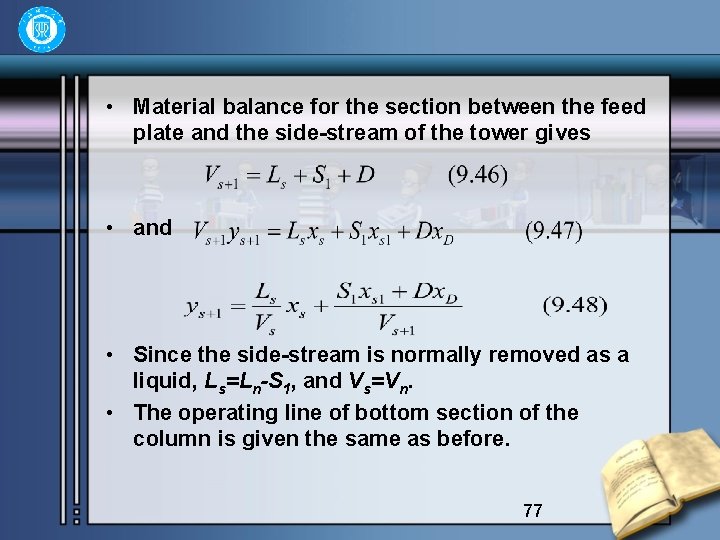
• Material balance for the section between the feed plate and the side-stream of the tower gives • and • Since the side-stream is normally removed as a liquid, Ls=Ln-S 1, and Vs=Vn. • The operating line of bottom section of the column is given the same as before. 77

• that is: • The effect of any additional side-stream or feed is to introduce an additional operating line for each the stream. In all other respects the method of calculation is identical with that for the straight separation of a binary mixture outlined earlier. 78

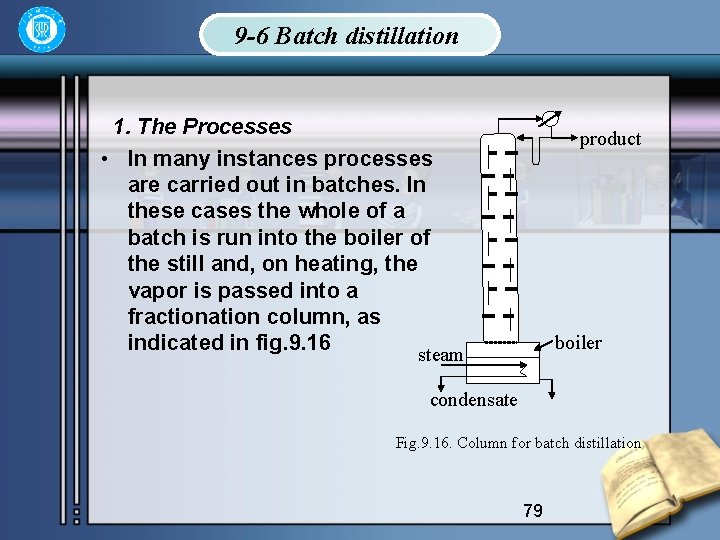
9 -6 Batch distillation 1. The Processes • In many instances processes are carried out in batches. In these cases the whole of a batch is run into the boiler of the still and, on heating, the vapor is passed into a fractionation column, as indicated in fig. 9. 16 steam product boiler condensate Fig. 9. 16. Column for batch distillation 79

2. The Futures • When the still is operating, since the top product will be relatively rich in the more volatile component, the liquid remaining in the still will become steadily weaker in this component. As a result, the purity of the top product will steadily fall. The processes are unsteady state. 80

• According to the purpose of operation, two methods of operation for batch distillation are used: • One is constant composition of the top product, the other is operating at constant reflux ratio which allows the composition of the top product to fall. • One of the added merits of batch distillation lies in the fact that more than one product may be obtained. The method of operating is particularly useful for handing small quantities of multi-comp -onent organic mixture. 81

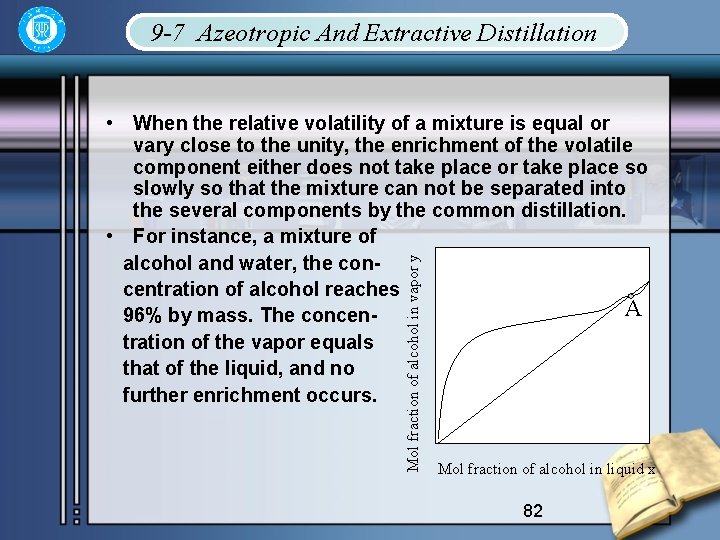
9 -7 Azeotropic And Extractive Distillation Mol fraction of alcohol in vapor y • When the relative volatility of a mixture is equal or vary close to the unity, the enrichment of the volatile component either does not take place or take place so slowly so that the mixture can not be separated into the several components by the common distillation. • For instance, a mixture of alcohol and water, the concentration of alcohol reaches A 96% by mass. The concentration of the vapor equals that of the liquid, and no further enrichment occurs. Mol fraction of alcohol in liquid x 82

• This mixture is called azeotrope, and cannot be separated by straightforward distillation. • The equilibrium curve crosses the diagonal at A. • The second type of problem occurs where the rel -ativity of a binary mixture is very low, in which case continuous distillation of the mixture to give nearly pure product will required high reflux ratios with corresponding high heat requirements, in addition, it will necessitate a tower of larger crosssection containing many trays. 83

• The principle of azeotropic distillation lies in the addition of a new substance to the mixture so as to form an azeotrope with one or more of the components in the mixture and as a result is present on the most of the plates of the column in appreciable concentration. The minimum boiling point ternary azeotrope is taken overhead, and such substance is called entrainer. 84

• The principle of extraction distillation lies in the addition of new material to the mixture so as to increase the relativity of the two key components, and thus make separation relatively easy. Furfural, which is a highly polar solvent, lowers the activity of butadiene more than it does for butanes and alters the relativity of the key components. The thirdly additional material is termed solvent. 85

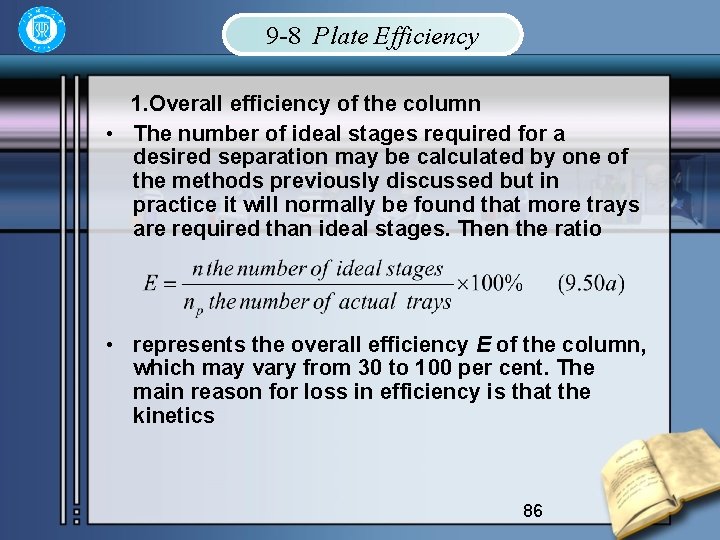
9 -8 Plate Efficiency 1. Overall efficiency of the column • The number of ideal stages required for a desired separation may be calculated by one of the methods previously discussed but in practice it will normally be found that more trays are required than ideal stages. Then the ratio • represents the overall efficiency E of the column, which may vary from 30 to 100 per cent. The main reason for loss in efficiency is that the kinetics 86

• for the rate of approach to equilibrium, and the flow pattern on the plate, may not permit equilib rium between the vapor and liquid to be attained. Some empirical equations have been developed from which values of efficiency may be calculated, but it is a considerable value. • Murpheree suggested that the proportion of liquid and vapor, and the physical properties of the mixtures on the trays will vary up the column, so the efficiency of individual plate is different each other, and conditions on individual trays must be examined. 87

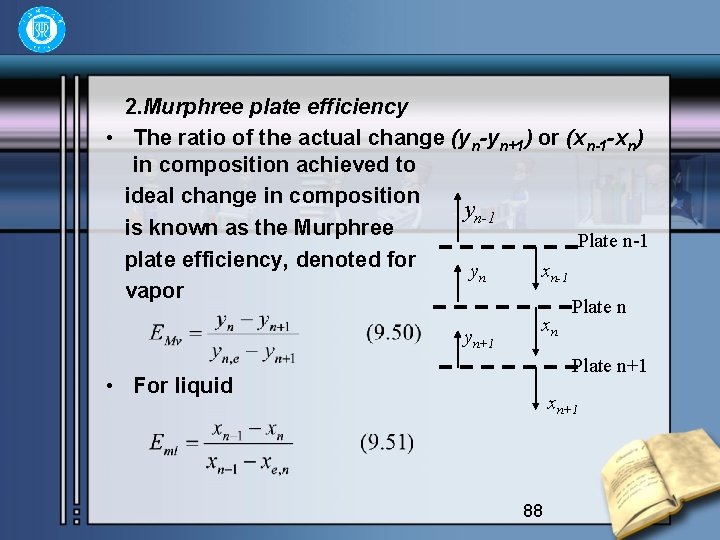
2. Murphree plate efficiency • The ratio of the actual change (yn-yn+1) or (xn-1 -xn) in composition achieved to ideal change in composition yn-1 is known as the Murphree Plate n-1 plate efficiency, denoted for yn xn-1 vapor yn+1 xn Plate n+1 • For liquid xn+1 88

• where ye, n is the composition of the vapor that would be in equilibrium with the liquid of actual composition xn leaving plate n. 3. Factors influencing plate efficiency • (1) plate operate properly. • (2) rate of mass transfer, depends on the flow parameter F 89