ERT 207 ANALYTICAL CHEMISTRY ALINA RAHAYU MOHAMED School


























- Slides: 40

ERT 207 ANALYTICAL CHEMISTRY ALINA RAHAYU MOHAMED School of Bioprocess Engineering University Malaysia Perlis 02600, Kangar Perlis email: alina@unimap. edu. my ERT 207 Analytical Chemistry 1

LECTURE 2 11 th July 2008 ERT 207 Analytical Chemistry 2

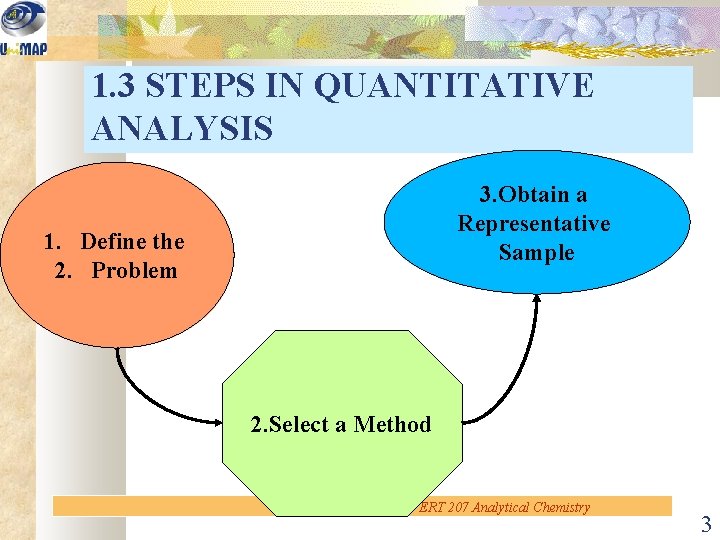
1. 3 STEPS IN QUANTITATIVE ANALYSIS 3. Obtain a Representative Sample 1. Define the 2. Problem 2. Select a Method ERT 207 Analytical Chemistry 3

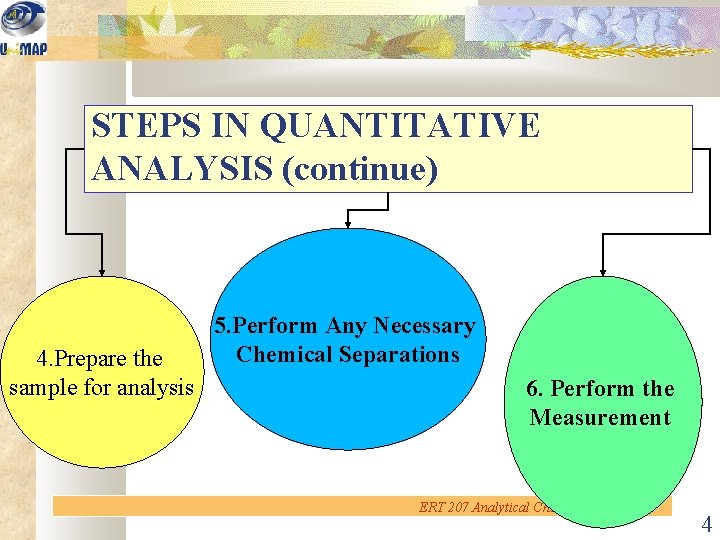
STEPS IN QUANTITATIVE ANALYSIS (continue) 4. Prepare the sample for analysis 5. Perform Any Necessary Chemical Separations 6. Perform the Measurement ERT 207 Analytical Chemistry 4

STEPS IN QUANTITATIVE ANALYSIS (continue) 7. Calculate the Results and Report ERT 207 Analytical Chemistry 5

1. Define the Problem Before we begin defining the problem for an analysis procedure, we must have some information; A)Who is the client(EPA, engineers) B)The purpose of analysis C)What type of sample to be analyzed ERT 207 Analytical Chemistry 6

1. Define the Problem Factors to consider: Ø What is the problem—what needs to be found? ØQualitative and/or quantitative? Ø What will the information be used for? Who will use it? Ø When will it be needed? ØHow accurate and precise does it have to be? ØWhat is the budget? ERT 207 Analytical Chemistry 7

Once the problem is defined, next questions: A) how sample is to be obtained B) how much is needed C)What separations may be required to eliminate interferences? ERT 207 Analytical Chemistry 8

How to select a Method? ERT 207 Analytical Chemistry 9

2. Select a Method Factors to be considered: q Sample type q Size of sample q Sample preparation needed q Concentration and range (sensitivity needed) q Selectivity needed (interferences) q Accuracy/precision needed q Tools/instruments available q Cost q Are methods available in the chemical literature? q Are standard methods available? ERT 207 Analytical Chemistry 10

3. Obtain a Representative Sample Factors • Sample type/homogeneity/size • Sampling statistics/errors ERT 207 Analytical Chemistry 11

The gross sample must be reduced in size to obtain a laboratory sample of several grams, from which a few grams to miligrams will be taken to be analyzed. ERT 207 Analytical Chemistry 12

Obtaining a representative sample is the first step of an analysis. The gross sample is several small portions of the sample. This is reduced to provide a laboratory sample. An aliquot of this sample is taken for the analysis sample. Sampling ERT 207 Analytical Chemistry 13

Some precautions should be taken during handling and storing samples to prevent or minimize contamination, loss, decomposition or matrix change. We must prevent contamination or alteration of the sample by (1)light (2)atmosphere (3) container. ERT 207 Analytical Chemistry 14

For example, some samples have to be protected from the atmosphere or light. It maybe an alkaline substance that will react with CO 2 in the air. Blood samples to be analyzed for CO 2 content shlould be protected from the atmosphere. ERT 207 Analytical Chemistry 15

Next example, corrosive sample may react with the container. In automobile exhaust SO 2 is lost by dissolving in condensed water vapour from the exhaust. ERT 207 Analytical Chemistry 16

4. Prepare the Sample for Analysis Factors • Solid, liquid, or gas? • Dissolve? • Ash or digest? • Chemical separation or masking of interferences needed? • Need to concentrate the analyte? • Need to change (derivatize) the analyte for detection? • Need to adjust solution conditions (p. H, add reagents)? ERT 207 Analytical Chemistry 17

4. Prepare the Sample for Analysis Step 1: Measure the amount being analyzed (volume or weight of the sample) Replicate samples are taken for analysis Why? ERT 207 Analytical Chemistry 18

1) to obatin statistical data on the precision of the analysis 2) to provide more reliable results. ERT 207 Analytical Chemistry 19

Step 2: Sample pretreatment Example: The organic materials sample are analyzed for inorganic constituents. The organic constituents maybe destroyed by dry ashing. How? ERT 207 Analytical Chemistry 20

The organic materials is slowly combusted in a furnace at 400 o. C to 700 o. C. Organic materials escape out, leaving behind an inorganic residue which is soluble in dilute acid. Aim of step 2: to remove unwanted constituents that make up the whole sample. ERT 207 Analytical Chemistry 21

Step 3: Optimizing sample condition Aim of step 3: to prepare sample for the next stage of analysis (The separation or measurement step) The solution condition is optimized. How? ERT 207 Analytical Chemistry 22

For example, the p. H may have to be adjusted or reagent is added to mask interference from other constituents. The analyte may have to be reacted with a reagent to convert it to a form suitable for measurement or separation. For example, a coloured product maybe formed that will be measured by spectrometry. ERT 207 Analytical Chemistry 23

5. Perform Any Necessary Chemical Separations • Distillation • Precipitation • Solvent extraction • Solid phase extraction • Chromatography (may be done as part of the measurement step) ERT 207 Analytical Chemistry 24

5. Perform Any Necessary Chemical Separations Why conduct chemical separation? 1. to eliminate interference 2. to provide suitable selectivity in the measurement 3. to preconcentrate the analyte for more sensitive or accurate measurement ERT 207 Analytical Chemistry 25

6. Perform the Measurement Factors • Calibration • Validation/controls/blanks • Replicates ERT 207 Analytical Chemistry 26

6. Perform the Measurement Methods of carrying out the measurements: 1. gravimetric analysis 2. volumetric analysis 3. instrumental analysis. What are these? ERT 207 Analytical Chemistry 27

7. Calculate the Results and Report • Statistical analysis (reliability) • Report results with limitations/accuracy information ERT 207 Analytical Chemistry 28

Tutorial Question 1 Close all of your notes! Will be discussed on Tuesday (15 th July 2008) for G 8 -G 13 at DJH room from 122 pm. Also, on 22 nd July 2008 , same place for G 1 -G 7. ERT 207 Analytical Chemistry 29

Basic tools in Analytical Chemistry ERT 207 Analytical Chemistry 30

Modern balances are electronic. They still compare one mass against another since they are calibrated with a known mass. Common balances are sensitive to 0. 1 mg. Fig. 2. 1. Electronic analytical balance. ERT 207 Analytical Chemistry 31

Volumetric flasks are calibrated to contain an accurate volume. See the inside back cover of the text for tolerances of Class A volumetric glassware. Fig. 2. 8. Volumetric. ERT flask. 207 Analytical Chemistry 32

Volumetric pipets accurately deliver a fixed volume. A small volume remains in the tip. Fig. 2. 9. Transfer or volumetric pipets. ERT 207 Analytical Chemistry 33

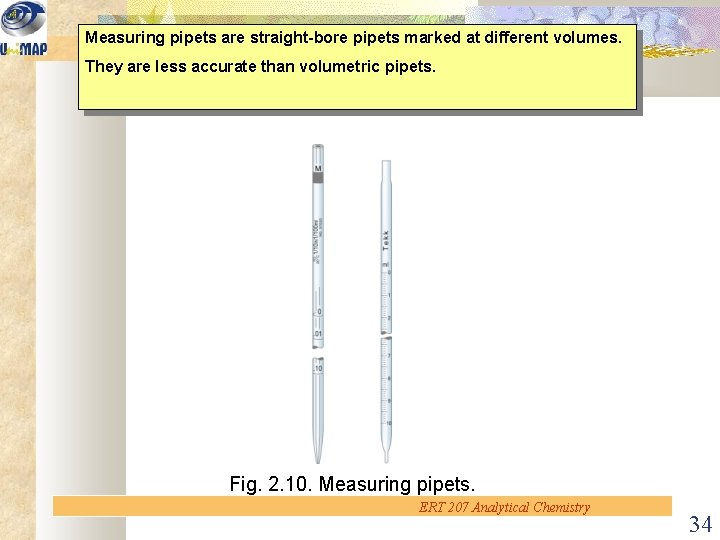
Measuring pipets are straight-bore pipets marked at different volumes. They are less accurate than volumetric pipets. Fig. 2. 10. Measuring pipets. ERT 207 Analytical Chemistry 34

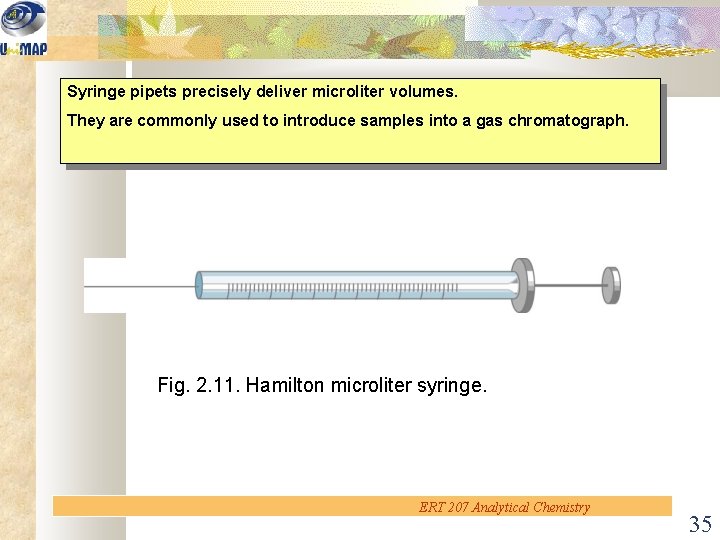
Syringe pipets precisely deliver microliter volumes. They are commonly used to introduce samples into a gas chromatograph. Fig. 2. 11. Hamilton microliter syringe. ERT 207 Analytical Chemistry 35

These syringe pipets can reproducibly deliver a selected volume. They come in fixed and variable volumes. The plastic tips are disposable. Fig. 2. 12 Single-channel and multichannel digital displacement pipets and microwell plates. ERT 207 Analytical Chemistry 36

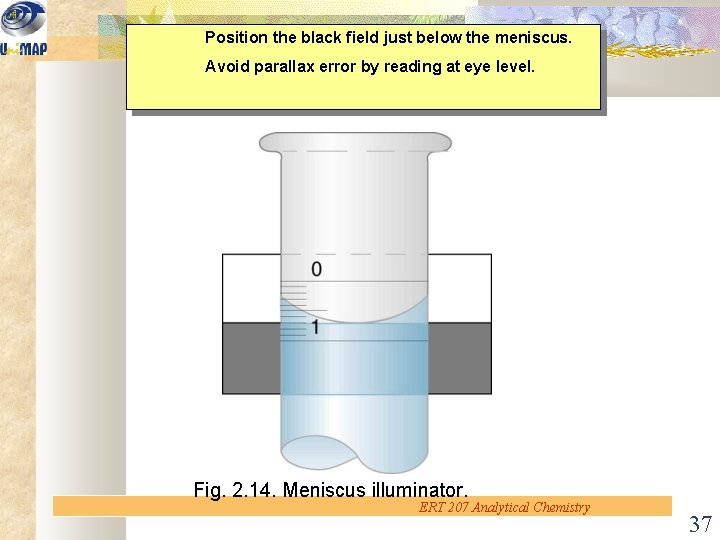
Position the black field just below the meniscus. Avoid parallax error by reading at eye level. Fig. 2. 14. Meniscus illuminator. ERT 207 Analytical Chemistry 37

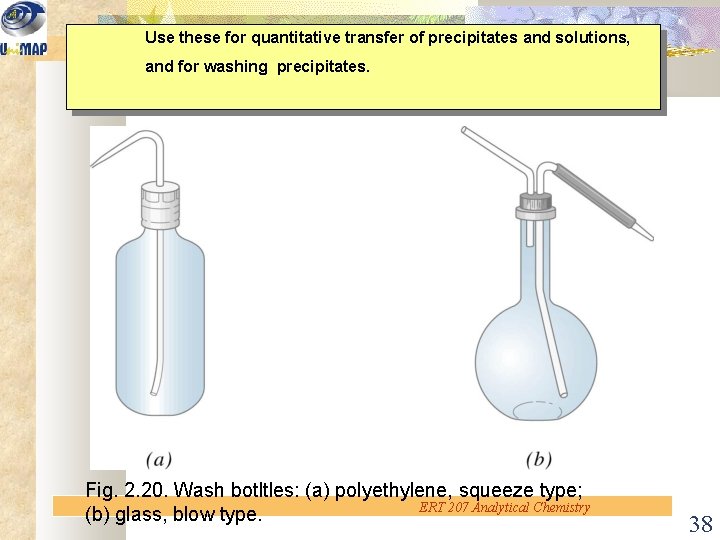
Use these for quantitative transfer of precipitates and solutions, and for washing precipitates. Fig. 2. 20. Wash botltles: (a) polyethylene, squeeze type; ERT 207 Analytical Chemistry (b) glass, blow type. 38

Next topic: Basic statistics ERT 207 Analytical Chemistry 39

Thank you Q& A session. ERT 207 Analytical Chemistry 40
 Pt = p.ert merupakan rumus dari pertumbuhan penduduk ….
Pt = p.ert merupakan rumus dari pertumbuhan penduduk …. The controllers chapter 8
The controllers chapter 8 Ert tool
Ert tool Ert diagram
Ert diagram Ert erp definition
Ert erp definition Ert erp definition
Ert erp definition Ert 1 programa
Ert 1 programa Ert products malaysia
Ert products malaysia Two way slab load distribution
Two way slab load distribution Iso/tc 207
Iso/tc 207 Article 207 tfeu
Article 207 tfeu Adil balghonaim
Adil balghonaim Csavarfej fajták
Csavarfej fajták Write an integer for each situation worksheet answers
Write an integer for each situation worksheet answers As a result of 207 years of pax romana, the roman empire
As a result of 207 years of pax romana, the roman empire Pg 207
Pg 207 Oae207
Oae207 2conf
2conf Csc 207
Csc 207 Oecd 207
Oecd 207 Ae 207
Ae 207 Acc 207
Acc 207 Analysis
Analysis Kesalahan dalam analisis
Kesalahan dalam analisis Pemeriaan
Pemeriaan Measuring apparatus in analytical chemistry
Measuring apparatus in analytical chemistry Amr validation
Amr validation Normal error curve in analytical chemistry
Normal error curve in analytical chemistry Redox reaction in alkaline medium
Redox reaction in alkaline medium Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Analytical chemistry definition
Analytical chemistry definition Q test in analytical chemistry
Q test in analytical chemistry Annual review of analytical chemistry
Annual review of analytical chemistry Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Analytical chemistry chapters
Analytical chemistry chapters Special purpose reagent chemicals
Special purpose reagent chemicals Analytical chemistry
Analytical chemistry Excellence in analytical chemistry
Excellence in analytical chemistry Difference between occlusion and mixed-crystal formation
Difference between occlusion and mixed-crystal formation Parikan tawon madu ngisep sekar
Parikan tawon madu ngisep sekar Apa gerakan penutup dari tari radap rahayu
Apa gerakan penutup dari tari radap rahayu