Environmental Sampling and Experimental Design Sampling Dependent on





















- Slides: 28

Environmental Sampling and Experimental Design

Sampling Dependent on: • Media – – Air Water Surfaces Food • Organisms – – – Viruses Bacteria Protozoans Fungi Algae By-products

Sampling Also Dependent on: • Detection Endpoint – Culture – Microscopy – Molecular Methods – Immunological Methods – Biochemical Methods • Logistics

Replication • Imperative for data interpretation • Should be multi-level – Experiment level – Assay level • 3 -5 replicates typical at experiment level • Minimum of duplication required at assay level; triplicate better

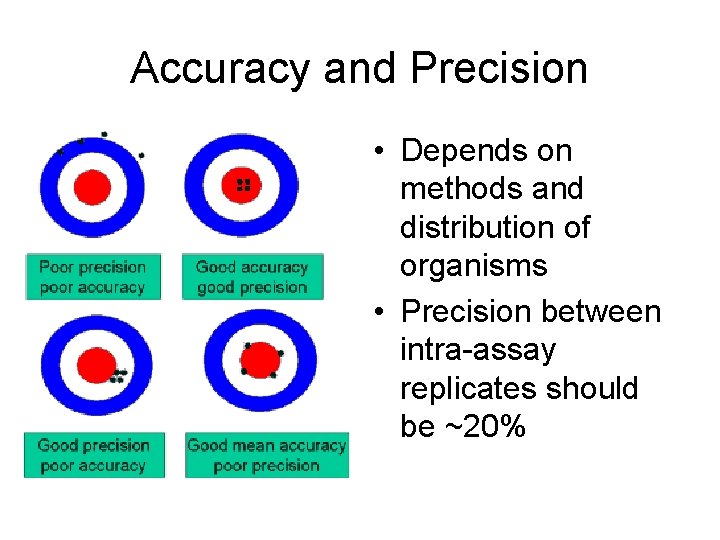
Accuracy and Precision • Depends on methods and distribution of organisms • Precision between intra-assay replicates should be ~20%

Controls • Negative Controls – Sterility Controls – Experimental Controls • Positive Controls – Bacteriostasis/Fungistasis Controls – Seeded Controls – Recovery Controls

Sampling Considerations What we want: • Fast • Sensitive • Specific • Easy to Perform • Reliable (Accurate/Precise) • Compatible with Downstream Detection What do we have? ? ?

The Challenge of Environmental Sampling for Pathogens • Variation in microbe type and distribution • Low microbe numbers: need to concentrate them • Non-random distribution and physical state of microbes of interest: aggregated, particleassociated, embedded, etc. • Volume considerations • Environmental factors may inhibit or interfere with downstream detection • Separate them from interfering and excess other material

Detection of Pathogens in The Environment • • Three main steps: (1) recovery and concentration, (2) purification and separation, and (3) assay and characterization.

Standard Methods • Water and Wastewaster – Standard Methods for the Examination of Water and Wastewater, 20 ed. , AWWA, APHA, WEF – EPA Methods • http: //www. epa. gov/nerlcwww/index. html • http: //www. epa. gov/waterscience/methods/ – ISO Methods – CEN/ISSS Methods – ASTM Methods

Standard Methods • Food – FDA Methods • http: //www. cfsan. fda. gov/~ebam/bam-toc. html • http: //www. cfsan. fda. gov/~comm/microbio. html – Codex Alimentarius standards – Standard Methods for the Examination of Dairy Products, 17 th ed. , APHA – NSSP – CEN/ISSS Methods – ISO Methods

Standard Methods • Disinfection and Disinfectant Efficacy Testing – EPA Methods • http: //www. epa. gov/oppbead 1/methods/atmpa 2 z. htm • http: //www. epa. gov/opptsfrs/publications/OPPTS_Harmonize d/885_Microbial_Pesticide_Test_Guidelines/Series/ – ASTM Methods – ISO Methods – CEN/ISS methods

Standard Methods • Air – ISO standards – CEN/ISS standards – ACGIH standards • Surfaces – ISO standards – ASTM methods – CEN standards

Good Laboratory Practice

GLP and GMP • GLP- Good Laboratory Practices • GMP- Good Manufacturing Practices

Where does GLP/GMP come from? • OECD- Organization for Economic Cooperation and Development – International organization tasked with assuring data or products of member states – Who belongs? • 30 Member Countries • Relations with 70 other countries • NGOS, and Civil Society

Member Countries • AUSTRALIA: 7 June 1971 AUSTRIA: 29 September 1961 BELGIUM: 13 September 1961 CANADA: 10 April 1961 CZECH REPUBLIC: 21 December 1995 DENMARK: 30 May 1961 FINLAND: 28 January 1969 FRANCE: 7 August 1961 GERMANY: 27 September 1961 GREECE: 27 September 1961 HUNGARY: 7 May 1996 ICELAND: 5 June 1961 IRELAND: 17 August 1961 ITALY: 29 March 1962 JAPAN: 28 April 1964 KOREA: 12 December 1996 • LUXEMBOURG: 7 December 1961 MEXICO: 18 May 1994 NETHERLANDS: 13 November 1961 NEW ZEALAND: 29 May 1973 NORWAY: 4 July 1961 POLAND: 22 November 1996 PORTUGAL: 4 August 1961 SLOVAK REPUBLIC: 14 December 2000 SPAIN: 3 August 1961 SWEDEN: 28 September 1961 SWITZERLAND: 28 September 1961 TURKEY: 2 August 1961 UNITED KINGDOM: 2 May 1961 UNITED STATES: 12 April 1961

How Enforced? • Left to authorities in individual countries • Who in the US? – FDA – EPA (TSCA and FIFRA)

What is Required? • • Overall QA/QC Manual QAPPs Biosafety and Chemical Hygiene Operational Controls Routine Maintenance and Calibration Training of Personnel Audit Schedule

QA/QC Manual Personnel • Staff Organization • Training Schedules • Personnel Files

QA/QC Manual Facilities • Physical Description – – – Size Floorplan Benchspace Lighting Storage • Security • Workflow • HVAC • Water • Environmental Monitoring • Maintenance Schedule

QA/QC Manual Biosafety/Chemical Hygiene – Gowning and PPE – Emergencies/Spills – Decontamination – SOPs – Description of Safety Practices

Equipment • Calibration and Maintenance – Autoclaves – Balances – Spectrophotometers – Centrifuges – Temperature Dependent Devices – Thermometers – Etc. • Relative vs. Traceable Calibration

Operational • • • Purchasing Inspection/Acceptance Tracking Sterility Media and Reagent Prep

QA/QC • • Staff Master Schedule Lab Notebooks SOPs Project Plans Reporting of Study Results Storage and Retention Audits

Data Management • Detailed plan for collection, recording, and maintenance of data • Specifies what data collected with observations • Specifies how and where data kept – Laboratory notebooks – Equipment/procedure logs – LIMs system • Specifies Data Retention and Storage – Frequently 3 -5 years on-site; upto 10 in off-site or archived storage

Keeping a Good Lab Notebook • Generally: – Be organized – Date (and sign) each new entry – Write everything down, no matter how trivial it may seem – Do not erase; rather strike through and initial corrections – Permanently affix additions to lab notebook – Have supervisor review and sign-off on data at least weekly. • When in Doubt write it out

Transferring Data from Lab Notebook to Electronic Database • Best to use LIMS with date stamping. • Location of original data should be recorded • Once input, data entry should be validated (ideally by another person; unrealistic) • Data analysis procedures should be annotated where possible