Engineering Materials Corrosion Is the deterioration of a





- Slides: 18

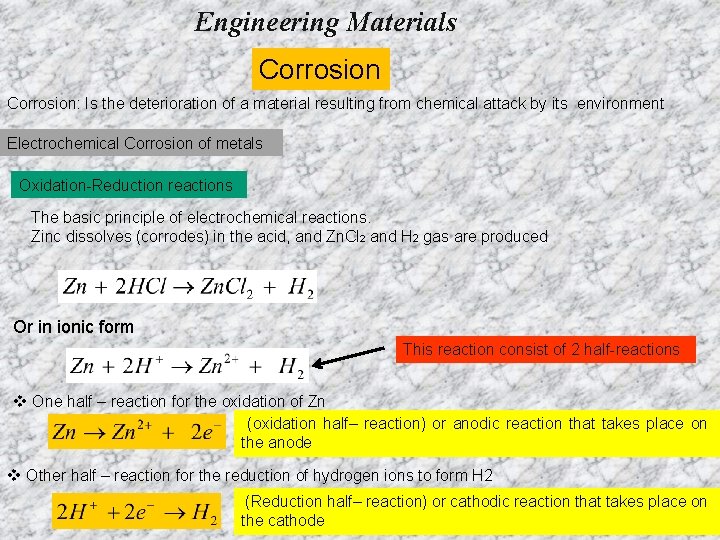
Engineering Materials Corrosion: Is the deterioration of a material resulting from chemical attack by its environment Electrochemical Corrosion of metals Oxidation-Reduction reactions The basic principle of electrochemical reactions. Zinc dissolves (corrodes) in the acid, and Zn. Cl 2 and H 2 gas are produced Or in ionic form This reaction consist of 2 half-reactions v One half – reaction for the oxidation of Zn (oxidation half– reaction) or anodic reaction that takes place on the anode v Other half – reaction for the reduction of hydrogen ions to form H 2 (Reduction half– reaction) or cathodic reaction that takes place on the cathode

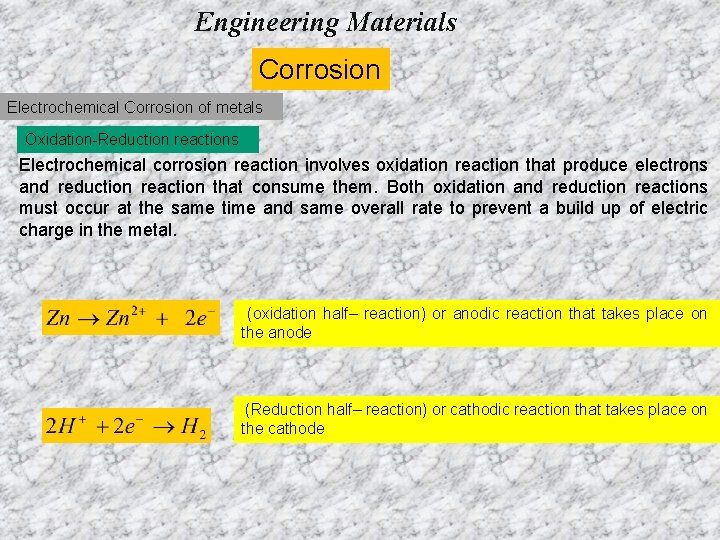
Engineering Materials Corrosion Electrochemical Corrosion of metals Oxidation-Reduction reactions Electrochemical corrosion reaction involves oxidation reaction that produce electrons and reduction reaction that consume them. Both oxidation and reduction reactions must occur at the same time and same overall rate to prevent a build up of electric charge in the metal. (oxidation half– reaction) or anodic reaction that takes place on the anode (Reduction half– reaction) or cathodic reaction that takes place on the cathode

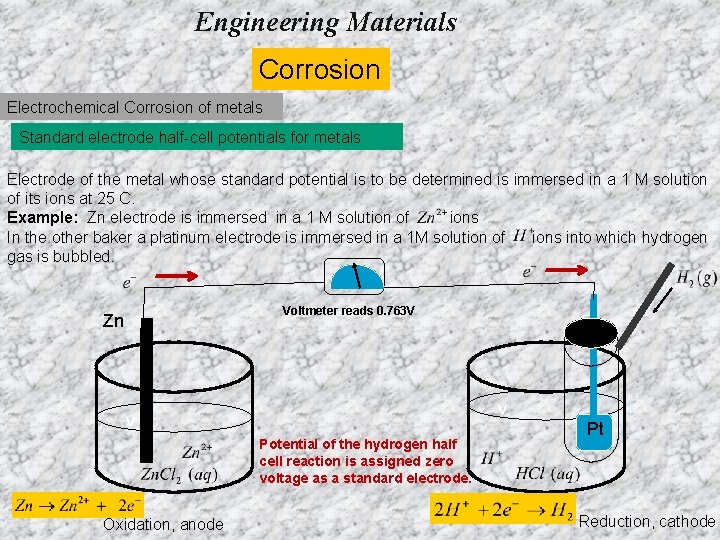
Engineering Materials Corrosion Electrochemical Corrosion of metals Standard electrode half-cell potentials for metals Electrode of the metal whose standard potential is to be determined is immersed in a 1 M solution of its ions at 25 C. Example: Zn electrode is immersed in a 1 M solution of ions In the other baker a platinum electrode is immersed in a 1 M solution of ions into which hydrogen gas is bubbled. Voltmeter reads 0. 763 V Zn v v Potential of the hydrogen half cell reaction is assigned zero voltage as a standard electrode. Oxidation, anode Pt Reduction, cathode

Engineering Materials Corrosion Electrochemical Corrosion of metals Standard electrode half-cell potentials for metals The following table lists the standard half-cell potentials of some selected metals. Table (page 723 ST electrod pot)

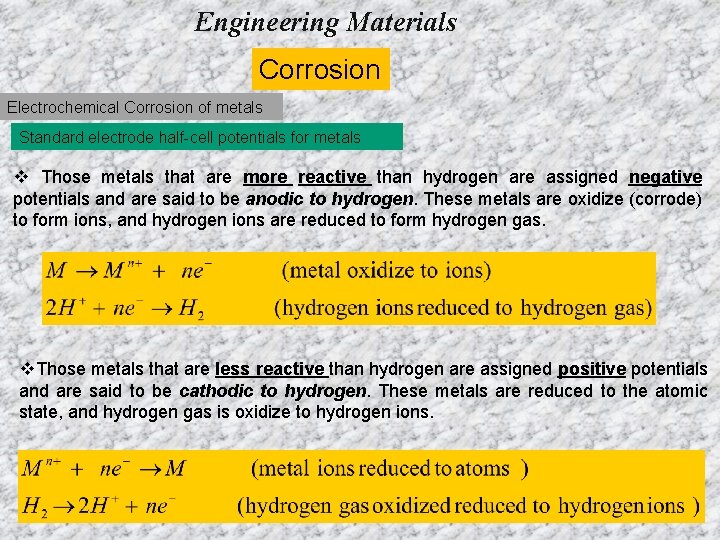
Engineering Materials Corrosion Electrochemical Corrosion of metals Standard electrode half-cell potentials for metals v Those metals that are more reactive than hydrogen are assigned negative potentials and are said to be anodic to hydrogen. These metals are oxidize (corrode) to form ions, and hydrogen ions are reduced to form hydrogen gas. v. Those metals that are less reactive than hydrogen are assigned positive potentials and are said to be cathodic to hydrogen. These metals are reduced to the atomic state, and hydrogen gas is oxidize to hydrogen ions.

Engineering Materials Corrosion Galvanic Cells Macroscopic Galvanic cells with electrolytes that are one molar Macroscopic Galvanic cells with electrolytes that are not one molar Macroscopic Galvanic cells with acid or alkaline electrolytes with no metal ions present Microscopic Galvanic cells corrosion of single electrolytes Concentration Galvanic cells created by differences in composition, structure, and stress

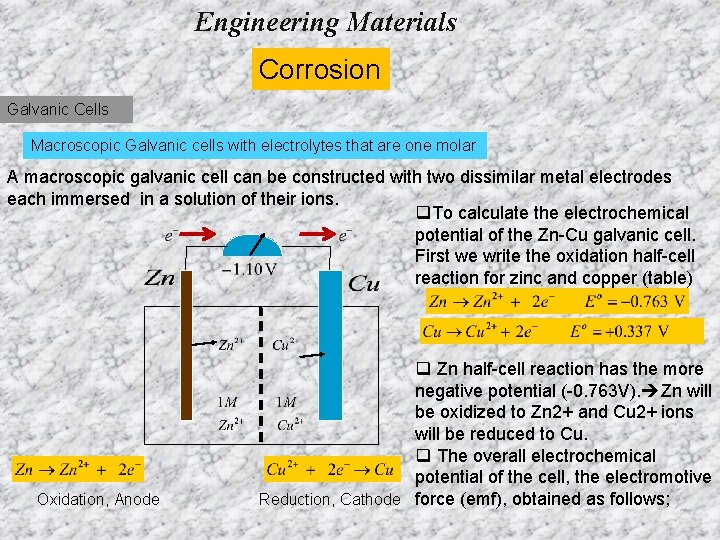
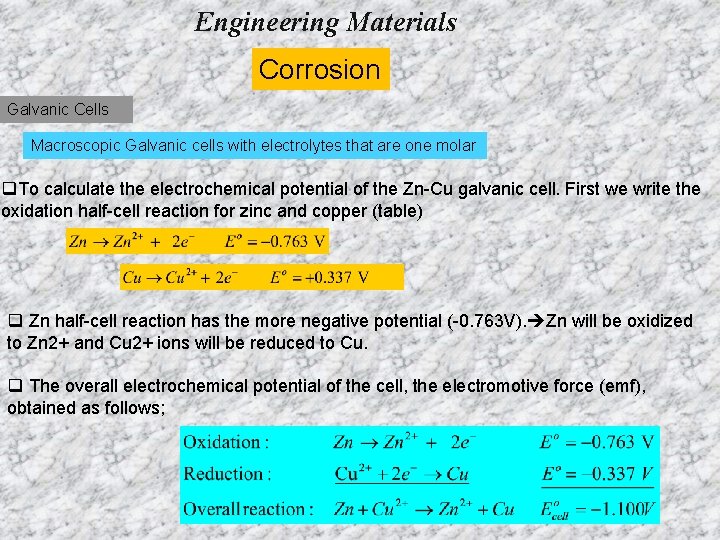
Engineering Materials Corrosion Galvanic Cells Macroscopic Galvanic cells with electrolytes that are one molar A macroscopic galvanic cell can be constructed with two dissimilar metal electrodes each immersed in a solution of their ions. q. To calculate the electrochemical potential of the Zn-Cu galvanic cell. First we write the oxidation half-cell reaction for zinc and copper (table) Oxidation, Anode q Zn half-cell reaction has the more negative potential (-0. 763 V). Zn will be oxidized to Zn 2+ and Cu 2+ ions will be reduced to Cu. q The overall electrochemical potential of the cell, the electromotive Reduction, Cathode force (emf), obtained as follows;

Engineering Materials Corrosion Galvanic Cells Macroscopic Galvanic cells with electrolytes that are one molar q. To calculate the electrochemical potential of the Zn-Cu galvanic cell. First we write the oxidation half-cell reaction for zinc and copper (table) q Zn half-cell reaction has the more negative potential (-0. 763 V). Zn will be oxidized to Zn 2+ and Cu 2+ ions will be reduced to Cu. q The overall electrochemical potential of the cell, the electromotive force (emf), obtained as follows;

Engineering Materials Corrosion Galvanic Cells Macroscopic Galvanic cells with electrolytes that are one molar Example: A galvanic cell consists of an electrode of zinc in a 1 M Zn. SO 4 solution and another of nickel in a 1 M Ni. SO 4 solution. The two electrodes are separated by a porous wall so that mixing of the solution is prevented. An external wire with a switch connects the two electrodes, when the switch is just closed: A- At which electrode does oxidation occur? B- Which electrode is the anode of the ell? C- Which electrode corrodes? D- What is the emf of this galvanic cell when the switch is just closed? (ans=-0. 513 V)

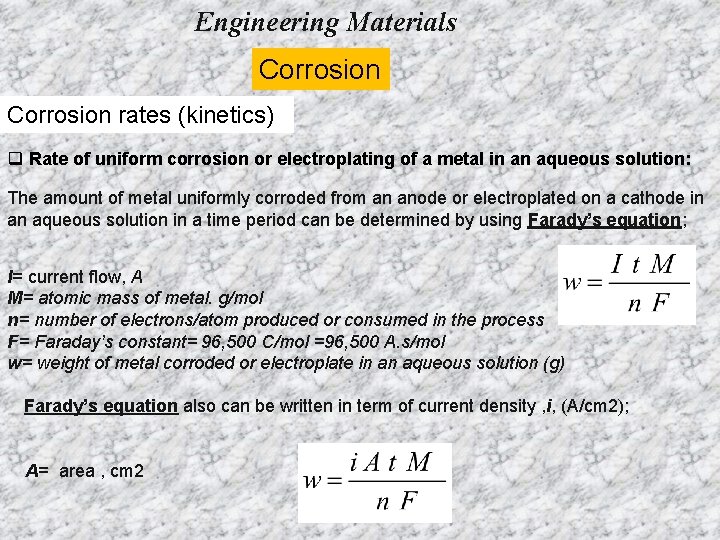
Engineering Materials Corrosion rates (kinetics) q Rate of uniform corrosion or electroplating of a metal in an aqueous solution: The amount of metal uniformly corroded from an anode or electroplated on a cathode in an aqueous solution in a time period can be determined by using Farady’s equation; I= current flow, A M= atomic mass of metal. g/mol n= number of electrons/atom produced or consumed in the process F= Faraday’s constant= 96, 500 C/mol =96, 500 A. s/mol w= weight of metal corroded or electroplate in an aqueous solution (g) Farady’s equation also can be written in term of current density , i, (A/cm 2); A= area , cm 2

Engineering Materials Corrosion rates (kinetics) Example 1: A copper electroplating process uses 15 A of current by chemically dissolving (corroding) a copper anode and electroplating a copper cathode. If it is assumed that there are no side reactions, how long will it take to corrode 8. 50 g of copper from the anode? Mcu=63. 5 g/mol (Ans=1722 s or 28. 7 min)

Engineering Materials Corrosion rates (kinetics) Example 2: A mild steel cylindrical tank 1 m high and 50 cm in diameter contains aerated water to the 60 cm level and shows a loss in weight due to corrosion of 304 g after six weeks. Calculate (a) the corrosion current and (b) the current density involved in the corrosion of the tank. Assume uniform corrosion on the tank’s inner surface and that the steel corrodes in the same manner as pure iron. (MFe=55. 85 g/mol). Ans a=0. 289 A, b=2. 53 x 10 -5 A/cm 2).

Engineering Materials Corrosion rates (kinetics) The units of corrosion rate could be; mdd= milligram weight loss per square decimeter per day. =mg/dm 2. d OR In terms of loss in depth of metal per unit time (mm/yr) or (mils/yr) Example 3: The wall of a steel tank containing aerated water is corroding at a rate of 54. 7 mdd. How long will it take for the wall thickness to decrease by 0. 50 mm? Fe density=7. 87 g/cm 3), Ans=719 days Example 4: A sample of zinc corrodes uniformly with a current density of 4. 27 x 10 -7 A/cm 2 in an aqueous solution. What is the corrosion rate of the zinc in milligrams per decimeter per day? The reaction for oxidation of zinc is Zn Zn 2+ +2 e-. (M of Zn=65. 38 g/mol) Ans=1. 25 mdd

Engineering Materials Corrosion Galvanic Cells Macroscopic Galvanic cells with electrolytes that are one molar Macroscopic Galvanic cells with electrolytes that are not one molar Macroscopic Galvanic cells with acid or alkaline electrolytes with no metal ions present Microscopic Galvanic cells corrosion of single electrolytes Concentration Galvanic cells created by differences in composition, structure, and stress

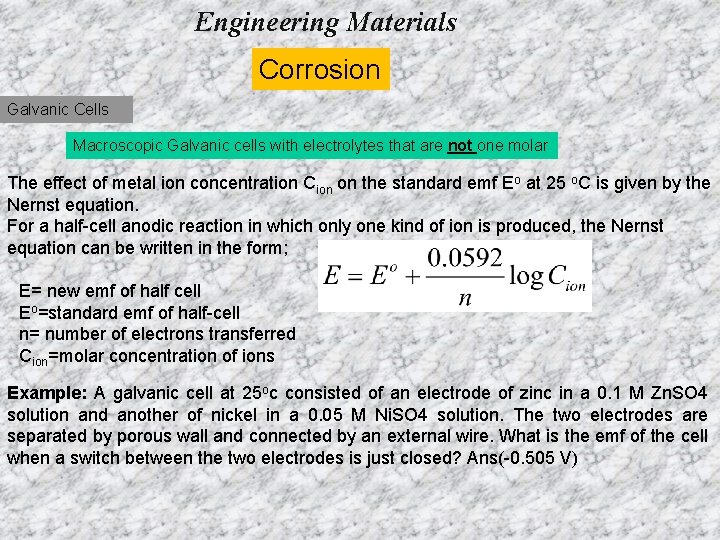
Engineering Materials Corrosion Galvanic Cells Macroscopic Galvanic cells with electrolytes that are not one molar The effect of metal ion concentration Cion on the standard emf Eo at 25 o. C is given by the Nernst equation. For a half-cell anodic reaction in which only one kind of ion is produced, the Nernst equation can be written in the form; E= new emf of half cell Eo=standard emf of half-cell n= number of electrons transferred Cion=molar concentration of ions Example: A galvanic cell at 25 oc consisted of an electrode of zinc in a 0. 1 M Zn. SO 4 solution and another of nickel in a 0. 05 M Ni. SO 4 solution. The two electrodes are separated by porous wall and connected by an external wire. What is the emf of the cell when a switch between the two electrodes is just closed? Ans(-0. 505 V)

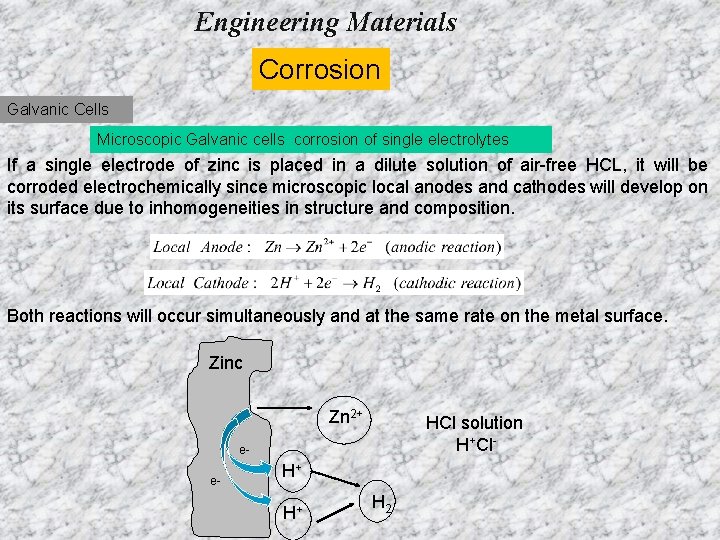
Engineering Materials Corrosion Galvanic Cells Microscopic Galvanic cells corrosion of single electrolytes If a single electrode of zinc is placed in a dilute solution of air-free HCL, it will be corroded electrochemically since microscopic local anodes and cathodes will develop on its surface due to inhomogeneities in structure and composition. Both reactions will occur simultaneously and at the same rate on the metal surface. Zinc Zn 2+ HCl solution H+Cl- ee- H+ H+ H 2

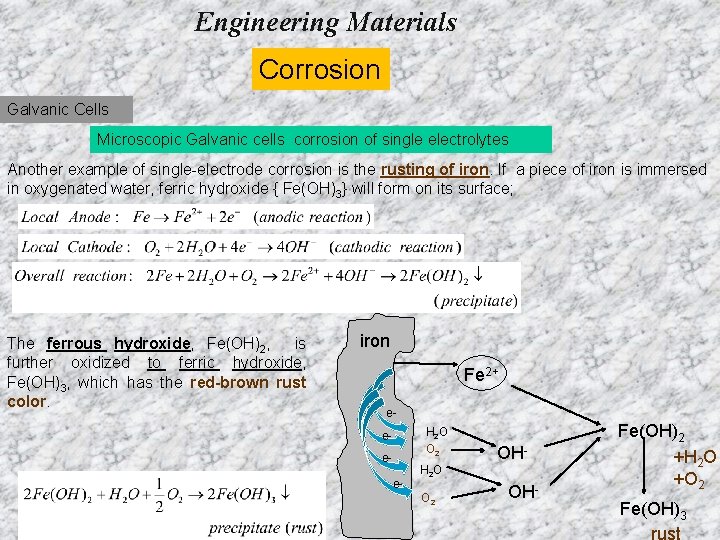
Engineering Materials Corrosion Galvanic Cells Microscopic Galvanic cells corrosion of single electrolytes Another example of single-electrode corrosion is the rusting of iron. If a piece of iron is immersed in oxygenated water, ferric hydroxide { Fe(OH)3} will form on its surface; The ferrous hydroxide, Fe(OH)2, is further oxidized to ferric hydroxide, Fe(OH)3, which has the red-brown rust color. iron Fe 2+ e- H 2 O O 2 eee- OH- H 2 O O 2 OH- Fe(OH)2 +H 2 O +O 2 Fe(OH)3

Engineering Materials Corrosion Galvanic Cells Microscopic Galvanic cells corrosion of single electrolytes Example: write the anodic and cathodic half-cell reactions for an iron electrode immersed in an oxygenated water solution.
 Wear vs deterioration in software engineering
Wear vs deterioration in software engineering Dry corrosion and wet corrosion
Dry corrosion and wet corrosion Dry corrosion and wet corrosion
Dry corrosion and wet corrosion Ninds-airen criteria
Ninds-airen criteria Osmophillic
Osmophillic Reason of food spoilage
Reason of food spoilage Cant stop the feeling go noodle
Cant stop the feeling go noodle 10 useful and harmful materials
10 useful and harmful materials Man made map
Man made map Differentiate adopting materials and adapting materials
Differentiate adopting materials and adapting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Manufacturing processes for engineering materials
Manufacturing processes for engineering materials Heat conductivity examples
Heat conductivity examples What is the engineering materials
What is the engineering materials Optical properties of engineering materials
Optical properties of engineering materials Materials: engineering, science, processing and design
Materials: engineering, science, processing and design Classification of engineering materials chart
Classification of engineering materials chart Engineering materials
Engineering materials Materials for engineering
Materials for engineering