Electrostatics Are you Positive Charges at rest are



















- Slides: 26

Electrostatics

Are you Positive? Charges at rest are referred to as static electricity. The distribution of charge and the interactions with other charged objects is electrostatics. Recall that the electron is the only particle around an atom that can be removed or added from the atom. Metals hold onto their electrons weakly and so electrons flow easily through them. An object becomes charged by the addition or removal of electrons.

Feeling negative? An object becomes negatively charged by gaining electrons. (There are more negative charges than there are protons (locked in the nucleus) in the material) An object becomes positively charged by losing electrons. (This leaves behind the positive protons in the nucleus so the object has more positive than negative charges. ) An object is neutral when it has an equal number of positive and negative charges.

Charging by Friction: the transfer of electrons between two different neutral objects when they are rubbed together charging by friction video

Charging by Friction When two objects rub together (like clothes in a dryer) then charges transfer between the clothes due to their different electron holding strengths. Some materials like to take electrons from other materials (like cotton and silk, polyester, rayon etc. ). Scientists tested many materials and organized them into a list based on electron holding strength called the Electrostatic Series.

Electrostatic Series: a short list tendency to attract electrons high low rubber polyethylene ebonite silk fur wool glass acetate relative charge negative positive

Using the electrostatic series: look up the two materials being rubbed together. The one higher on the series will become negative, the other will become positive tendency to attract electrons high low rubber polyethylene ebonite silk fur wool glass acetate relative charge negative positive

Using the electrostatic series: look up the two materials being rubbed together. The one higher on the series will become negative, the other will become positive tendency to attract electrons high low rubber polyethylene ebonite silk fur wool glass acetate relative charge negative positive e. g. ebonite rubbed with fur

Using the electrostatic series: look up the two materials being rubbed together. The one higher on the series will become negative, the other will become positive tendency to attract electrons high low rubber polyethylene ebonite silk fur wool glass acetate relative charge negative positive e. g. ebonite rubbed with fur -ebonite becomes negative because it is further up on the series

Using the electrostatic series: look up the two materials being rubbed together. The one higher on the series will become negative, the other will become positive Try these: a) rubber rubbed with silk b) wool rubbed on glass c) polyethylene rubbed with glass

Using the electrostatic series: look up the two materials being rubbed together. The one higher on the series will become negative, the other will become positive Try these: a) rubber rubbed with silk rubber = negative silk = positive b) wool rubbed on glass c) polyethylene rubbed with glass

Using the electrostatic series: look up the two materials being rubbed together. The one higher on the series will become negative, the other will become positive Try these: a) rubber rubbed with silk rubber = negative silk = positive b) wool rubbed on glass wool = negative glass = positive c) polyethylene rubbed with glass

Using the electrostatic series: look up the two materials being rubbed together. The one higher on the series will become negative, the other will become positive Try these: a) rubber rubbed with silk rubber = negative silk = positive b) wool rubbed on glass wool = negative glass = positive c) polyethylene rubbed with glass polyethylene = negative glass = positive

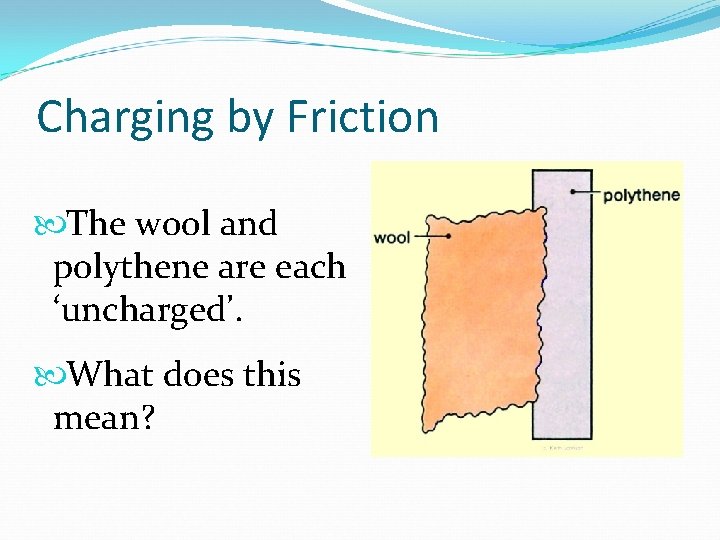
Charging by Friction The wool and polythene are each ‘uncharged’. What does this mean?

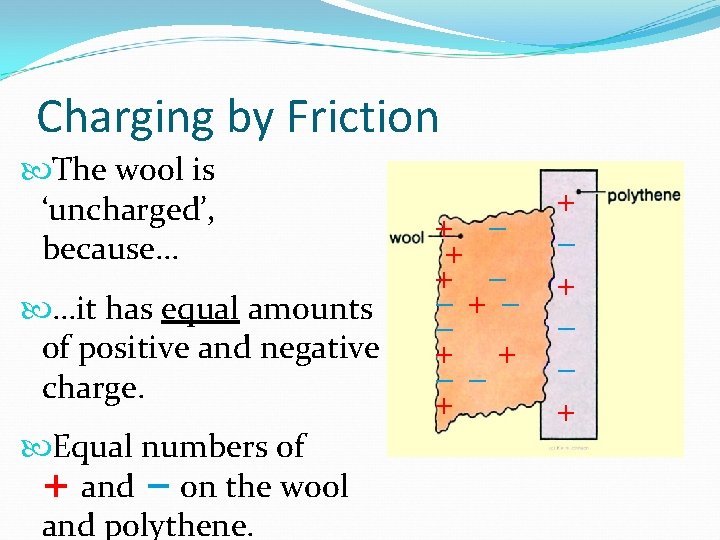
Charging by Friction The wool is ‘uncharged’, because… …it has equal amounts of positive and negative charge. Equal numbers of + and − on the wool and polythene. + − + + − −+− − + + − + − − +

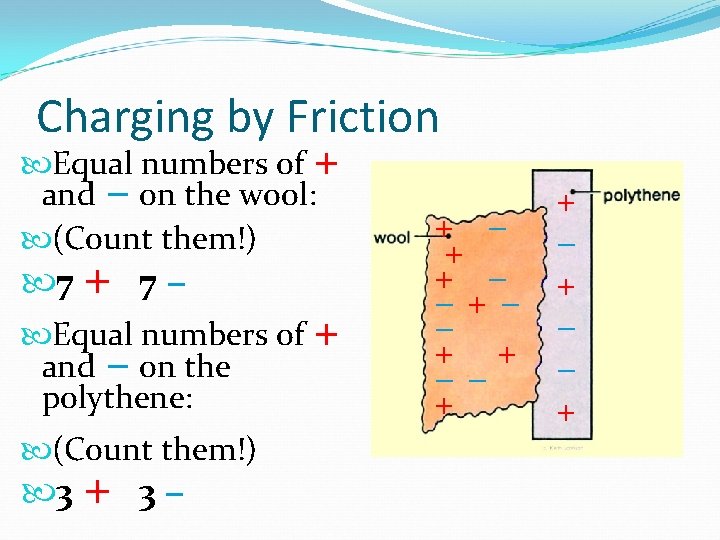
Charging by Friction Equal numbers of + and − on the wool: (Count them!) 7 + 7 − Equal numbers of + and − on the polythene: (Count them!) 3 + 3 − + + − −+− − + + − + − − +

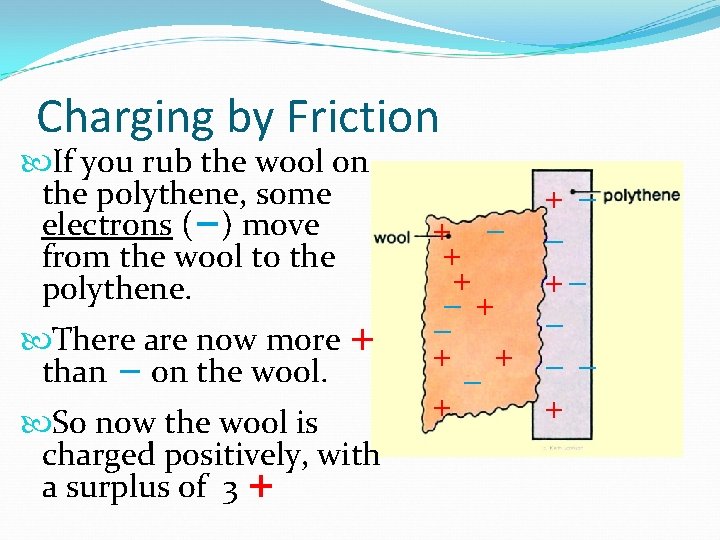
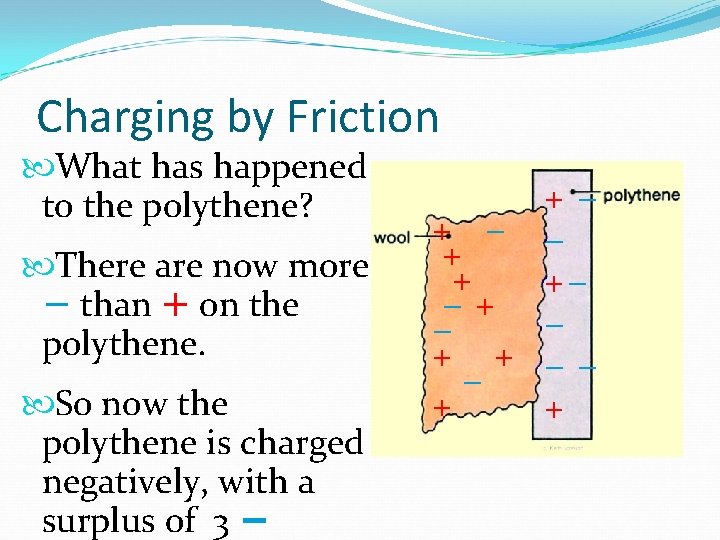
Charging by Friction If you rub the wool on the polythene, some electrons (−) move from the wool to the polythene. There are now more + than − on the wool. So now the wool is charged positively, with a surplus of 3 + + −+ − + +− − −− +

Charging by Friction What has happened to the polythene? There are now more − than + on the polythene. So now the polythene is charged negatively, with a surplus of 3 − + + −+ − + +− − −− +

Charging by Friction Both objects are now equally charged, with opposite charges because electrons − (only) have moved.

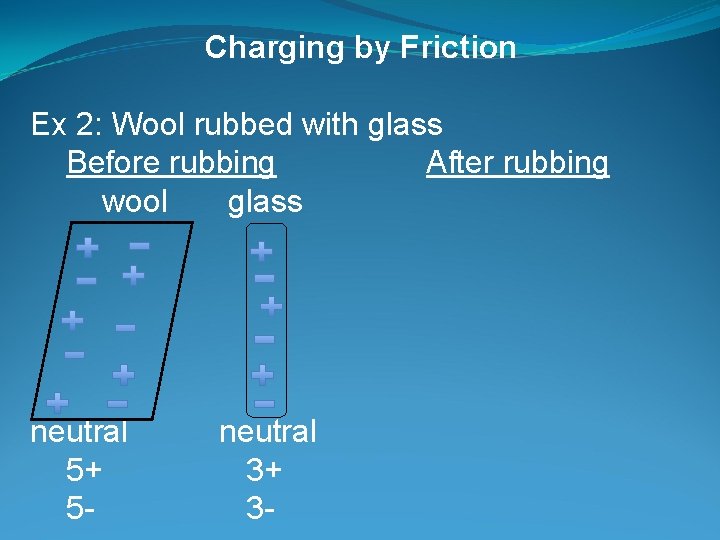
Charging by Friction Ex 2: Wool rubbed with glass Before rubbing After rubbing wool glass neutral 5+ 5 - neutral 3+ 3 -

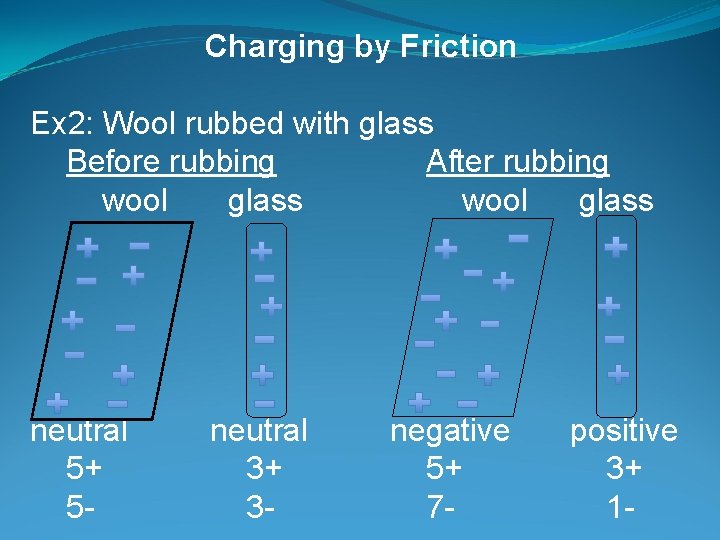
Charging by Friction Ex 2: Wool rubbed with glass Before rubbing After rubbing wool glass neutral 5+ 5 - neutral 3+ 3 - negative 5+ 7 - positive 3+ 1 -

Charging by Friction try this one: ebonite rubbed with fur

Charging by Friction try this one: ebonite rubbed with fur Before rubbing After rubbing fur ebonite neutral 5+ 5 - neutral 3+ 3 -

Charging by Friction try this one: ebonite rubbed with fur Before rubbing After rubbing fur ebonite neutral 5+ 5 - neutral 3+ 3 - positive 5+ 3 - negative 3+ 5 -

Law of Electric Charges 1) Opposite Charges attract (Static cling, paint, etc. ) 2) Like Charges Repel (hair with Van de Graaff generator. ) 3) Neutral objects can be attracted to charged objects by Induced Charge Separation. (Balloon stuck to wall)

Class/home work Page 471 #3 -8 Page 477 #1, 2, 8