Electrostatics and Nuclear Processes From The Physics Classroom







- Slides: 13

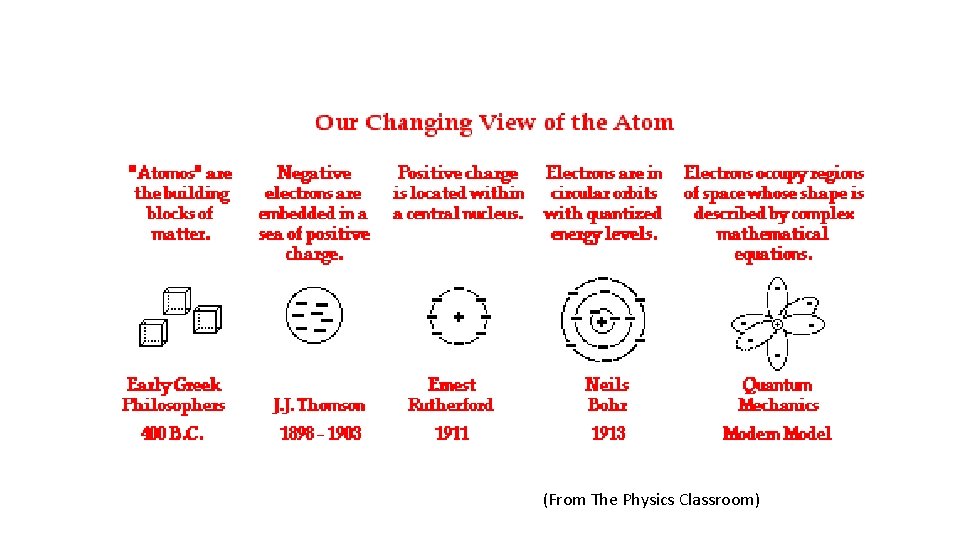
Electrostatics and Nuclear Processes

(From The Physics Classroom)


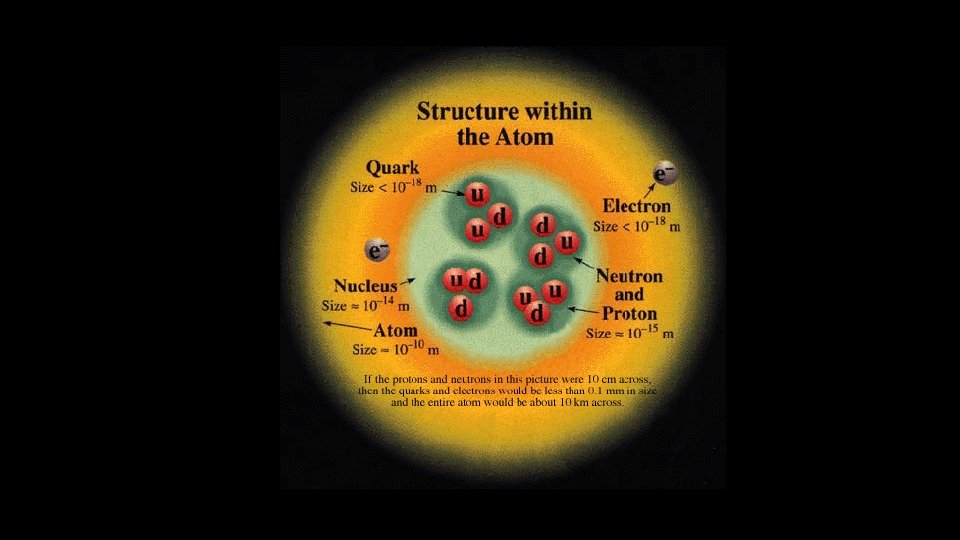
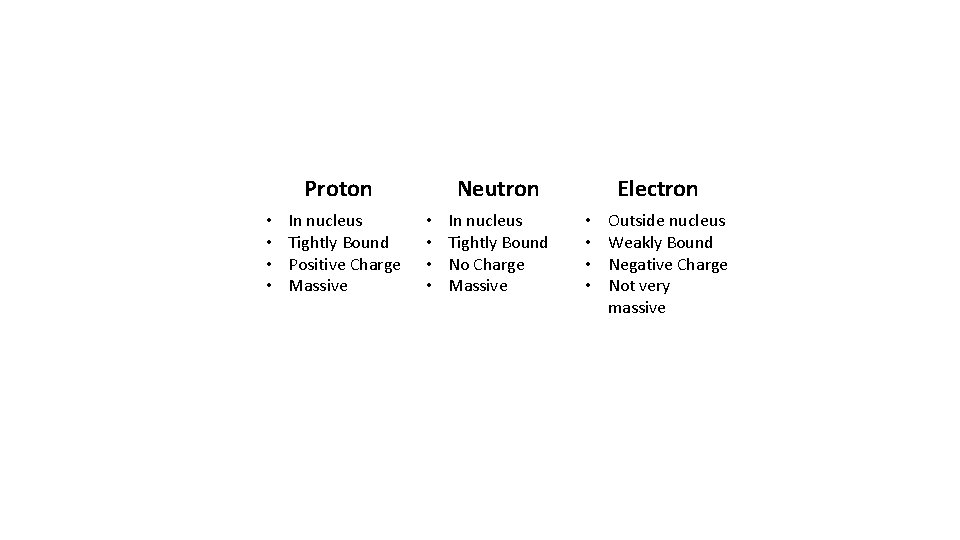
Proton • • In nucleus Tightly Bound Positive Charge Massive Neutron • • In nucleus Tightly Bound No Charge Massive Electron • • Outside nucleus Weakly Bound Negative Charge Not very massive

The charge on a single electron is -1. 6 x 10 -19 Coulomb. The charge on a single proton is +1. 6 x 10 -19 Coulomb. The quantity of charge on an object reflects the amount of imbalance between electrons and protons on that object.

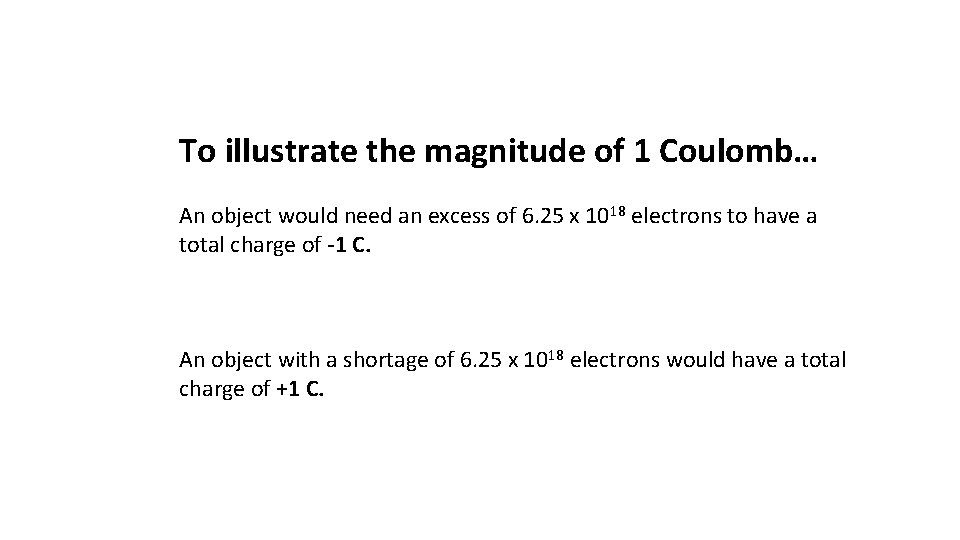
To illustrate the magnitude of 1 Coulomb… An object would need an excess of 6. 25 x 1018 electrons to have a total charge of -1 C. An object with a shortage of 6. 25 x 1018 electrons would have a total charge of +1 C.

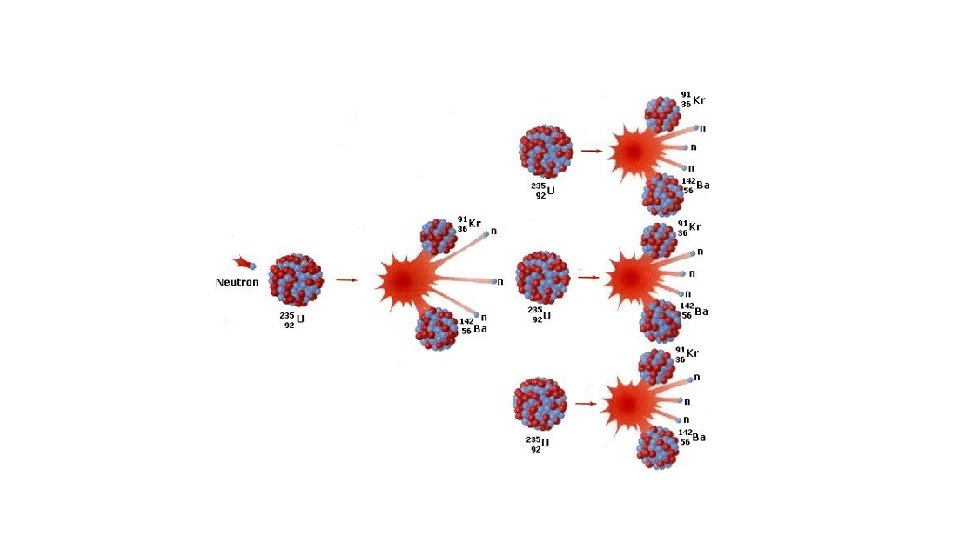
Nuclear Fission Nuclear Fusion Definition Fission is the splitting of a large atom into two or more smaller ones. Fusion is the fusing of two or more lighter atoms into a larger one. Natural occurrence of the process Fission reaction does not normally Fusion occurs in stars, such as the occur in nature. sun. Few radioactive particles are produced by fusion reaction, but if a fission "trigger" is used, radioactive particles will result from that. By-products of the reaction Fission produces many highly radioactive particles. Conditions Critical mass of the substance and High density, high temperature high-speed neutrons are required. environment is required. Extremely high energy is required to bring two or more protons close enough that nuclear forces overcome their electrostatic repulsion. Energy Requirement Takes little energy to split two atoms in a fission reaction. Energy Released The energy released by fission is a million times greater than that The energy released by fusion is released in chemical reactions, three to four times greater than but lower than the energy released by fission. released by nuclear fusion.

Where does the energy come from? If you measure the masses of all the atoms and subatomic particles you start with and all the atoms and subatomic particles you end up with, and then compare the two, you find that there's some "missing" mass. (called “mass defect”) Matter actually disappears during the nuclear reaction. The missing matter is converted into energy.

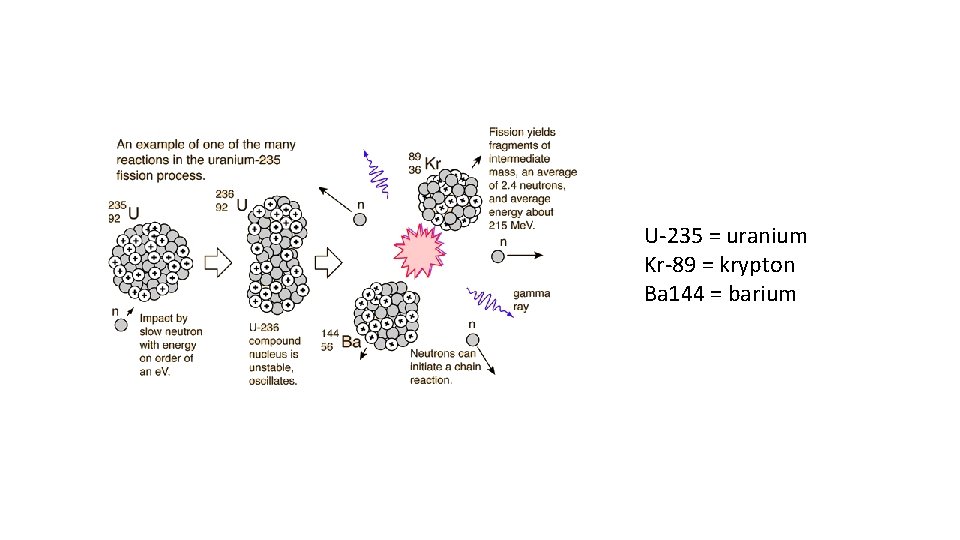
U-235 = uranium Kr-89 = krypton Ba 144 = barium


How is energy created in fusion?


High temperature The high temperature gives the input atoms enough energy to overcome the electrical repulsion between the protons. • Fusion requires temperatures about 100 million Kelvin (approximately six times hotter than the sun's core). • At these temperatures, elements become plasma, not gas. Plasma is a highenergy state of matter in which all the electrons are stripped from atoms and move freely about. High pressure Pressure squeezes the atoms together. They must be within 1 x 10 -15 meters of each other to fuse.