ELECTROPHORETIC METHODS Principles of Electrophoresis is the process



- Slides: 18

ELECTROPHORETIC METHODS

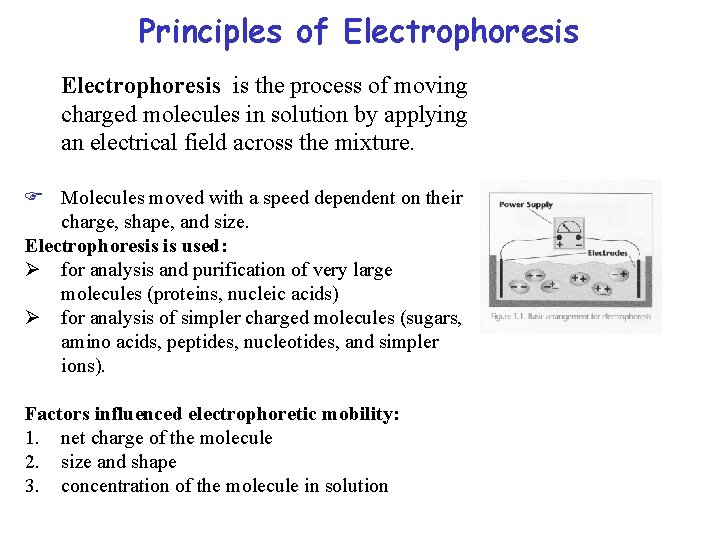
Principles of Electrophoresis is the process of moving charged molecules in solution by applying an electrical field across the mixture. F Molecules moved with a speed dependent on their charge, shape, and size. Electrophoresis is used: Ø for analysis and purification of very large molecules (proteins, nucleic acids) Ø for analysis of simpler charged molecules (sugars, amino acids, peptides, nucleotides, and simpler ions). Factors influenced electrophoretic mobility: 1. net charge of the molecule 2. size and shape 3. concentration of the molecule in solution

Electrophoresis is carried out by applying a thin layer Aqueous protein solution is immobilized in a solid hydrophilic support, Solid matrix with poreswhich are used: • Paper • Cellulose acetate • Different gels – polyacrylamide, agarose, starch Molecules in the sample move through porous matrix at different velocity. Electrophoresis can be one dimensional (i. e. one plane of separation) or two dimensional. • One dimensional electrophoresis is used for most routine protein and nucleic acid separations. • Two dimensional separation of proteins is used for finger printing , and when properly constructed can be extremely accurate in resolving all of the proteins present within a cell (greater than 1, 500). Most common stabilizing media - polyacrylamide or agarose gels

Zone electrophoresis Much simple method • greater resolution • require small sample support medium - acetate cellulose Protein separation depends on : • Type and number of ionizable side chains of amino acids - R. • Size of net charge (pozitive or negative). • negatively charged proteins move towards the anode • positively charged proteins move towards the cathode

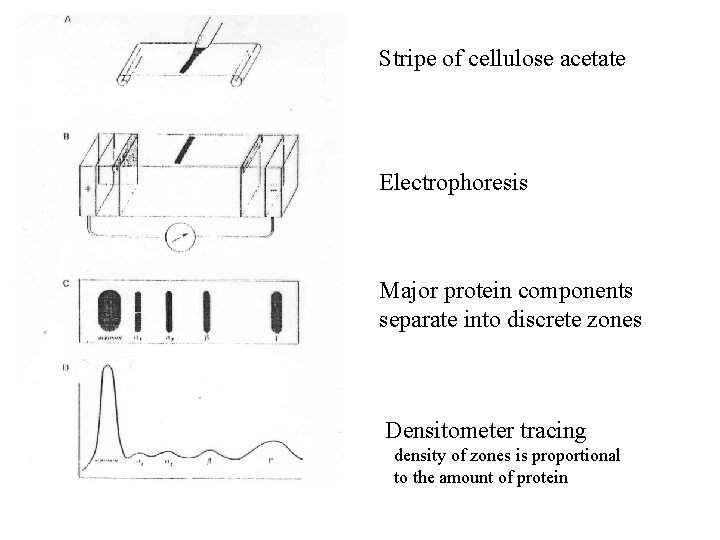
Stripe of cellulose acetate Electrophoresis Major protein components separate into discrete zones Densitometer tracing density of zones is proportional to the amount of protein

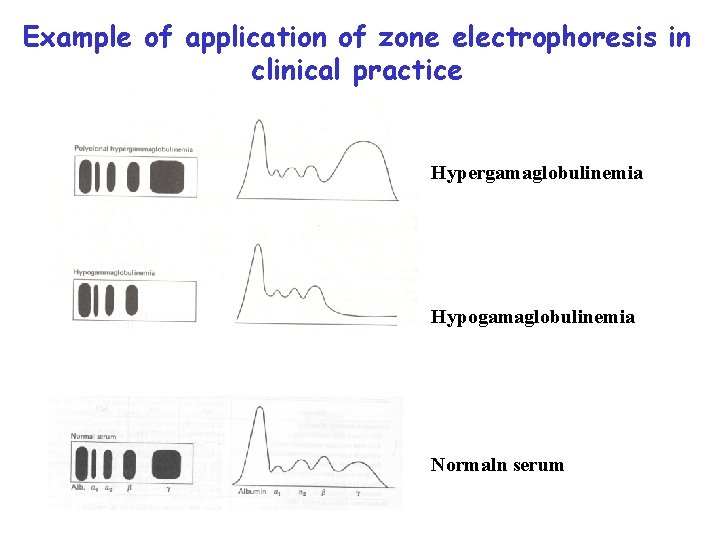
Example of application of zone electrophoresis in clinical practice Hypergamaglobulinemia Hypogamaglobulinemia Normaln serum

Gel electrophoresis Gel is a colloid in a solid form (99% is water) • Gels act as a "molecular sieve • Macromolecules are forced to move through the pores when the electrical current is applied. • The rate of migration through the electric field depends on: - the strength of the field, size and shape of the molecules, - relative hydrophobicity of the samples, and on the ionic strength - temperature of the buffer in which the molecules are moving. • After staining, the separated macromolecules in each lane can be seen in a series of bands spread from one end of the gel to the other.

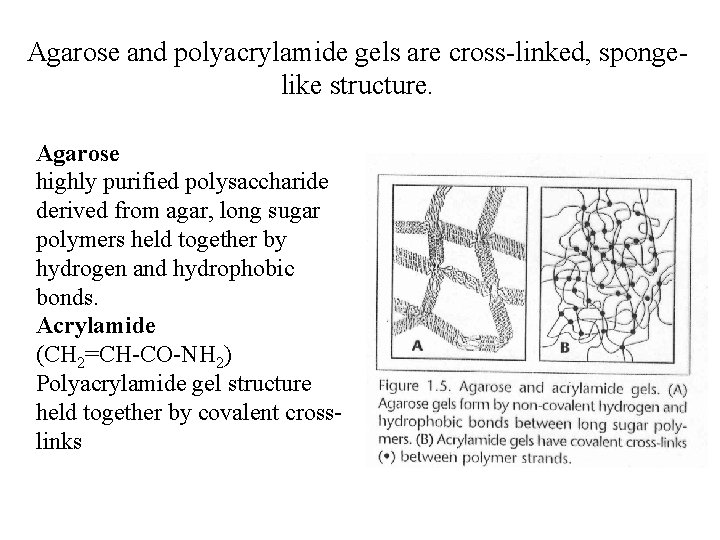
Agarose and polyacrylamide gels are cross-linked, spongelike structure. Agarose highly purified polysaccharide derived from agar, long sugar polymers held together by hydrogen and hydrophobic bonds. Acrylamide (CH 2=CH-CO-NH 2) Polyacrylamide gel structure held together by covalent crosslinks

SDS-polyacrylamide gel electrophoresis (SDSPAGE) • • Sodium dodecyl supfate (SDS, also called lauryl sulfate) - anionic detergent Molecules in solution with SDS are denaturated and have a net negative charge (p. H range 7 – 10) A polypeptide chain binds amounts of SDS in proportion to its relative molecuar mass. The negative charges on SDS destroy most of the complex structure of proteins, and are strongly attracted toward an anode (positively-charged electrode) in an electric field.

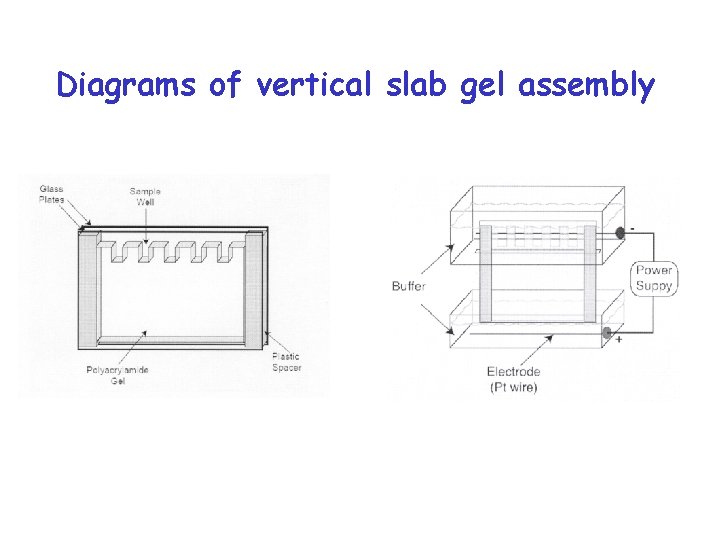
Diagrams of vertical slab gel assembly

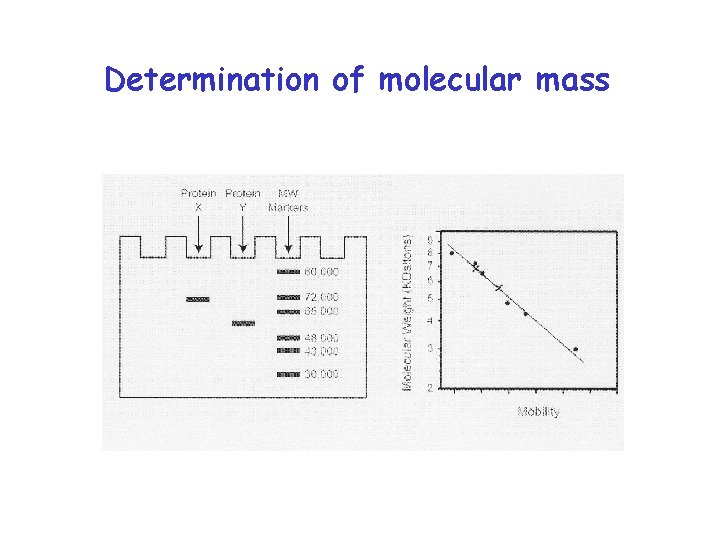
Determination of molecular mass

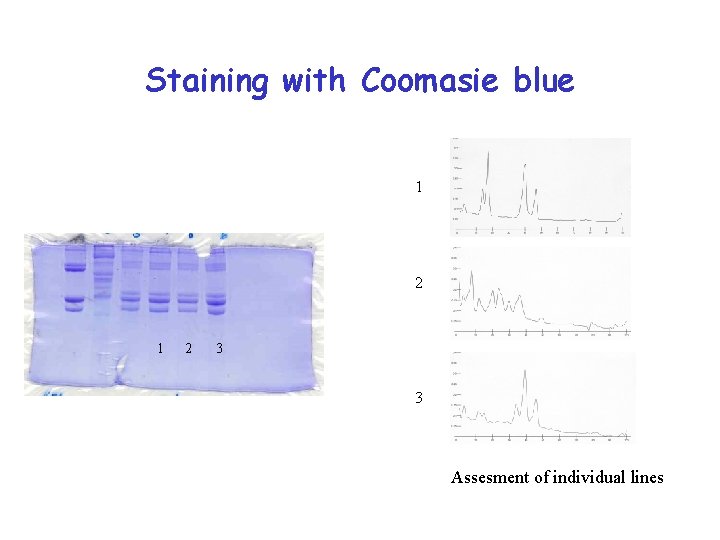
Staining with Coomasie blue 1 2 3 3 Assesment of individual lines

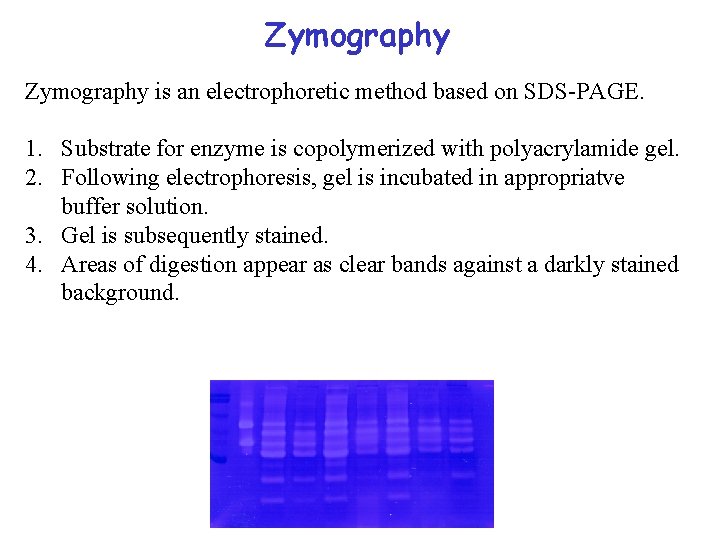
Zymography is an electrophoretic method based on SDS-PAGE. 1. Substrate for enzyme is copolymerized with polyacrylamide gel. 2. Following electrophoresis, gel is incubated in appropriatve buffer solution. 3. Gel is subsequently stained. 4. Areas of digestion appear as clear bands against a darkly stained background.

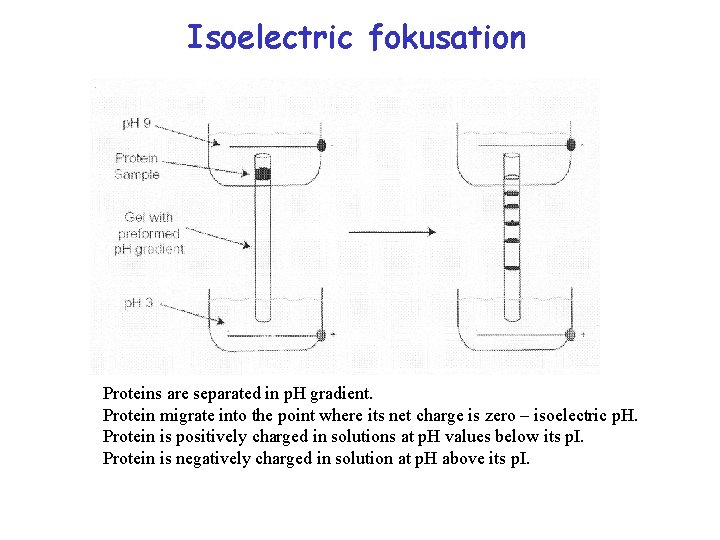
Isoelectric fokusation Proteins are separated in p. H gradient. Protein migrate into the point where its net charge is zero – isoelectric p. H. Protein is positively charged in solutions at p. H values below its p. I. Protein is negatively charged in solution at p. H above its p. I.

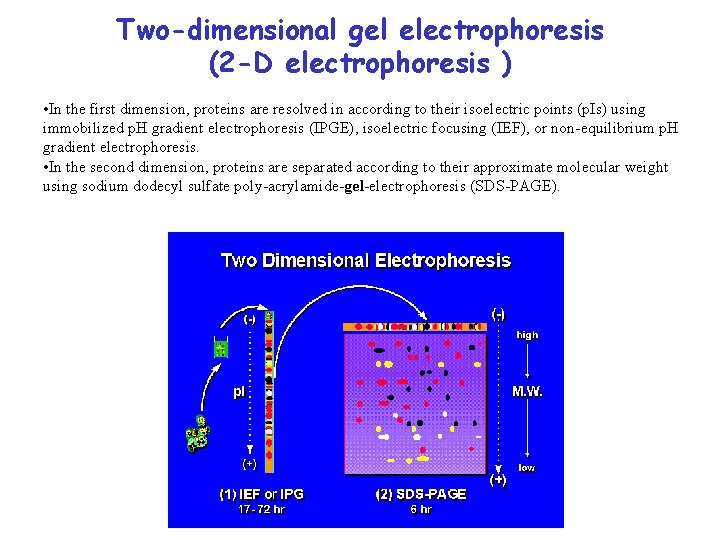
Two-dimensional gel electrophoresis (2 -D electrophoresis ) • In the first dimension, proteins are resolved in according to their isoelectric points (p. Is) using immobilized p. H gradient electrophoresis (IPGE), isoelectric focusing (IEF), or non-equilibrium p. H gradient electrophoresis. • In the second dimension, proteins are separated according to their approximate molecular weight using sodium dodecyl sulfate poly-acrylamide-gel-electrophoresis (SDS-PAGE).

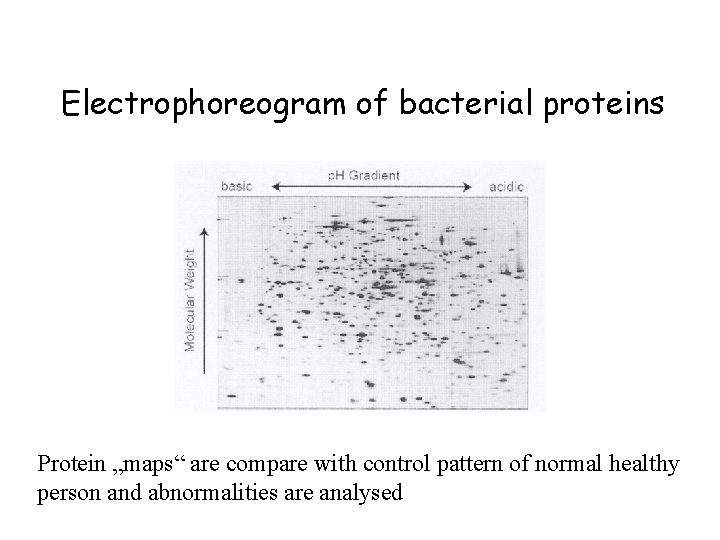
Electrophoreogram of bacterial proteins Protein „maps“ are compare with control pattern of normal healthy person and abnormalities are analysed

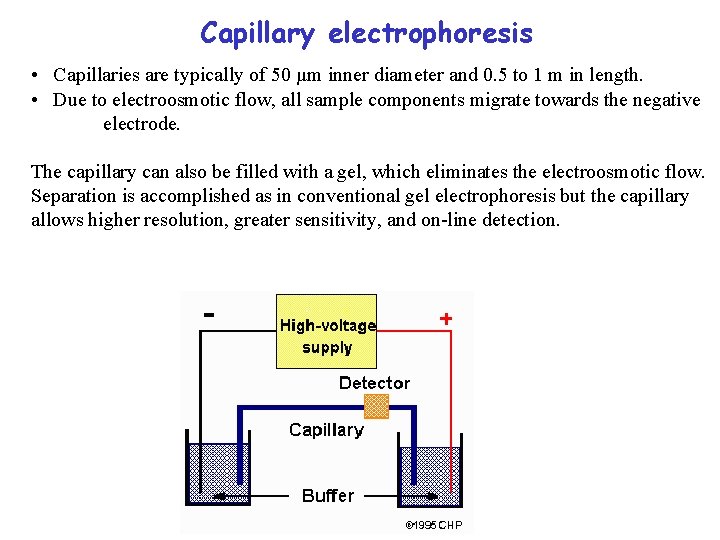
Capillary electrophoresis • Capillaries are typically of 50 µm inner diameter and 0. 5 to 1 m in length. • Due to electroosmotic flow, all sample components migrate towards the negative electrode. The capillary can also be filled with a gel, which eliminates the electroosmotic flow. Separation is accomplished as in conventional gel electrophoresis but the capillary allows higher resolution, greater sensitivity, and on-line detection.

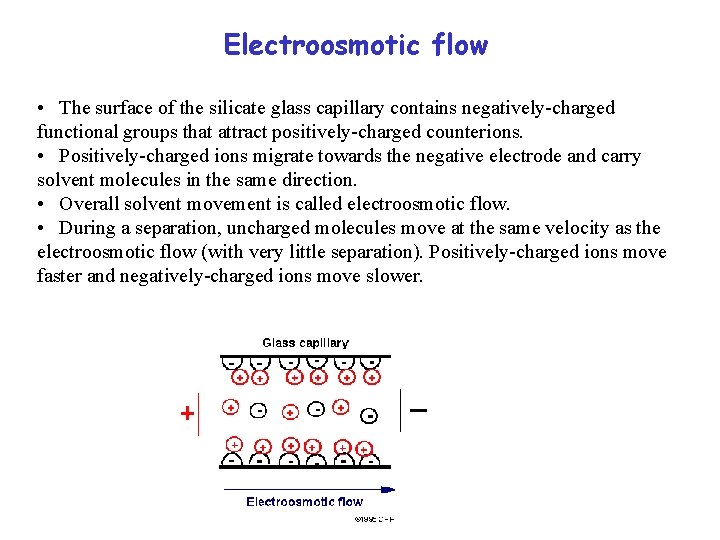
Electroosmotic flow • The surface of the silicate glass capillary contains negatively-charged functional groups that attract positively-charged counterions. • Positively-charged ions migrate towards the negative electrode and carry solvent molecules in the same direction. • Overall solvent movement is called electroosmotic flow. • During a separation, uncharged molecules move at the same velocity as the electroosmotic flow (with very little separation). Positively-charged ions move faster and negatively-charged ions move slower.