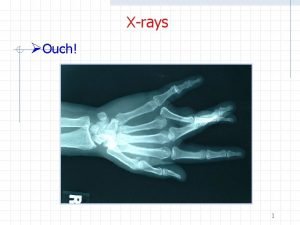
Electromagnetic Radiation and XRays Its of no use
![Electromagnetic Radiation and X-Rays "It's of no use whatsoever[. . . ] this is Electromagnetic Radiation and X-Rays "It's of no use whatsoever[. . . ] this is](https://slidetodoc.com/presentation_image_h2/1d5aaa7ac321327a9bba9a020d0ec30f/image-1.jpg)






- Slides: 23
![Electromagnetic Radiation and XRays Its of no use whatsoever this is Electromagnetic Radiation and X-Rays "It's of no use whatsoever[. . . ] this is](https://slidetodoc.com/presentation_image_h2/1d5aaa7ac321327a9bba9a020d0ec30f/image-1.jpg)
Electromagnetic Radiation and X-Rays "It's of no use whatsoever[. . . ] this is just an experiment that proves Maestro Maxwell was right - we just have these mysterious electromagnetic waves that we cannot see with the naked eye. But they are there. " Heinrich Hertz 1

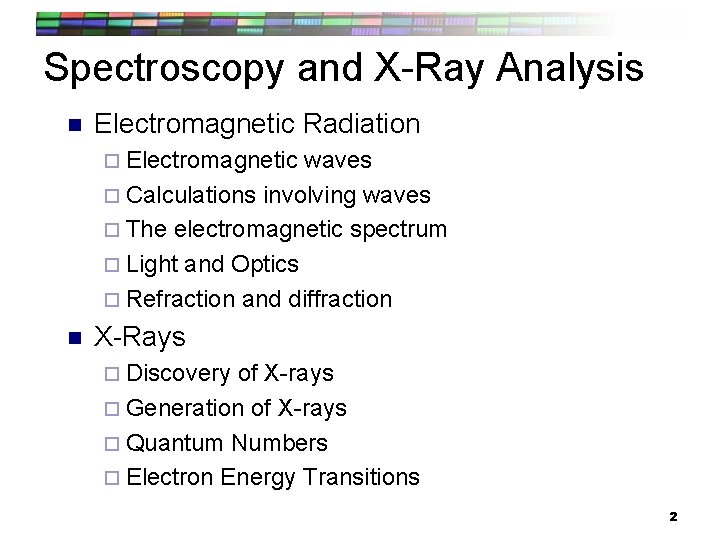
Spectroscopy and X-Ray Analysis n Electromagnetic Radiation ¨ Electromagnetic waves ¨ Calculations involving waves ¨ The electromagnetic spectrum ¨ Light and Optics ¨ Refraction and diffraction n X-Rays ¨ Discovery of X-rays ¨ Generation of X-rays ¨ Quantum Numbers ¨ Electron Energy Transitions 2

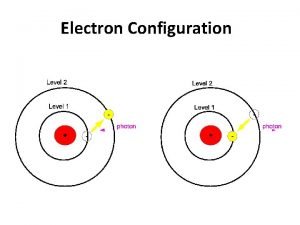
The Electromagnetic Waves Insert electromagnetic wave image here Light waves are self propagating waves that consist of both an electronic and magnetic component. 3

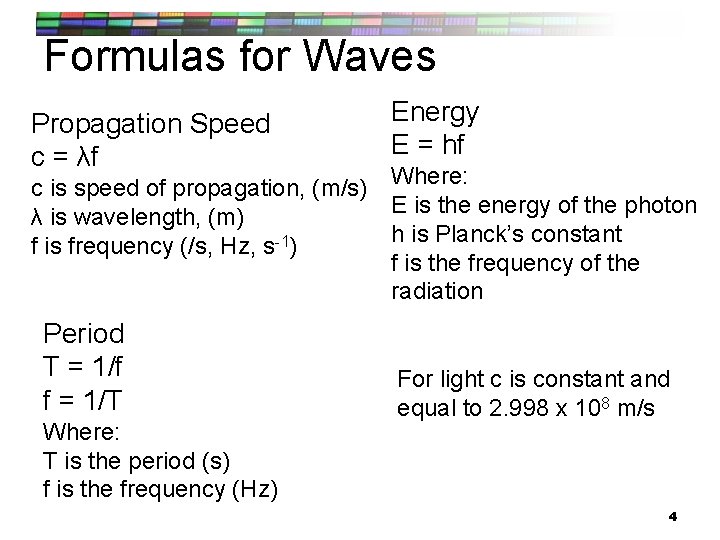
Formulas for Waves Propagation Speed c = λf Energy E = hf c is speed of propagation, (m/s) Where: E is the energy of the photon λ is wavelength, (m) h is Planck’s constant f is frequency (/s, Hz, s-1) f is the frequency of the radiation Period T = 1/f f = 1/T Where: T is the period (s) f is the frequency (Hz) For light c is constant and equal to 2. 998 x 108 m/s 4

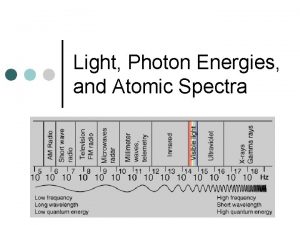
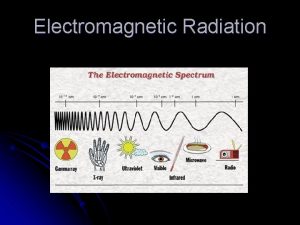
The Electromagnetic Spectrum Insert electromagnetic spectrum picture here 5

EM Radiation Activity n You will each be assigned one of the following types of electromagnetic radiation. Look it up. Report the following information for it: ¨ Wavelength ¨ How it is generated ¨ What it are some common uses n Gamma rays, X-rays, Ultraviolet radiation, Light, Infra-red radiation, Microwaves, Radio waves (FM, AM, ELF), Gravity waves. 6

Calculations Calculate the frequency of a red laser pointer light with wavelength 655 nm. 7

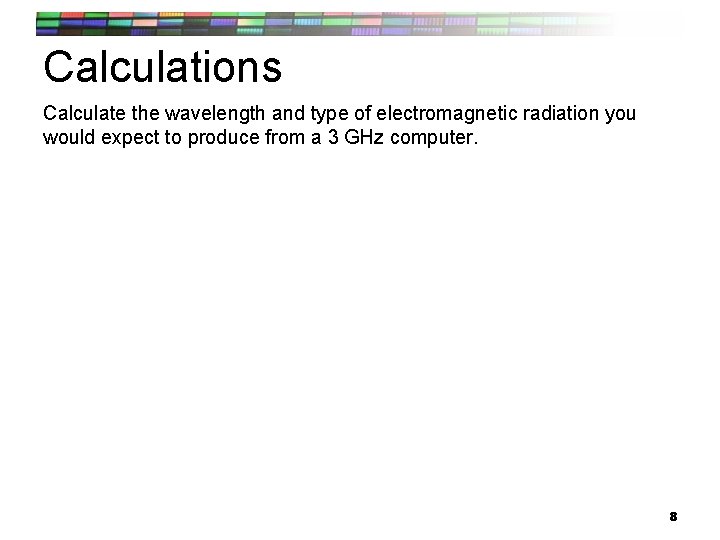
Calculations Calculate the wavelength and type of electromagnetic radiation you would expect to produce from a 3 GHz computer. 8

Calculations A common unit in spectroscopy is the “wave number” which is usually defined as the number of waves per cm. How many wave cycles per cm (wave numbers) would you expect to find in radiation produced from a microwave oven operating at a frequency of 2450 MHz? 9

Calculations Copper emits a kα X-ray of 8. 04 ke. V. What would the wavelength be? 10

Light and Optics n Electromagnetic radiation ¨ What we see as light is part of the electromagnetic spectrum. ¨ Photon: a unit of electromagnetic energy (light). Photons have no electric charge, they have zero “rest mass” but they do have momentum and energy. ¨ http: //hyperphysics. phy-astr. gsu. edu/hbase/emwav. html#c 1 http: //en. wikipedia. org/wiki/Electromagnetic_radiation 11

Discovery of X-rays 1895 Insert Wilhelm Roentgen image here Insert image of the first X-ray here Wilhelm Röntgen http: //en. wikipedia. org/wiki/X-ray 12

X-ray Tube Insert X-ray tube image here 13

Two methods for generating X-rays Bremsstrahlung / Braking Insert image Ionization / Characteristic Insert image http: //www. antonine-education. co. uk/Physics_A 2/Options/Module_6/Topic_7/topic_7_x. htm 14

X-Ray Analysis n n n Quantum numbers Electron Shells Allowed electron transitions Insert image http: //www 4. nau. edu/microanalysis/Microprobe/Probe. html 15

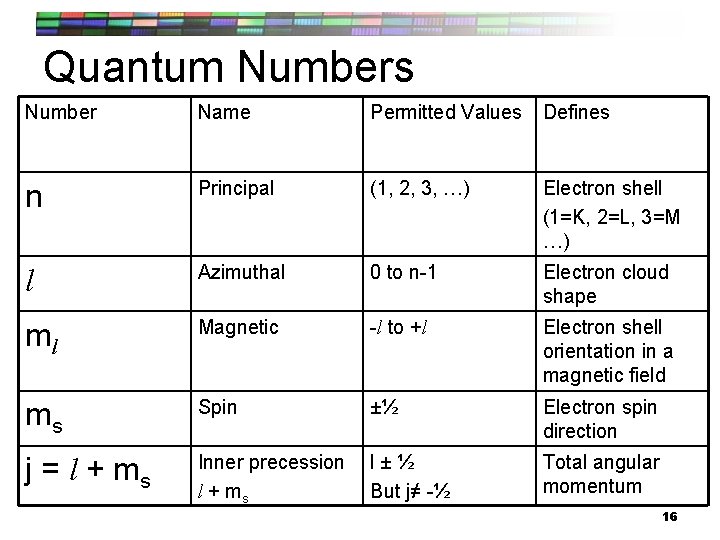
Quantum Numbers Number Name Permitted Values Defines n Principal (1, 2, 3, …) Electron shell (1=K, 2=L, 3=M …) l Azimuthal 0 to n-1 Electron cloud shape ml Magnetic -l to +l Electron shell orientation in a magnetic field ms Spin ±½ Electron spin direction j = l + ms Inner precession l + ms l±½ But j≠ -½ Total angular momentum 16

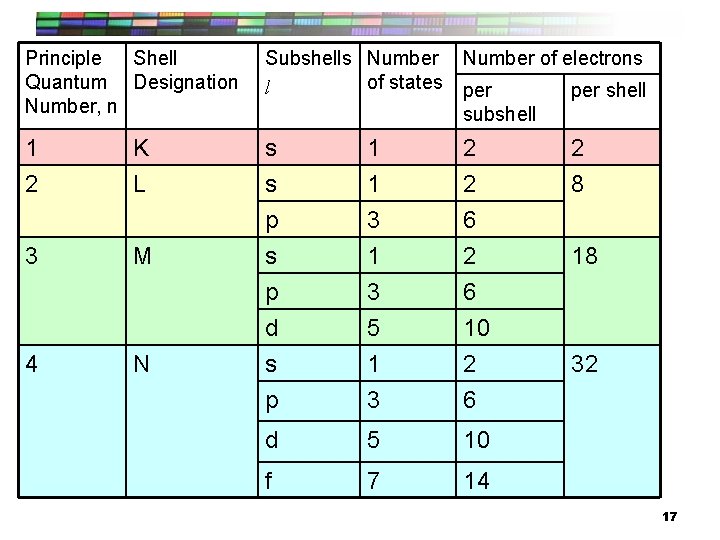
Principle Shell Quantum Designation Number, n Subshells Number of states l Number of electrons per subshell per shell 1 2 s s 1 1 2 2 2 8 p s p d s p 3 1 3 5 1 3 6 2 6 10 2 6 d 5 10 f 7 14 K L 3 M 4 N 18 32 17

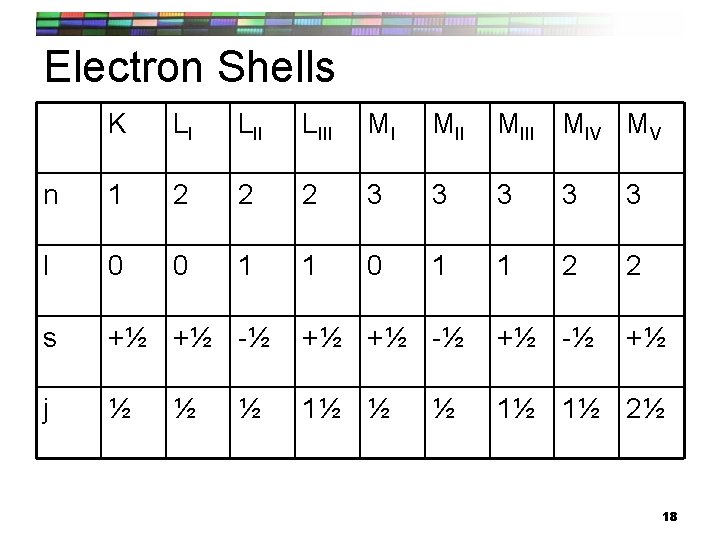
Electron Shells K LI LIII MI MIII MIV MV n 1 2 2 2 3 3 3 l 0 0 1 1 2 2 s +½ +½ -½ j ½ 1½ 1½ 2½ ½ +½ 18

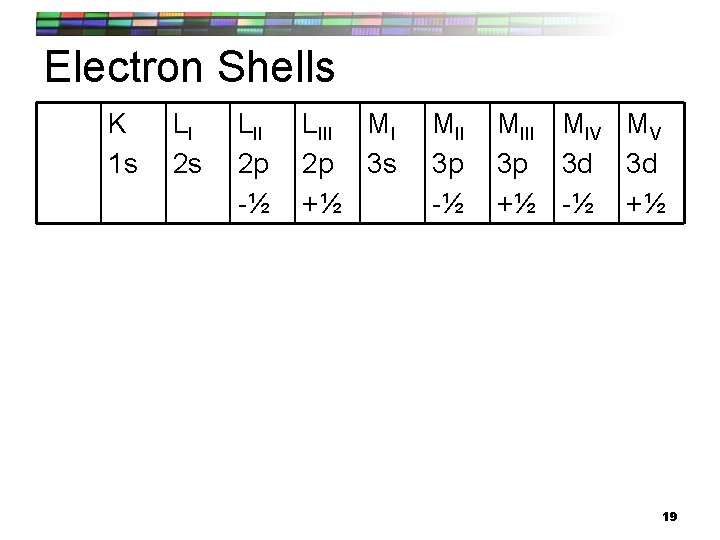
Electron Shells K 1 s LI 2 s LII 2 p -½ LIII MI 2 p 3 s +½ MII 3 p -½ MIII MIV MV 3 p 3 d 3 d +½ -½ +½ 19

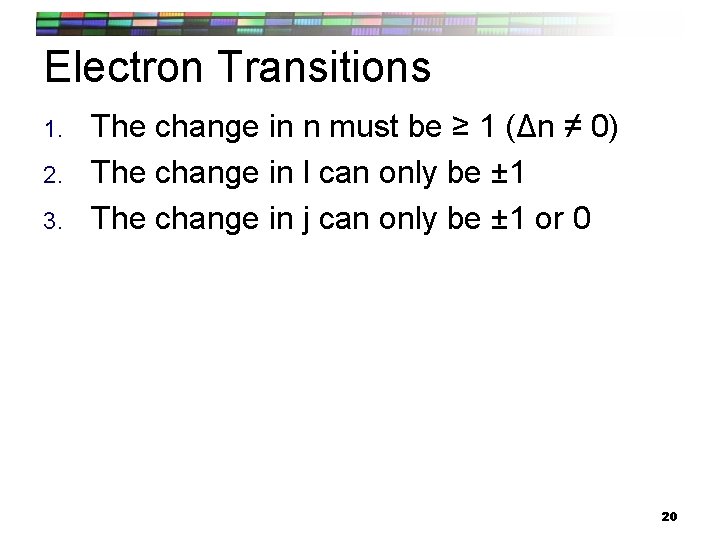
Electron Transitions 1. 2. 3. The change in n must be ≥ 1 (Δn ≠ 0) The change in l can only be ± 1 The change in j can only be ± 1 or 0 20

Calculation 1. 2. 3. The change in n must be ≥ 1 (Δn ≠ 0) The change in l can only be ± 1 The change in j can only be ± 1 or 0 2 p +½ to 1 s Quantu m# n l ml ms j Δ 21

Example of Electron Transitions Insert image 22

Spectroscopy and X-Ray Analysis n Electromagnetic Radiation ¨ Electromagnetic waves ¨ Calculations involving waves ¨ The electromagnetic spectrum ¨ Light and Optics ¨ Refraction and diffraction n X-Rays ¨ Discovery of X-rays ¨ Generation of X-rays ¨ Quantum Numbers ¨ Electron Energy Transitions 23
 Frequency of xrays
Frequency of xrays Gamma ray
Gamma ray Jfk jr plane crash photos
Jfk jr plane crash photos How were xrays discovered
How were xrays discovered Intensity of an em wave
Intensity of an em wave Facts about electromagnetic radiation
Facts about electromagnetic radiation Wavelength of electromagnetic radiation formula
Wavelength of electromagnetic radiation formula Types of radiation in the electromagnetic spectrum
Types of radiation in the electromagnetic spectrum Intensity of electromagnetic radiation
Intensity of electromagnetic radiation Which telescope detects invisible electromagnetic radiation
Which telescope detects invisible electromagnetic radiation Electromagnetic waves are transverse waves true or false
Electromagnetic waves are transverse waves true or false Electromagnetic frequency
Electromagnetic frequency When electromagnetic radiation of wavelength 300
When electromagnetic radiation of wavelength 300 Sub orbitals
Sub orbitals I comb its hair and love its shining eyes
I comb its hair and love its shining eyes Its halloween its halloween the moon is full and bright
Its halloween its halloween the moon is full and bright Lesser of two evils propaganda examples
Lesser of two evils propaganda examples When a train increases its velocity its momentum
When a train increases its velocity its momentum Sunny rainy snowy windy cloudy
Sunny rainy snowy windy cloudy If its square its a sonnet
If its square its a sonnet Its not easy but its worth it
Its not easy but its worth it How you use ict today and how you will use it tomorrow
How you use ict today and how you will use it tomorrow Similarities of mechanical waves and electromagnetic waves
Similarities of mechanical waves and electromagnetic waves Mechanical waves and electromagnetic waves similarities
Mechanical waves and electromagnetic waves similarities