Effective Nuclear Charge Zeff In a manyelectron atom










- Slides: 16

Effective Nuclear Charge (Zeff) – In a many-electron atom, each electron is attracted to the positively charged nucleus and repelled by the other negatively charged electrons. – Zeff takes both of these factors into account and represents an estimate of the net electric field experienced by an electron. – Zeff = Z – S – Z = number of protons in the nucleus. – S = screening constant; the number of core electrons that screen the outer electrons from the positive charge in the nucleus.

• Estimate the effective nuclear charge experienced by the outer electron in each of the following atoms: – Potassium – Fluorine – Silicon

Effective nuclear charge Increases (b/c number of protons increases)

Atomic Radius – represents one half the distance between the nuclei of atoms held together by a bond. Increases Decreases

As we move from left to right across a period, the atomic radius decreases. Why does this occur? • as we move from left to right, atomic number increases. • Since there are more protons in the nucleus, the effective nuclear charge increases. • This increases the attractive force felt by the outer e- making the atom smaller.

As we move from the top of a group to the bottom of a group, the atomic radius increases. Why does this occur? • as we move down a group, the principal quantum number (n) increases. • This means that the outer e- are more likely to be found in higher energy orbitals further from the nucleus.

Ionic Radii • When atoms gain an electron(s), they become negatively charged anions. Anions are larger than the corresponding neutral atom. X– will be larger than X because: • Negatively charged ions have more electrons in the electron cloud. • Each electron repels the other negatively charged electrons, causing the electron cloud to “spread out”. • More electron-electron repulsions leads to a larger radius than a neutral atom would have.

Ionic Radii • When atoms lose an electron(s), they become positively charged cations. Cations are smaller than the corresponding neutral atom. M+ will be smaller than M because: • Positively charged ions have less electrons in the electron cloud. • Less electrons means there will be fewer electron-electron repulsions, making the ion smaller than a neutral atom would be.

Ionization Energy – the energy required to remove an electron from the ground state of a gaseous atom or ion. Decreases Increases

As we move from left to right across a period, the ionization energy increases. Why does this occur? • as we move from left to right, atomic number increases. • Since there are more protons in the nucleus, the effective nuclear charge increases. • This increases the attractive force felt by the outer e- making it harder to remove.

As we move from the top of a group to the bottom of a group, the ionization energy decreases. Why does this occur? • as we move down a group, the principal quantum number (n) increases. • This means that the outer e- are more likely to be found in higher energy orbitals further from the nucleus. • This makes them less attracted to the nucleus so they take less energy to remove.

Successive Ionization Energies • Removing a second (or third, etc. ) electron always requires even more energy than removing the first electron. • This occurs because the atom gets smaller each time an electron is removed and this makes the outer electrons closer to the nucleus (and more strongly attracted to it).

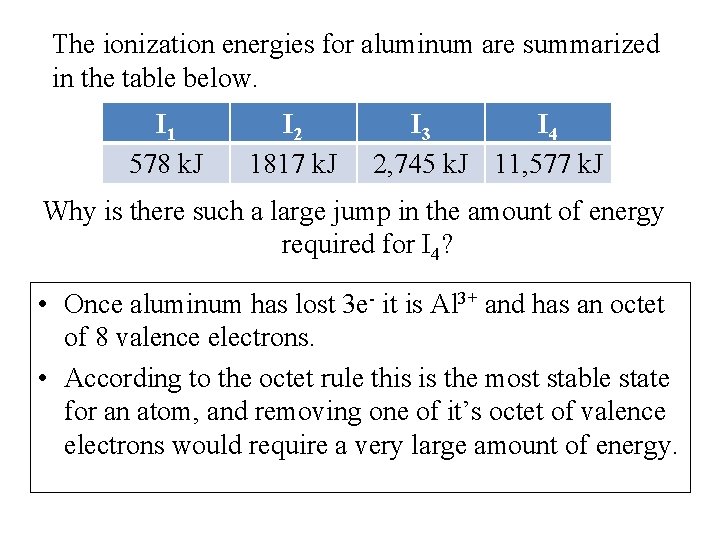
The ionization energies for aluminum are summarized in the table below. I 1 578 k. J I 2 1817 k. J I 3 I 4 2, 745 k. J 11, 577 k. J Why is there such a large jump in the amount of energy required for I 4? • Once aluminum has lost 3 e- it is Al 3+ and has an octet of 8 valence electrons. • According to the octet rule this is the most stable state for an atom, and removing one of it’s octet of valence electrons would require a very large amount of energy.

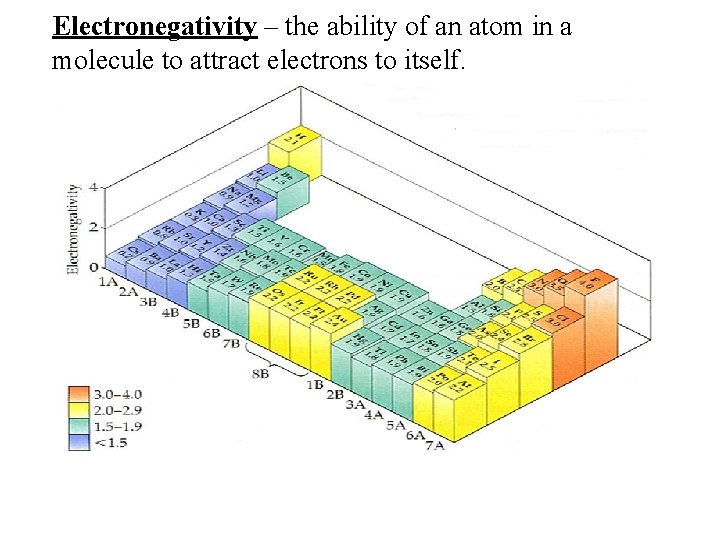
Electronegativity – the ability of an atom in a molecule to attract electrons to itself.

Electronegativity – the ability of an atom in a molecule to attract electrons to itself. Decreases Increases

• Electronegativity tends to increase as we move from left to right across a period and tends to decrease as we move down a group. • The most electronegative elements have highly negative electron affinities (attraction for additional electrons) and high ionization energies (tendency to hold onto their own electrons). • Fluorine (F) is the most electronegative element. • Does not apply to noble gases – since already have full outer shell and will not attract more electrons.