Dual HispanoItalian spinout from Aromics SL and Naxospharma



- Slides: 15

Dual Hispano-Italian spin-out from Aromics SL and Naxospharma SRL Company Overview info@aesistherapeutics. eu

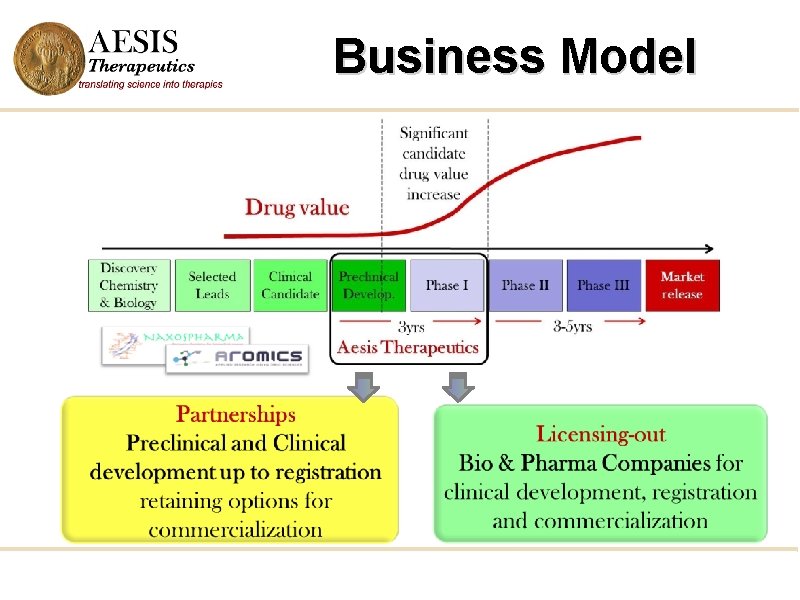
Business Model

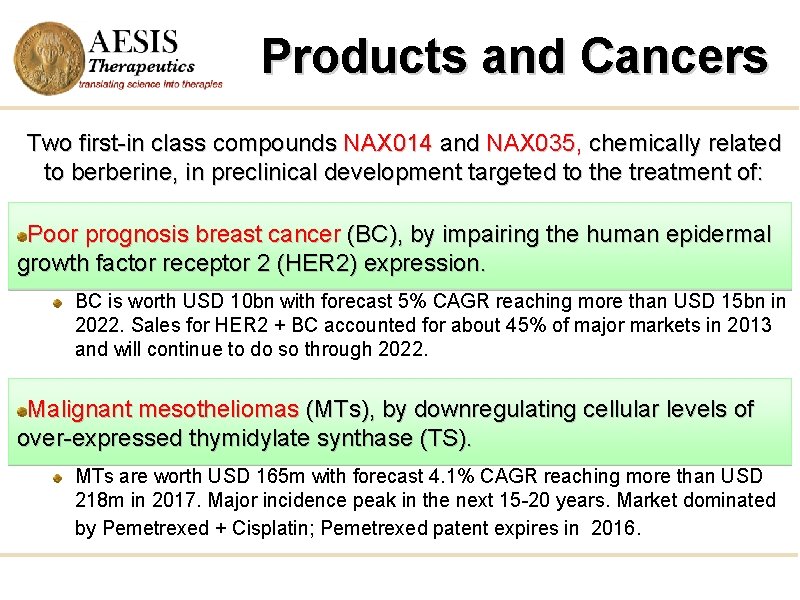
Products and Cancers Two first-in class compounds NAX 014 and NAX 035, chemically related to berberine, in preclinical development targeted to the treatment of: Poor prognosis breast cancer (BC), by impairing the human epidermal growth factor receptor 2 (HER 2) expression. BC is worth USD 10 bn with forecast 5% CAGR reaching more than USD 15 bn in 2022. Sales for HER 2 + BC accounted for about 45% of major markets in 2013 and will continue to do so through 2022. Malignant mesotheliomas (MTs), by downregulating cellular levels of over-expressed thymidylate synthase (TS). MTs are worth USD 165 m with forecast 4. 1% CAGR reaching more than USD 218 m in 2017. Major incidence peak in the next 15 -20 years. Market dominated by Pemetrexed + Cisplatin; Pemetrexed patent expires in 2016.

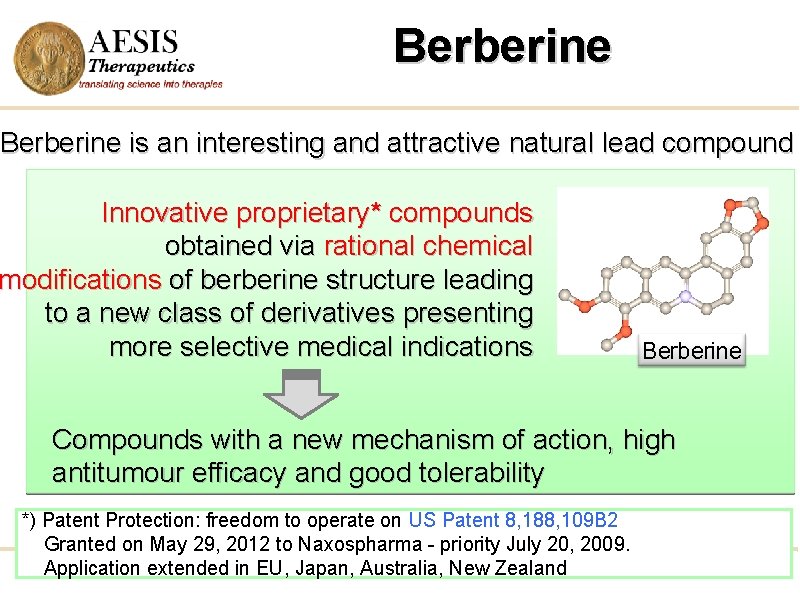
Berberine is an interesting and attractive natural lead compound Innovative proprietary* compounds obtained via rational chemical modifications of berberine structure leading to a new class of derivatives presenting more selective medical indications Berberine Compounds with a new mechanism of action, high antitumour efficacy and good tolerability *) Patent Protection: freedom to operate on US Patent 8, 188, 109 B 2 Granted on May 29, 2012 to Naxospharma - priority July 20, 2009. Application extended in EU, Japan, Australia, New Zealand

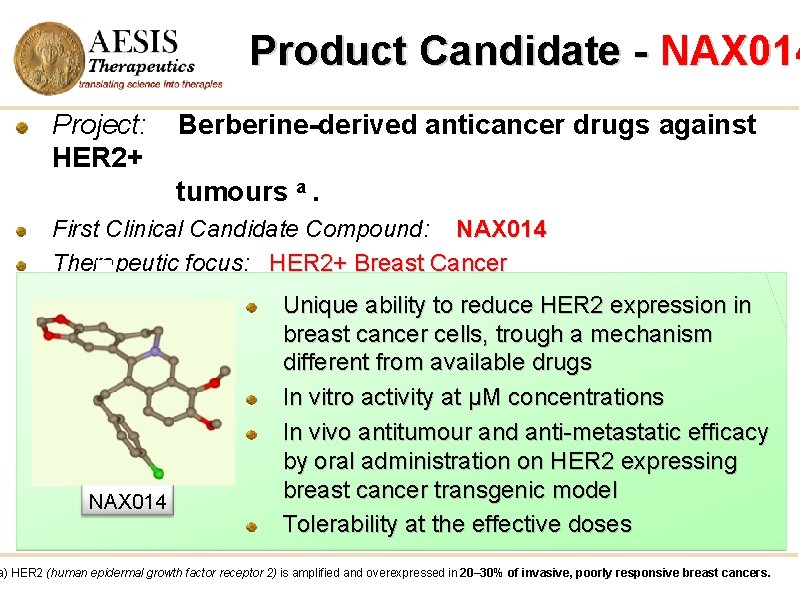
Product Candidate - NAX 014 Project: HER 2+ Berberine-derived anticancer drugs against tumours a. First Clinical Candidate Compound: NAX 014 Therapeutic focus: HER 2+ Breast Cancer NAX 014 Unique ability to reduce HER 2 expression in breast cancer cells, trough a mechanism different from available drugs In vitro activity at µM concentrations In vivo antitumour and anti-metastatic efficacy by oral administration on HER 2 expressing breast cancer transgenic model Tolerability at the effective doses a) HER 2 (human epidermal growth factor receptor 2) is amplified and overexpressed in 20– 30% of invasive, poorly responsive breast cancers.

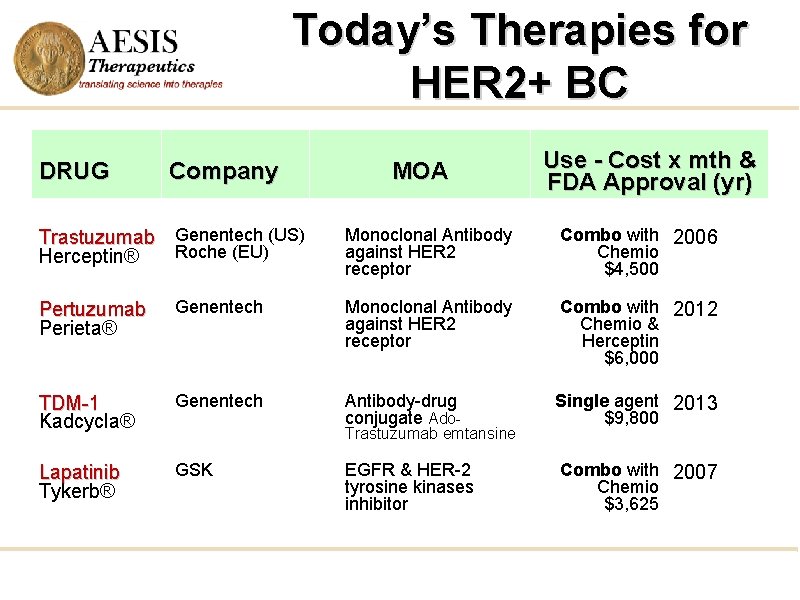
Today’s Therapies for HER 2+ BC DRUG Company Trastuzumab Genentech (US) Roche (EU) Herceptin® MOA Use - Cost x mth & FDA Approval (yr) Monoclonal Antibody against HER 2 receptor Combo with 2006 Chemio $4, 500 Pertuzumab Perieta® Genentech Monoclonal Antibody against HER 2 receptor Combo with 2012 Chemio & Herceptin $6, 000 TDM-1 Kadcycla® Genentech Antibody-drug conjugate Ado- Single agent 2013 $9, 800 Lapatinib Tykerb® GSK EGFR & HER-2 tyrosine kinases inhibitor Combo with 2007 Chemio $3, 625 Trastuzumab emtansine

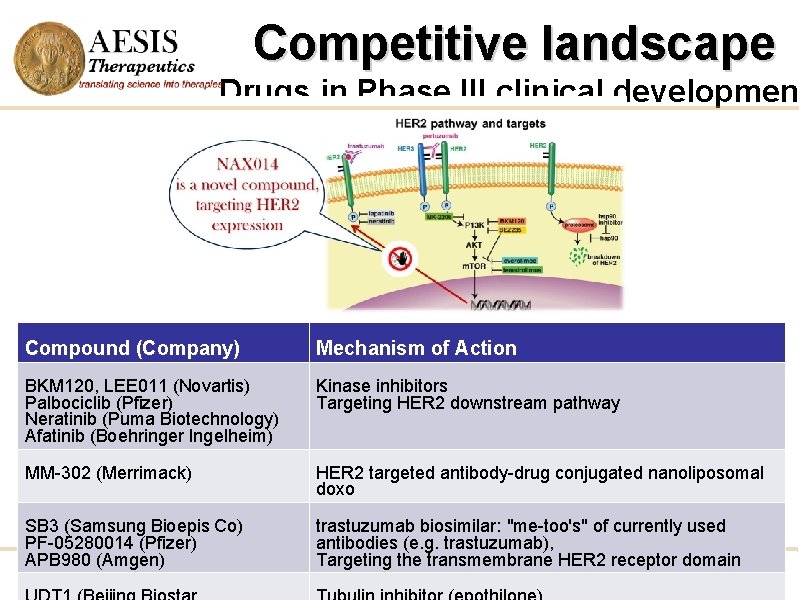
Competitive landscape Drugs in Phase III clinical development Compound (Company) Mechanism of Action BKM 120, LEE 011 (Novartis) Palbociclib (Pfizer) Neratinib (Puma Biotechnology) Afatinib (Boehringer Ingelheim) Kinase inhibitors Targeting HER 2 downstream pathway MM-302 (Merrimack) HER 2 targeted antibody-drug conjugated nanoliposomal doxo SB 3 (Samsung Bioepis Co) PF-05280014 (Pfizer) APB 980 (Amgen) trastuzumab biosimilar: "me-too's" of currently used antibodies (e. g. trastuzumab), Targeting the transmembrane HER 2 receptor domain

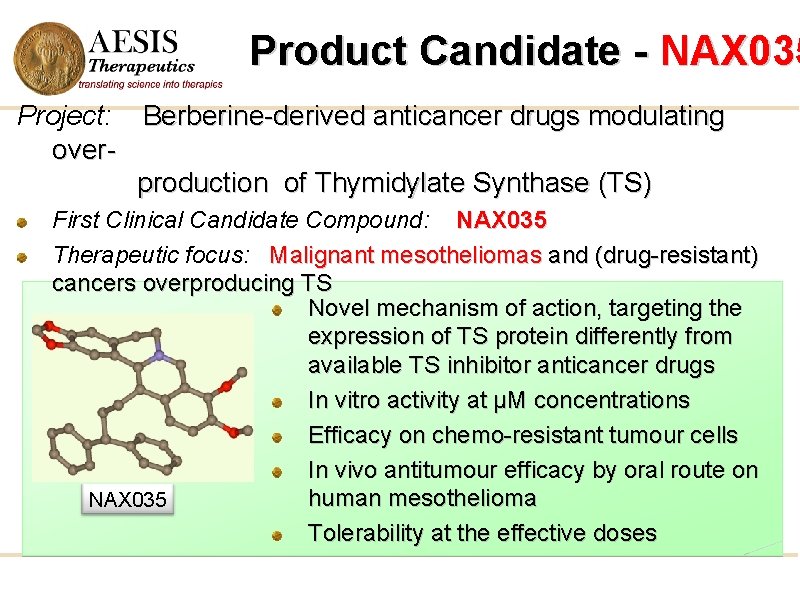
Product Candidate - NAX 035 Project: Berberine-derived anticancer drugs modulating overproduction of Thymidylate Synthase (TS) First Clinical Candidate Compound: NAX 035 Therapeutic focus: Malignant mesotheliomas and (drug-resistant) cancers overproducing TS Novel mechanism of action, targeting the expression of TS protein differently from available TS inhibitor anticancer drugs In vitro activity at µM concentrations Efficacy on chemo-resistant tumour cells In vivo antitumour efficacy by oral route on human mesothelioma NAX 035 Tolerability at the effective doses

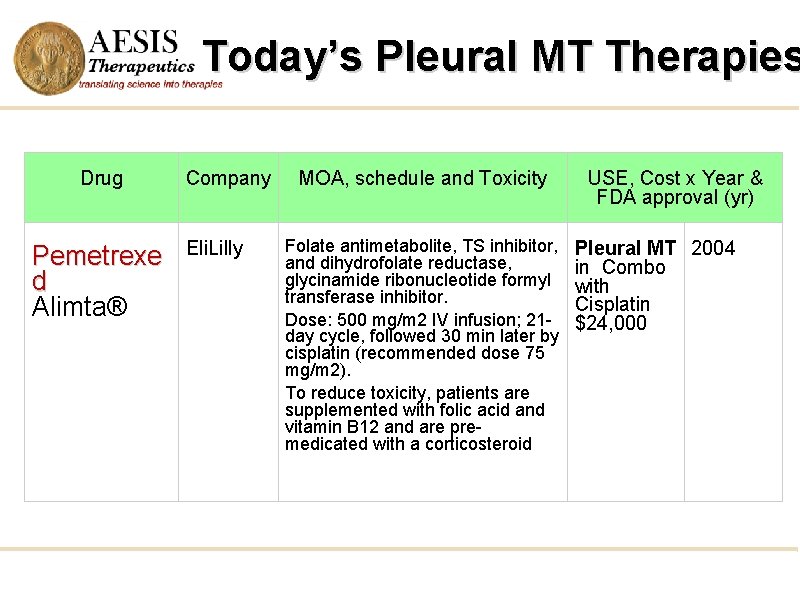
Today’s Pleural MT Therapies Drug Pemetrexe d Alimta® Company Eli. Lilly MOA, schedule and Toxicity Folate antimetabolite, TS inhibitor, and dihydrofolate reductase, glycinamide ribonucleotide formyl transferase inhibitor. Dose: 500 mg/m 2 IV infusion; 21 day cycle, followed 30 min later by cisplatin (recommended dose 75 mg/m 2). To reduce toxicity, patients are supplemented with folic acid and vitamin B 12 and are premedicated with a corticosteroid USE, Cost x Year & FDA approval (yr) Pleural MT 2004 in Combo with Cisplatin $24, 000

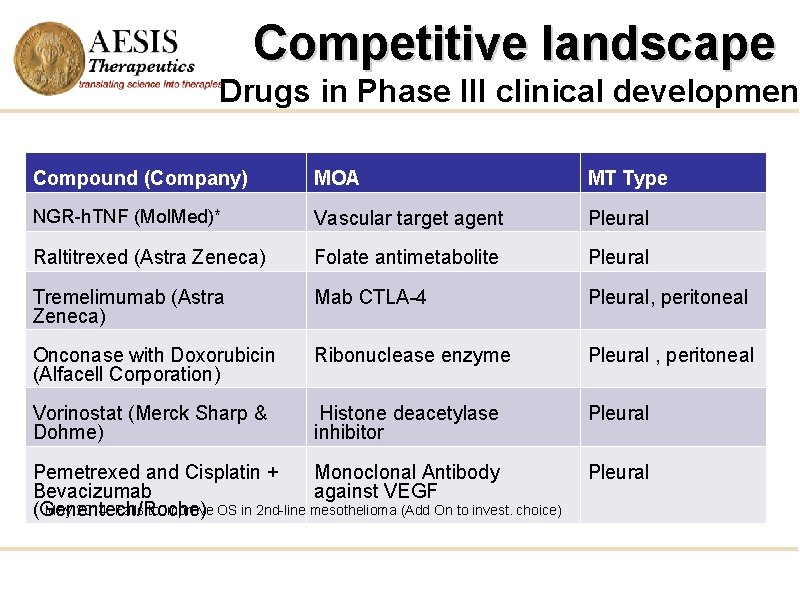
Competitive landscape Drugs in Phase III clinical development Compound (Company) MOA MT Type NGR-h. TNF (Mol. Med)* Vascular target agent Pleural Raltitrexed (Astra Zeneca) Folate antimetabolite Pleural Tremelimumab (Astra Zeneca) Mab CTLA-4 Pleural, peritoneal Onconase with Doxorubicin (Alfacell Corporation) Ribonuclease enzyme Pleural , peritoneal Vorinostat (Merck Sharp & Dohme) Histone deacetylase inhibitor Pleural Pemetrexed and Cisplatin + Monoclonal Antibody Bevacizumab against VEGF May 2014: Fails to improve OS in 2 nd-line mesothelioma (Add On to invest. choice) (Genentech/Roche) Pleural

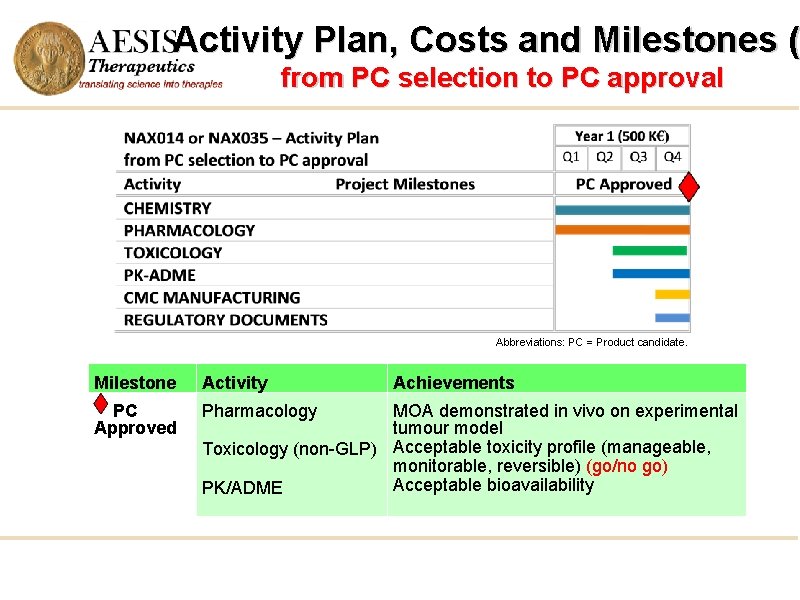
Activity Plan, Costs and Milestones ( from PC selection to PC approval Abbreviations: PC = Product candidate. Milestone Activity PC Approved Pharmacology Achievements MOA demonstrated in vivo on experimental tumour model Toxicology (non-GLP) Acceptable toxicity profile (manageable, monitorable, reversible) (go/no go) Acceptable bioavailability PK/ADME

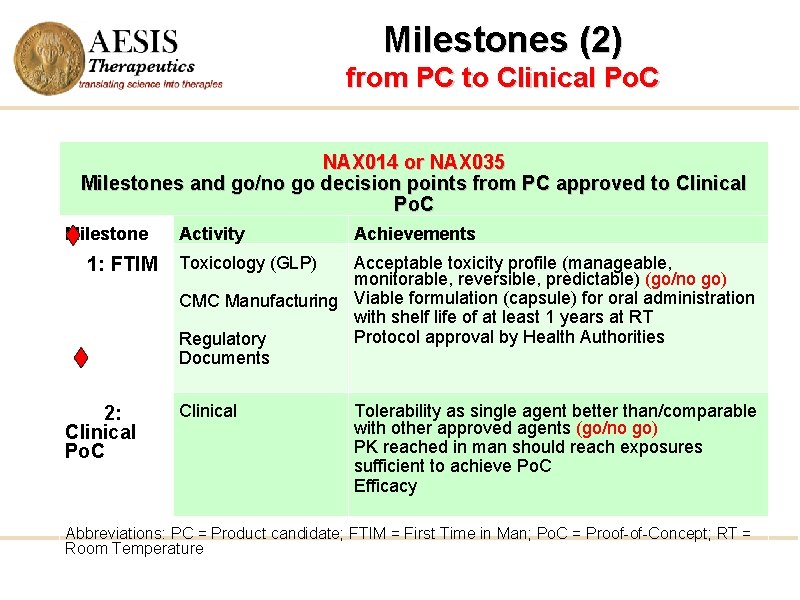
Milestones (2) from PC to Clinical Po. C NAX 014 or NAX 035 Milestones and go/no go decision points from PC approved to Clinical Po. C Milestone 1: FTIM 2: Clinical Po. C Activity Achievements Toxicology (GLP) Acceptable toxicity profile (manageable, monitorable, reversible, predictable) (go/no go) CMC Manufacturing Viable formulation (capsule) for oral administration with shelf life of at least 1 years at RT Protocol approval by Health Authorities Regulatory Documents Clinical Tolerability as single agent better than/comparable with other approved agents (go/no go) PK reached in man should reach exposures sufficient to achieve Po. C Efficacy Abbreviations: PC = Product candidate; FTIM = First Time in Man; Po. C = Proof-of-Concept; RT = Room Temperature

Management Team Management & Co-founders Cristina Geroni, CEO • 35+yr Oncology R&D Farmitalia, Pharmacia, Pfizer • Licensing-out oncology drugs • >55 patents Carmela Salvatore , COO • 15+yr preclinical Oncology R&D Menarini Ricerche • Development of oncology clinical candidates Paolo Lombardi, CSO • 35+yr Oncology R&D Farmitalia, Menarini, IBI, Chrysalon • CEO of Naxospharma (Italy) • Discovered Aromasin for BC therapy (global market) • >60 patents Carmen Plasencia. • CEO of Aromics (Barcelona, Spain) • biochemistry, biomedicine, molecular biology, proteomics, genomics in oncology research Narcis Clavell, • Engineer, MBA • Co-owner of Aromics (Barcelona, Spain) • Founder and owner of ATEKNEA SOLUTIONS S. A. Advisor Federico M Arcamone • 40+yr Oncology R&D Farmitalia, Menarini Ricerche • Discovered anticancer drugs doxorubicin, idarubicin, epirubicin (global market) • Board of Naxospharma • >100 patents we-know-how-to-do-because-we-have-alreadydone-it

Company profile City: Jesi (AN) Country: ITALY Industry: Healthcare Sector: Pharmaceuticals Subsector: ONCOLOGY Founded in: 2013 Skilled Team with a track record of achievements in Oncology R&D Innovative compounds to overcome current therapies drawbacks c. geroni@aesistherapeutics.

 Syngenta corn herbicides
Syngenta corn herbicides Dual language immersion programs pros cons
Dual language immersion programs pros cons Types of adc
Types of adc Compound bar chart worksheet
Compound bar chart worksheet When using dual symbols in a logic diagram
When using dual symbols in a logic diagram Dual mode and multimode operation in os
Dual mode and multimode operation in os Negative and positive voltage from a dual power supply
Negative and positive voltage from a dual power supply What does compounding the brakes mean
What does compounding the brakes mean Phsc porter campus
Phsc porter campus Nova dual enrollment
Nova dual enrollment Eku merit scholarships
Eku merit scholarships Ggc dual enrollment
Ggc dual enrollment System call in os
System call in os Valencia dual enrollment classes
Valencia dual enrollment classes Esfinter vesical interno
Esfinter vesical interno Ung dual enrollment classes
Ung dual enrollment classes