Division of Sponsored Programs Who are we and






















- Slides: 29

Division of Sponsored Programs: Who are we and how to we impact Clinical Trials? Academy for Research Professionals Series January 11, 2018 Presented by: Caitlin Flaherty J. D. & Katherine Gonzales B. A.

Today’s Agenda • Overview of DSP’s role in clinical trial studies • CDA/CTA Definitions • UIRIS Routing Forms • The DSP Process • Issues in CTAs • Accelerated Research Agreements • Resources

Meet our DSP Clinical Trial Staff: Jessica Boyle (Associate Director) Confidential Disclosure Agreement (CDA) Reviewers: Carrie Damon Melissa Shriver Clinical Trial Agreements (CTA) Contract Reviewers: Kathie Gonzales Caite Flaherty Adwin Hesseltine Linda Jones Loren Le. Clair See also: DSP Staff Directory https: //dsp. research. uiowa. edu/dsp-staff-directory

What is DSP & What do We Do? • Division of Sponsored Programs (DSP) reviews all external funding agreements for research that involve UI staff and/or occur on the UI or UIHC campuses. We are a resource for campus. • Review and negotiate contract terms to ensure compliance with Iowa law, federal law & UI policies • DSP is the signature authority for all externally funded research agreements *Note* A Principal Investigator (PI) is not an authorized signatory

Confidential Disclosure Agreement (CDA) • A Confidential Disclosure Agreement (CDA) or a Non-Disclosure Agreement (NDA) is a binding contract to safeguard confidential information • CDAs are signed prior to a Sponsor disclosing their Protocol for review or a Feasibility Study completion • Please note any real Sponsor deadlines on the UIRIS routing form (e. g. , site visit scheduled? ) Note on Investigator-Initiated Trials: If your PI is interested in proposing a study Protocol they develop, contact DSP to have a mutual CDA drafted or to add mutual confidentiality terms.

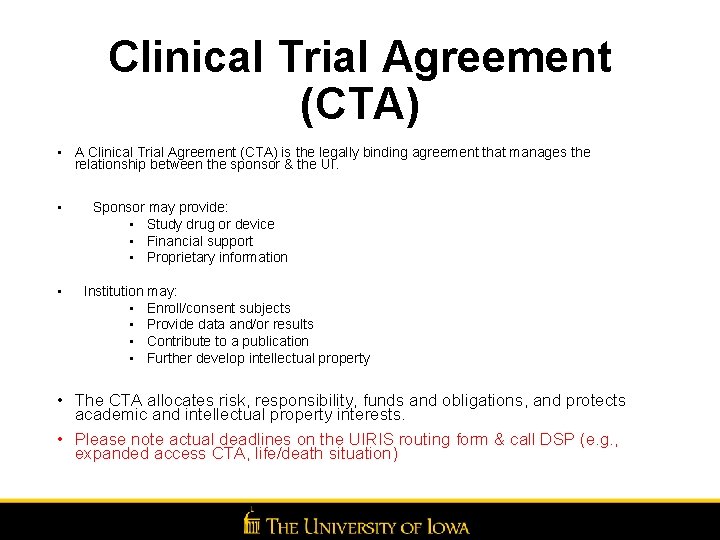
Clinical Trial Agreement (CTA) • A Clinical Trial Agreement (CTA) is the legally binding agreement that manages the relationship between the sponsor & the UI. • • Sponsor may provide: • Study drug or device • Financial support • Proprietary information Institution may: • Enroll/consent subjects • Provide data and/or results • Contribute to a publication • Further develop intellectual property • The CTA allocates risk, responsibility, funds and obligations, and protects academic and intellectual property interests. • Please note actual deadlines on the UIRIS routing form & call DSP (e. g. , expanded access CTA, life/death situation)

Getting Started with a CDA or CTA • Every CDA and CTA requires a UIRIS routing form: ØCDAs – use non-monetary routing from ØCTAs – use proposal routing form See also: University of Iowa Research Information System (UIRIS), https: //dsp. research. uiowa. edu/university-iowa-research-information-system-uiris

UIRIS Routing Form: Proposal and Non-Monetary V 3 - https: //uiris. uiowa. edu/dashboard V 2 -- https: //uiris. research. uiowa. edu/ 8

UIRIS Routing Form Start a new Routing form Search Unsubmitted Submitted Voided Approved Links to templates Editors 9

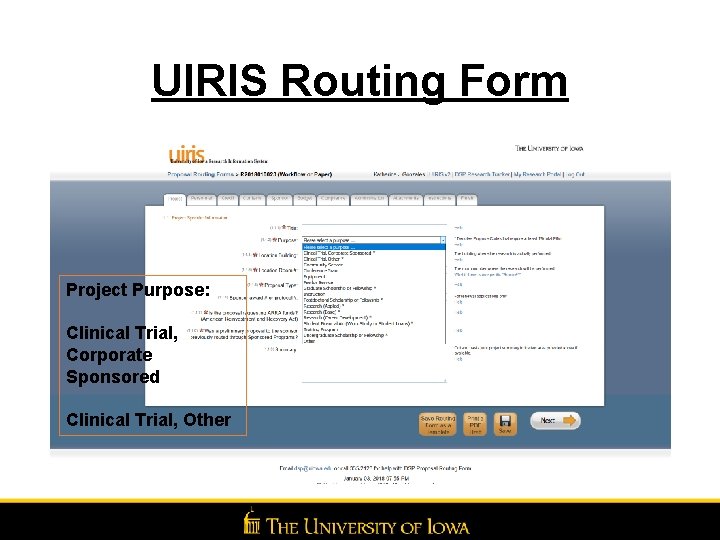
UIRIS Routing Form Project Purpose: Clinical Trial, Corporate Sponsored Clinical Trial, Other

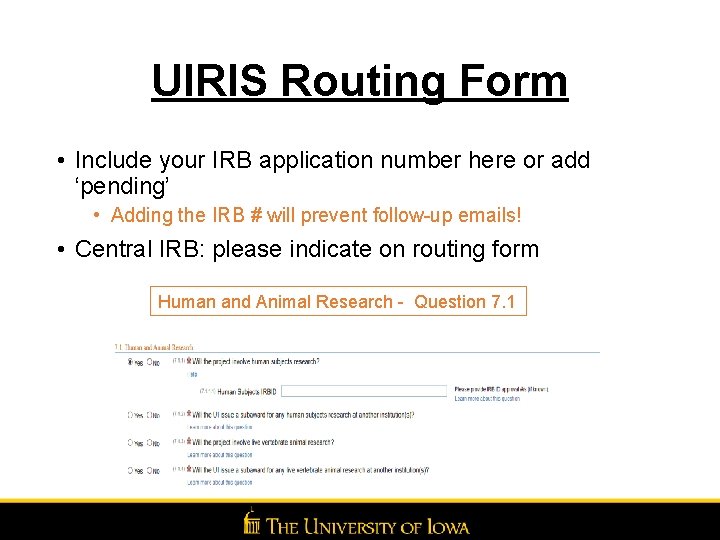
UIRIS Routing Form • Include your IRB application number here or add ‘pending’ • Adding the IRB # will prevent follow-up emails! • Central IRB: please indicate on routing form Human and Animal Research - Question 7. 1

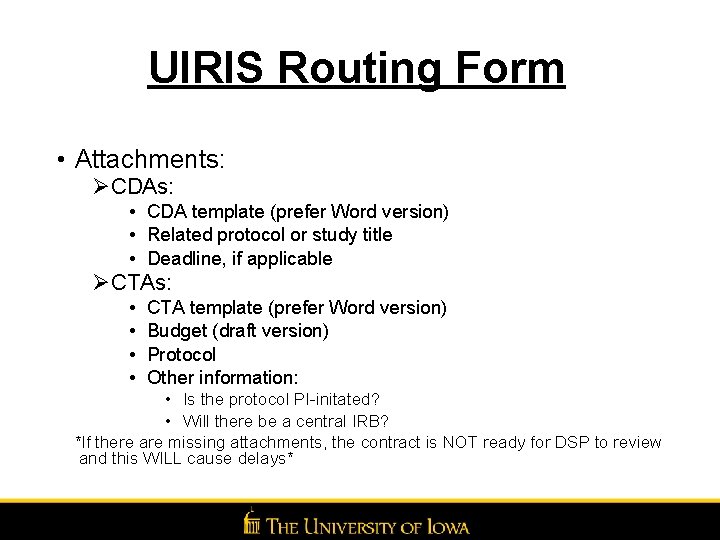
UIRIS Routing Form • Attachments: ØCDAs: • CDA template (prefer Word version) • Related protocol or study title • Deadline, if applicable ØCTAs: • • CTA template (prefer Word version) Budget (draft version) Protocol Other information: • Is the protocol PI-initated? • Will there be a central IRB? *If there are missing attachments, the contract is NOT ready for DSP to review and this WILL cause delays*

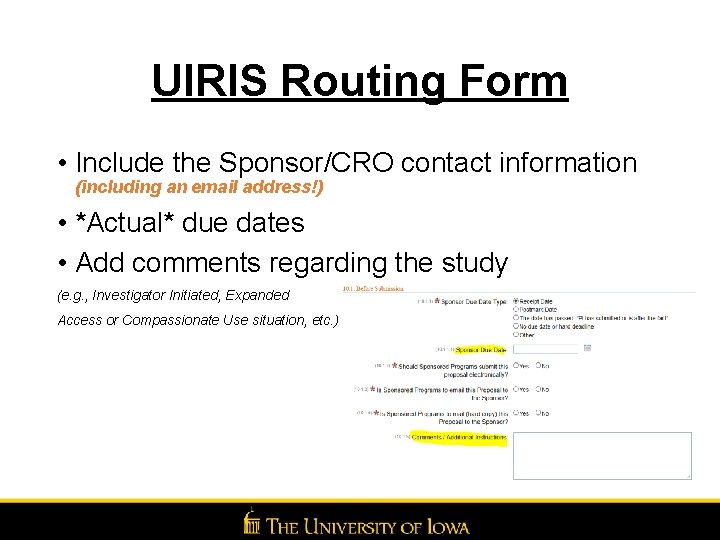
UIRIS Routing Form • Include the Sponsor/CRO contact information (including an email address!) • *Actual* due dates • Add comments regarding the study (e. g. , Investigator Initiated, Expanded Access or Compassionate Use situation, etc. )

UIRIS Routing Form • Submit a completed Proposal or Non. Monetary Routing form to DSP a minimum of 5 Business Days in advance of a deadline! *Note: Most Agreements will take time (average 2 -6 weeks) to negotiate, excluding budget and IRB reviews.

The DSP Process: CTA Reviews • Three Simultaneous Processes: Ø Contract Negotiation (DSP negotiates with sponsor) Ø Budget Negotiation (PI/Coordinator negotiate with sponsor) Ø IRB & Other Committee Approvals (PI/Coordinator work with HSO/committees and sponsor) All of these process take time and depend on the complexity of your study. Route the CTA to DSP to begin negotiations while working with the IRB.

The DSP Process: • Investigator Initiated Study (IIS) • UI Principal Investigator is an author on the Protocol • Either the Sponsor approached the PI for the study or the PI proposed an idea to the Sponsor • Notify the IRB! • Notify DSP by adding a comment on the Routing Form or emailing dsp-contracts@uiowa. edu and reference the study. • IIS agreements typically take longer to negotiate - route as soon as possible.

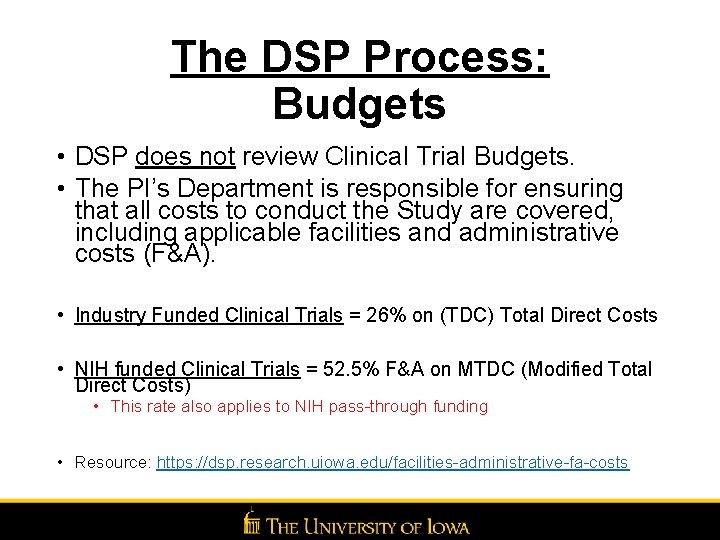
The DSP Process: Budgets • DSP does not review Clinical Trial Budgets. • The PI’s Department is responsible for ensuring that all costs to conduct the Study are covered, including applicable facilities and administrative costs (F&A). • Industry Funded Clinical Trials = 26% on (TDC) Total Direct Costs • NIH funded Clinical Trials = 52. 5% F&A on MTDC (Modified Total Direct Costs) • This rate also applies to NIH pass-through funding • Resource: https: //dsp. research. uiowa. edu/facilities-administrative-fa-costs

The DSP Process: • Requirements for completion of a clinical trial agreement: Ø Agreement on contract terms (DSP Contract Reviewer) Ø Agreement on the budget (PI and Study Coordinator lead) Ø If applicable: Ø Conflicts of Interest in Research office approval Ø Export Control review and/or approval

The DSP Process: • Requirements for release of contract and MFK: Ø Fully signed contract and IRB approval for the study Ø AAAN is issued and MFK assigned ØCommon Delay: DSP does not have your IRB number. Ø Submit your IRB number (even if still pending) on your routing form or send to dsp-contracts@uiowa. edu as soon as possible.

Issues in CTAs: • Liability and Indemnification • Subject Injury • Publication • AAHRPP • Working with Contract Research Organizations (CRO) Read your Contract!

Issues in CTAs: AAHRPP ØThe Association for the Accreditation of Human Research Protection Programs, Inc. , (AAHRPP) is an independent accrediting body that protects the rights and welfare of research participants and promotes scientifically meritorious and ethically sound research by fostering and advancing the ethical and professional conduct of persons and organizations that engage in research with human participants.

Issues in CTAs: Working with Contract Research Organizations • What and Why: Many sponsors contract with a CRO to manage the conduct of a study. Some CROs negotiate terms of the CTA. • Delay of the negotiation process - CRO is a ‘middle man’ • Often a Letter of Indemnification (or LOI) is required from sponsor if CRO is signing the CTA

Issues in CTAs: • Central IRB required or requested from Study Sponsor or Protocol • Will non-UI Employees be involved in the Study? • Inform DSP and HSO • Was there a change in the Protocol? • Did the change affect the Budget? • Do not hesitate to contact the Sponsor to revise the budget in an amendment to the Contract! • Review the CTA for payment terms and invoicing requirements – contact DSP with any questions.

Accelerated Research Agreements • Master CDA or CTA agreements • DSP currently has: • 12 Master CDAs • 41 Master CTAs • For inquiries regarding a Master Agreement, contact DSP

Accelerated Research Agreements • Accelerated Clinical Trial Agreement (ACTA) • Accelerated Confidential Disclosure Agreement (ACDA) Created to facilitate relationships with Sponsors/CROs and expedite the contract process • See https: //ctsacentral. org/tools/acta/ for more information

Other Agreements • DSP also reviews: • Grant Proposals to the federal government, industry sponsors, private foundations or non-profit organizations • Material Transfer Agreements (MTA) • Data Use Agreements (DUA) • Equipment Loan Agreements (for research purposes or in connection with external funding)

Resources • DSP Research Tracker • Find the DSP contact in DSP who is negotiating your agreement • Easily find access to the status of the Agreement • ‘DSP Review’=DSP reviewer is still waiting on documents or is still reviewing the agreement • ‘Negotiating’=DSP has emailed edits to the Sponsor • ‘Pending Signature’ (either PI, UI or Sponsor)=Agreement is out for signature • ‘Held for IRB’= `IRB approval has not been rec’d from HSO or UI department has not responded to an email from DSP • ‘Pending GAO Action’=Agreement is final but MFK has not yet been assigned.

Resources • Excellent "How to" information: https: //dsp. research. uiowa. edu/how • DSP Research Tracker-https: //uiris. uiowa. edu/combined_log/main/view • Look up the submission/negotiation status of your routing form. • Find your DSP contact for your Study. • Access your final contract or award information • Training-https: //dsp. research. uiowa. edu/training-opportunities • Sign up for our Listservs! • RAD-Research Administrator Dispatch/Grant Bulletin https: //dsp. research. uiowa. edu/sign-dsp-newsletters-research-administration-dispatch-rad-andgrant-bulletin-gb

Questions? DSP 2 Gilmore Hall 112 N. Capitol Street The University of Iowa City, Iowa 52242 (319) 335 -2123 Dsp-contracts@uiowa. edu https: //dsp. research. uiowa. edu/ Staff Directory: https: //dsp. research. uiowa. edu/dsp-staff-directory
 Ui division of sponsored programs
Ui division of sponsored programs Insidan region jh
Insidan region jh Ndsu sponsored programs
Ndsu sponsored programs Cpmcd full form
Cpmcd full form Short division vs long division
Short division vs long division Short division
Short division Synthetic division examples with answers
Synthetic division examples with answers Synthetic division examples with answers
Synthetic division examples with answers Srs tamu
Srs tamu Who introduce the house bill 393?
Who introduce the house bill 393? Dbhds sponsored residential regulations
Dbhds sponsored residential regulations Usc sponsored projects accounting
Usc sponsored projects accounting Uci sponsored projects
Uci sponsored projects Usc sponsored projects accounting
Usc sponsored projects accounting Manufacturer sponsored wholesaler franchise system
Manufacturer sponsored wholesaler franchise system Government sponsored recreation
Government sponsored recreation Manufacturer sponsored retailer franchise system
Manufacturer sponsored retailer franchise system The agency sponsored by the united nations that compiles
The agency sponsored by the united nations that compiles Ptao uva
Ptao uva Who sponsored vasco nunez de balboa
Who sponsored vasco nunez de balboa Who sponsored vasco nunez de balboa
Who sponsored vasco nunez de balboa Manuel roxas economic adviser
Manuel roxas economic adviser National programmes related to child health and welfare pdf
National programmes related to child health and welfare pdf Monitoring and evaluation of family planning programs
Monitoring and evaluation of family planning programs Programmers use backdoors to debug and test programs.
Programmers use backdoors to debug and test programs. Steps in designing hrd programs
Steps in designing hrd programs Motivational methods and programs
Motivational methods and programs Developing pricing strategies and programs
Developing pricing strategies and programs Dual language immersion programs pros cons
Dual language immersion programs pros cons Ppc seo and affiliate programs
Ppc seo and affiliate programs