Diagnostic methods in parasitology Examination of feces microscopy















- Slides: 17

Diagnostic methods in parasitology

Examination of feces

microscopy u The microscope should be equipped with a micrometer eyepiece which essential to measure the size of parasites. It should also include contributory findings ﺍﻟﻤﺤﺴﻮﺑﺔ ﺍﻟﻨﺘﺎﺋﺞ such as the presence of Charcot-Leyden crystals and cellular exudate ﺍﻻﻓﺮﺍﺯﺍﺕ ﺍﻟﺨﻠﻮﻳﺔ

WET MOUNT PREPARATION u The unstained wet film is the standard preparation and is made by emulsifying a small quantity of feces in a drop of saline placed on a slide and applying a cover slip on top, avoiding air bubbles u A proper preparation should be just dense enough for newspaper print to be read through it. If the feces contain mucus, it is advisable to prepare films using the mucus part

WET MOUNT PREPARATION Ø It is a fast, simple, procedure and provides a quick answer when positive Ø It provides an estimate of the parasitic burden Ø Results should be confirmed by permanent stained smears

u Wet saline mounts are particularly useful for detecting live motile trophozoites of E. histolytica, B. coli and G. lamblia. Eggs of helminthes are also readily seen u Eosin 1%aqueous solution and Iodine staining can be used for staining wet films

Thick smears u Valuable in surveys for intestinal helminth eggs. the method described by Kato and Miura in 1954 is known as the Kato thick smear technique.

The Kato thick smear technique u About 50 gm feces is taken on a slide and covered with a special wettable cellophane coverslip soaked in glycerine containing aqueous malachite green. The preparation is left for about an hour at room temperature in which period the glycerine clears the feces enabling the helminth eggs to be seen distinctly. The method is not useful for diagnosis of protozoa or helminth larvae

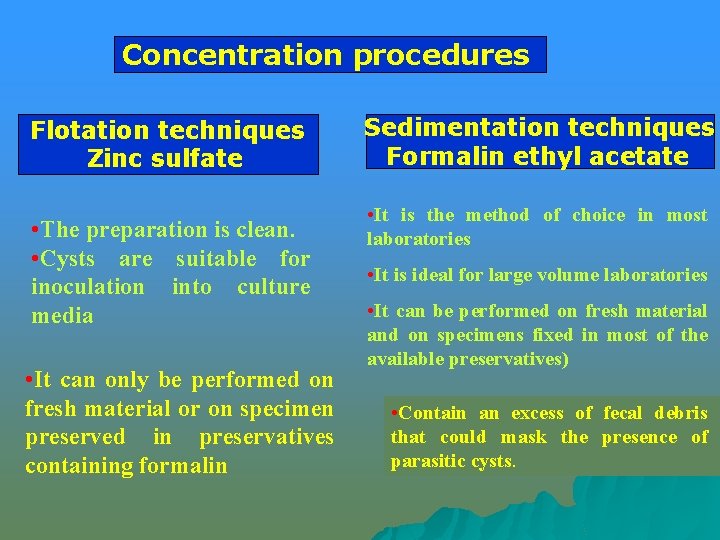
Concentration procedures 1 - Flotation method where the feces is suspended in solution of high specific gravity so that parasitic eggs and cysts float up and get concentrated at the surface. 2 - Sedimentation method where the feces is suspended in a solution with low specific gravity so that the eggs and cysts get sedimented at the bottom, either spontaneously or by centrifugation

Concentration procedures Flotation techniques Zinc sulfate • The preparation is clean. • Cysts are suitable for inoculation into culture media • It can only be performed on fresh material or on specimen preserved in preservatives containing formalin Sedimentation techniques Formalin ethyl acetate • It is the method of choice in most laboratories • It is ideal for large volume laboratories • It can be performed on fresh material and on specimens fixed in most of the available preservatives) • Contain an excess of fecal debris that could mask the presence of parasitic cysts.

The permanent stained smear is recommended for use with every stool specimen submitted for a routine parasite examination. 1 - used for identification of protozoan trophozoites and cysts and for confirmation of species 2 - It provides laboratories with a permanent record 3 - The slides can be sent to a reference laboratory when organisms with unusual morphology are encountered, or when identification is difficult

Problems encountered in the staining of protozoan cysts u The specimen is too old u The smears are too dense u The smears are allowed to dry before fixation or fixation is inadequate.

u The most important stains for permanent stained smear are: u 1 -Iron haematoxylin stain u 2 -Trichrome stain

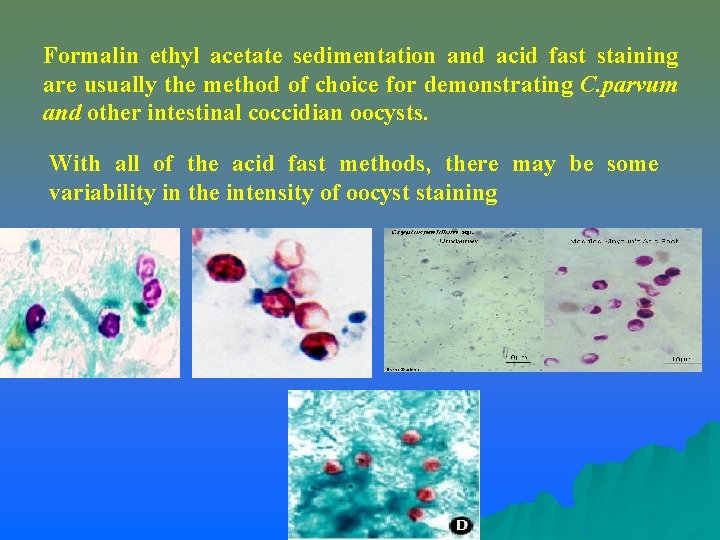
Formalin ethyl acetate sedimentation and acid fast staining are usually the method of choice for demonstrating C. parvum and other intestinal coccidian oocysts. With all of the acid fast methods, there may be some variability in the intensity of oocyst staining Modified Zeil-Neelsen

Fecal Culture u. Not used for routine diagnosis, but for species identification as for example in differentiation detween Ancylostoma and Necator.

Definition of Harada-Mori culture u Harada-Mori culture Method uses a strips of filter paper on which feces is smeared in the middle third. The paper strip are kept in conical centrifuge tubes with water at the bottom in which the strip dip for the purpose of culturing and recovering nematode larvae (Strongyloides stercoralis, hookworms).

Harada-Mori culture for the purpose of culturing and recovering nematode larvae (Strongyloides stercoralis, hookworms
 Microscopic urine analysis
Microscopic urine analysis Microscopy methods
Microscopy methods Clamping
Clamping Calprotectine waarde 5
Calprotectine waarde 5 Methods of fresh tissue examination
Methods of fresh tissue examination Introduction to parasitology
Introduction to parasitology Parasitology
Parasitology Toxoplaxmosis
Toxoplaxmosis Helminthology notes
Helminthology notes Parasitology
Parasitology Definition of metazoa
Definition of metazoa Parasitology
Parasitology Ancylostoma
Ancylostoma Sedimentation technique for stool concentration
Sedimentation technique for stool concentration Parasitology
Parasitology Father of veterinary parasitology
Father of veterinary parasitology Medical
Medical Zinc sulphate flotation technique procedure
Zinc sulphate flotation technique procedure