Curriculum Vitae Dr Prayudi Santoso Sp PDKP M


















- Slides: 43

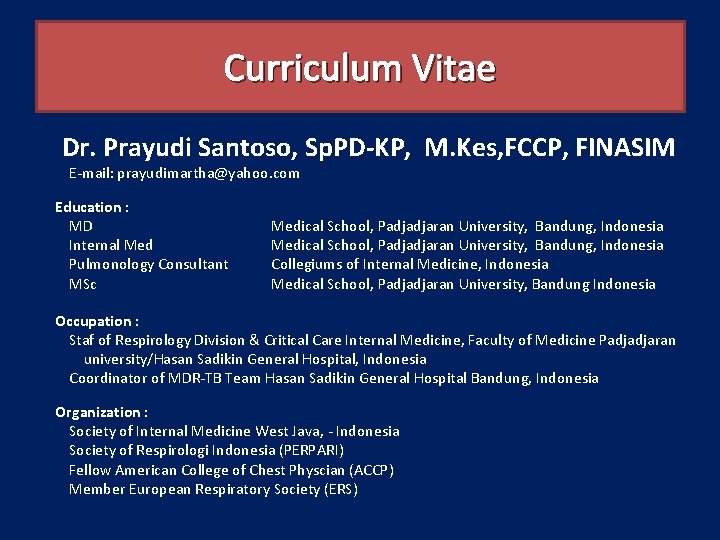
Curriculum Vitae Dr. Prayudi Santoso, Sp. PD-KP, M. Kes, FCCP, FINASIM E-mail: prayudimartha@yahoo. com Education : MD Internal Med Pulmonology Consultant MSc Medical School, Padjadjaran University, Bandung, Indonesia Collegiums of Internal Medicine, Indonesia Medical School, Padjadjaran University, Bandung Indonesia Occupation : Staf of Respirology Division & Critical Care Internal Medicine, Faculty of Medicine Padjadjaran university/Hasan Sadikin General Hospital, Indonesia Coordinator of MDR-TB Team Hasan Sadikin General Hospital Bandung, Indonesia Organization : Society of Internal Medicine West Java, - Indonesia Society of Respirologi Indonesia (PERPARI) Fellow American College of Chest Physcian (ACCP) Member European Respiratory Society (ERS)

Penggunaan Antibiotik yang Rasional dalam Penanganan Infeksi CAP dan AECB Prayudi Santoso Departemen Ilmu Penyakit Dalam, Divisi Respirologi dan Kritis Respirasi, RSHS, FK UNPAD Bandung, Indonesia 2016 prayudimartha@yahoo. com

Pneumonia n Infectious /inflammatory disorders of the lung parenchyma n Pathology : pulmonary alveoli and or interstitium are filled with WBC, RBC and fibrin

In Indonesia based on Indonesia Health Profiles on 2009: • Pneumonia is one of the 10 most common diseases in hospitals leading to highest crude fatality rate of 7. 6%, while the respiratory tract infection rate is 0. 43%. 3

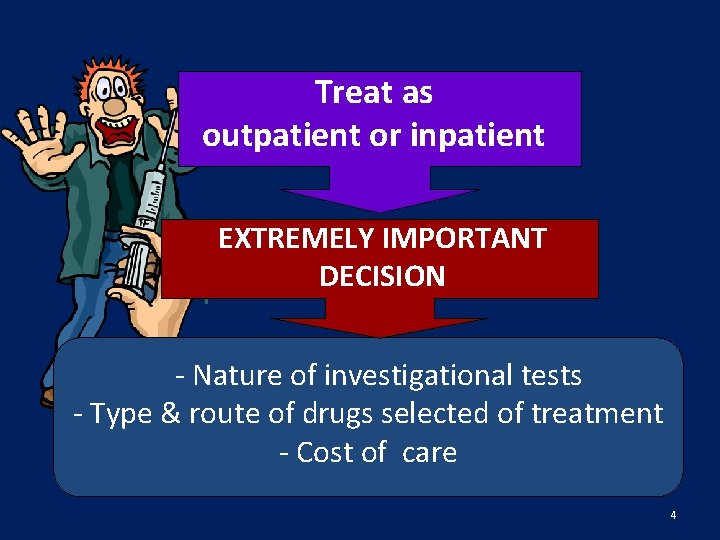
Treat as outpatient or inpatient EXTREMELY IMPORTANT DECISION - Nature of investigational tests - Type & route of drugs selected of treatment - Cost of care 4

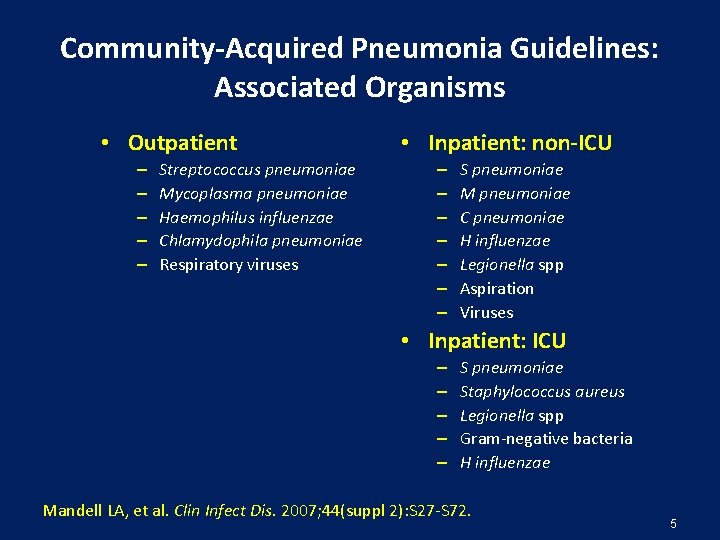
Community-Acquired Pneumonia Guidelines: Associated Organisms • Outpatient – – – Streptococcus pneumoniae Mycoplasma pneumoniae Haemophilus influenzae Chlamydophila pneumoniae Respiratory viruses • Inpatient: non-ICU – – – – S pneumoniae M pneumoniae C pneumoniae H influenzae Legionella spp Aspiration Viruses • Inpatient: ICU – – – S pneumoniae Staphylococcus aureus Legionella spp Gram-negative bacteria H influenzae Mandell LA, et al. Clin Infect Dis. 2007; 44(suppl 2): S 27 -S 72. 5

S. pneumoniae Is the Most Commonly Identified Organism Overall Causing CAP (8 Asian Countriesa) Pathogens isolated (N=390) No. of isolates (%) S. pneumoniae 114 (29. 2) Klebsiella pneumoniae 60 (15. 4) Hemophilus influenzae 59 (15. 1) Pseudomonas aeruginosa 26 (6. 7) Staphylococcus aureus 19 (4. 9) Mycobacterium tuberculosis 13 (3. 3) Moraxella catarrhalis 12 (3. 1) Other pathogens 77 (19. 7) Mycoplasma pneumoniae 61/556 (11. 0) Chlamydia pneumoniae 55/411 (13. 4) Legionella pneumoniae 7/648 (1. 1) a. South Korea, China, Taiwan, Hong Kong, India, Singapore, Vietnam, and the Philippines. Study by ANSORP (Asian Network for Surveillance of Resistant Pathogens Study Group). Adapted from Song J-H et al. Int J Antimicrob Agents. 2008; 31(2): 107 -114.

Atypical Pathogens are Commonly Implicated in Hospitalized Community - Acquired Pneumonia (CAP) 22% of CAP cases are caused by Atypical Pathogens Streptococcu s pneumoniae Atypical pathogens: Legionella spp Chlamydia spp Mycoplasma spp Staphylococcus aureus Other Haemophilus influenzae and Moraxella catarrhalis Aerobic gram-negative rods (Adapted from Eron JJ et al. Hospital Formulary, 1994. ) • Due to the difficulty of culturing atypical bacteria, the prevalence of these organisms in CAP is likely to be underestimated (File TM et al. Infect Dis Clin North Am, 1998)

Etiology Pneumonia Atypical Patients (n) Mp (%) Cp (%) Lp (%) Bohte et al, 1994 (Dutch) 334 6 3 2 Marrie et al, 1996 (Canada) 149 26 14 1 Neill et al, 1996 (New Zealand) 251 16 3 11 Steinhoff et al, 1996 (Germany) 237 9 11 2 Lieberman et al, 1996 (Israel) 346 29 18 16 File et al, 1997 (USA) 456 9 22 2 Sopena et al, 1998 (Spain) 173 3 24 28 Ngeow et al, 2005 (Asia) 1374 12, 2 4, 7 6, 6 Mp= Mycoplasma pneumonia Cp= Chlamydia pneumonia LP = Legionella pneumonia Lieberman D. Clin Chest Med 1999; 20: 489 -97 Marrie TJ, et al. Am J Med 1996; 101: 508 -15 File TM, et al. Antimicrob Agents Chemother 1997; 41: 1965 -72 Sopena N, et al. Chest 1998; 113: 1195 -200 Steinhoff D, et al. Clin Infect Dis 1996; 22: 958 -64 Lieberman D, et al. Eur Respir J 1996; 9: 2630 -4 Neill AM, et al. Thorax 1996; 51: 1010 -6 Bohte R, et al. Thorax 1995; 50: 543 -7 Ngeow YF, et al. Int J Infect Dis 2005 May; 9: 144 -53

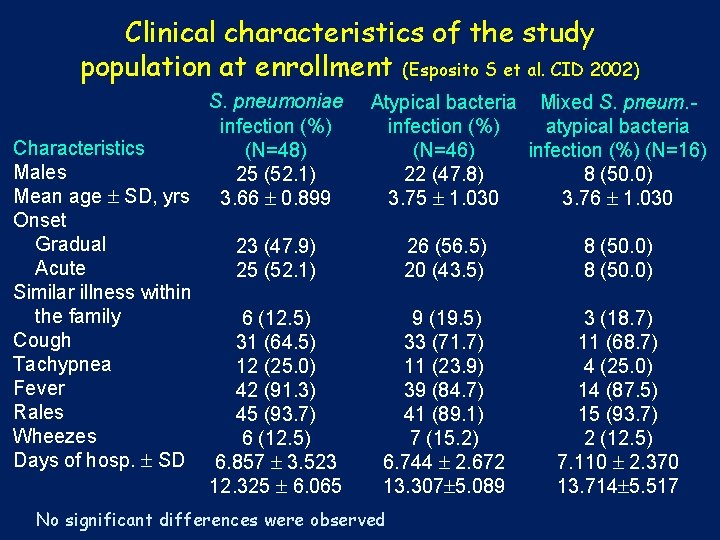
Clinical characteristics of the study population at enrollment (Esposito S et al. CID 2002) S. pneumoniae infection (%) Characteristics (N=48) Males 25 (52. 1) Mean age SD, yrs 3. 66 0. 899 Onset Gradual 23 (47. 9) Acute 25 (52. 1) Similar illness within the family 6 (12. 5) Cough 31 (64. 5) Tachypnea 12 (25. 0) Fever 42 (91. 3) Rales 45 (93. 7) Wheezes 6 (12. 5) Days of hosp. SD 6. 857 3. 523 12. 325 6. 065 Atypical bacteria Mixed S. pneum. infection (%) atypical bacteria (N=46) infection (%) (N=16) 22 (47. 8) 8 (50. 0) 3. 75 1. 030 3. 76 1. 030 26 (56. 5) 8 (50. 0) 20 (43. 5) 8 (50. 0) 9 (19. 5) 3 (18. 7) 33 (71. 7) 11 (68. 7) 11 (23. 9) 4 (25. 0) 39 (84. 7) 14 (87. 5) 41 (89. 1) 15 (93. 7) 7 (15. 2) 2 (12. 5) 6. 744 2. 672 7. 110 2. 370 13. 307 5. 089 13. 714 5. 517 No significant differences were observed

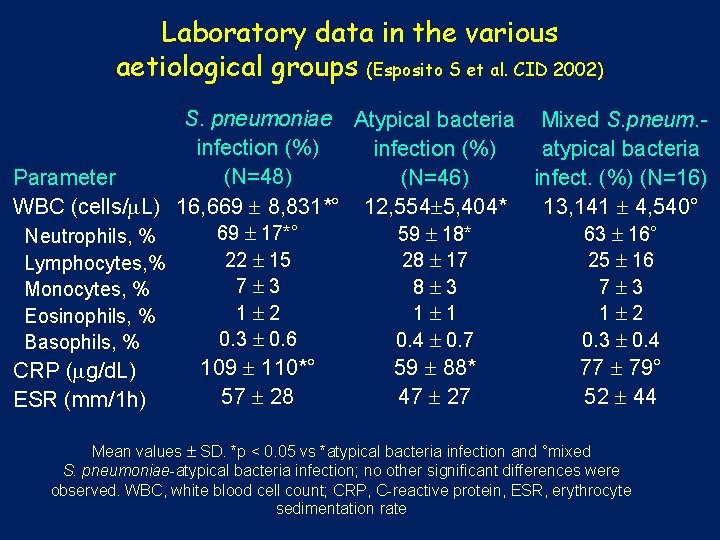
Laboratory data in the various aetiological groups (Esposito S et al. CID 2002) S. pneumoniae Atypical bacteria Mixed S. pneum. infection (%) atypical bacteria (N=48) Parameter (N=46) infect. (%) (N=16) WBC (cells/ L) 16, 669 8, 831*° 12, 554 5, 404* 13, 141 4, 540° 69 17*° 59 18* 63 16° Neutrophils, % Lymphocytes, % Monocytes, % Eosinophils, % Basophils, % CRP ( g/d. L) ESR (mm/1 h) 22 15 7 3 1 2 0. 3 0. 6 28 17 8 3 1 1 0. 4 0. 7 25 16 7 3 1 2 0. 3 0. 4 109 110*° 57 28 59 88* 47 27 77 79° 52 44 Mean values SD. *p < 0. 05 vs *atypical bacteria infection and °mixed S. pneumoniae-atypical bacteria infection; no other significant differences were observed. WBC, white blood cell count; CRP, C-reactive protein, ESR, erythrocyte sedimentation rate

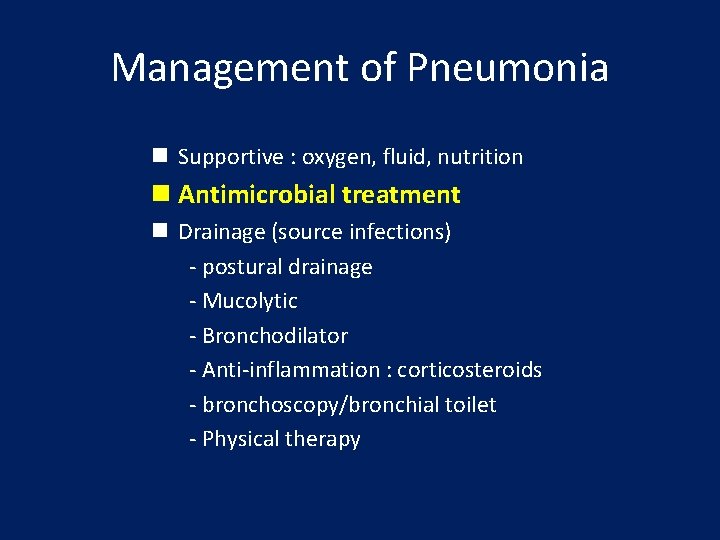
Management of Pneumonia n Supportive : oxygen, fluid, nutrition n Antimicrobial treatment n Drainage (source infections) - postural drainage - Mucolytic - Bronchodilator - Anti-inflammation : corticosteroids - bronchoscopy/bronchial toilet - Physical therapy

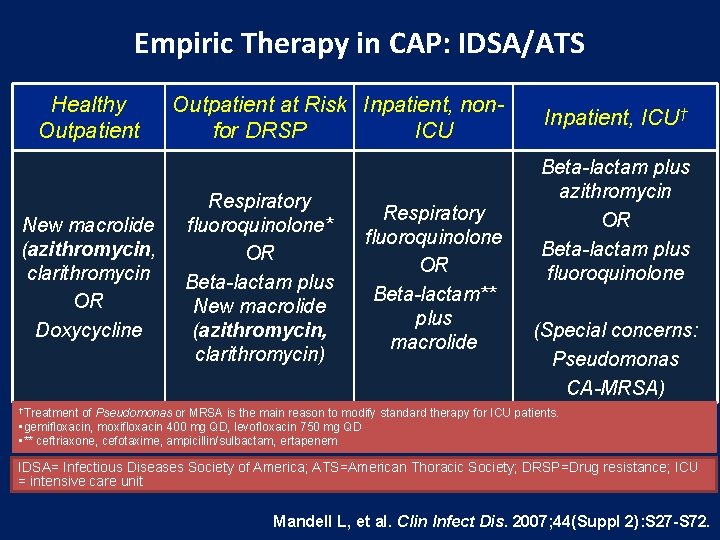
Empiric Therapy in CAP: IDSA/ATS Healthy Outpatient New macrolide (azithromycin, clarithromycin OR Doxycycline Outpatient at Risk Inpatient, nonfor DRSP ICU Respiratory fluoroquinolone* OR Beta-lactam plus New macrolide (azithromycin, clarithromycin) Respiratory fluoroquinolone OR Beta-lactam** plus macrolide Inpatient, ICU† Beta-lactam plus azithromycin OR Beta-lactam plus fluoroquinolone (Special concerns: Pseudomonas CA-MRSA) †Treatment of Pseudomonas or MRSA is the main reason to modify standard therapy for ICU patients. • gemifloxacin, moxifloxacin 400 mg QD, levofloxacin 750 mg QD • ** ceftriaxone, cefotaxime, ampicillin/sulbactam, ertapenem IDSA= Infectious Diseases Society of America; ATS=American Thoracic Society; DRSP=Drug resistance; ICU = intensive care unit Mandell L, et al. Clin Infect Dis. 2007; 44(Suppl 2): S 27 -S 72.

Empiric Therapy in CAP: Australia Mild (outpatient) Amoxicillin (Penicillin allergy: New macrolide (azithromycin, clarithromycin) Inpatient, non-ICU Inpatient, ICU Penicillin (Pen allergy: ceftriaxone) AND PLUS Gentamicin Doxycyline or New AND macrolide New macrolide (azithromycin, clarithromycin) www. safetyandquality. gov. au/. . . /3. 3 -Adult-Pneumonia-CPG-CAP-HAP

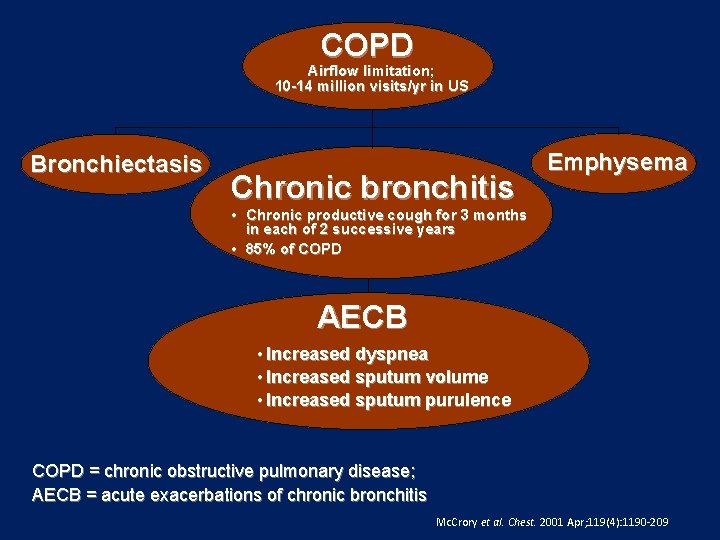
COPD Airflow limitation; 10 -14 million visits/yr in US Bronchiectasis Chronic bronchitis Emphysema • Chronic productive cough for 3 months in each of 2 successive years • 85% of COPD AECB • Increased dyspnea • Increased sputum volume • Increased sputum purulence COPD = chronic obstructive pulmonary disease; AECB = acute exacerbations of chronic bronchitis Mc. Crory et al. Chest. 2001 Apr; 119(4): 1190 -209

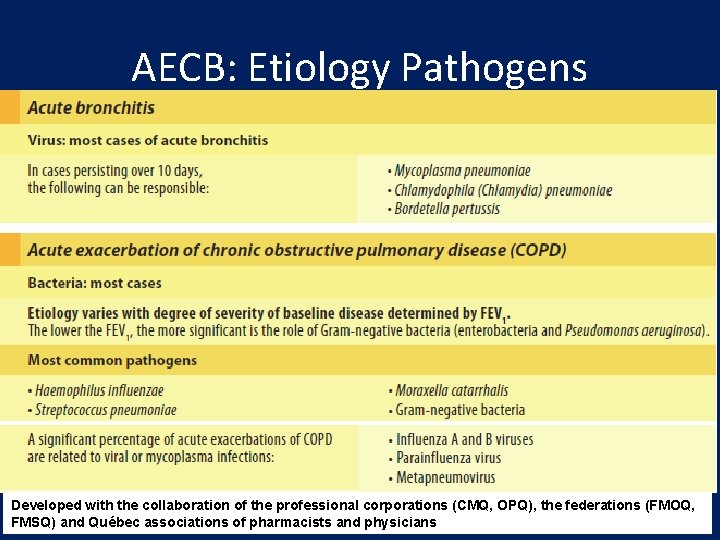
AECB: Etiology Pathogens Developed with the collaboration of the professional corporations (CMQ, OPQ), the federations (FMOQ, FMSQ) and Québec associations of pharmacists and physicians

AECB Stratification Increased dyspnea Increased Sputum purulence Type I: all three symptoms Treat Type II: two symptoms Probably Treat if include Purulence Type III: one symptom No treat Anthonisen NR et al. Ann Intern Med. 1987; 106: 196.

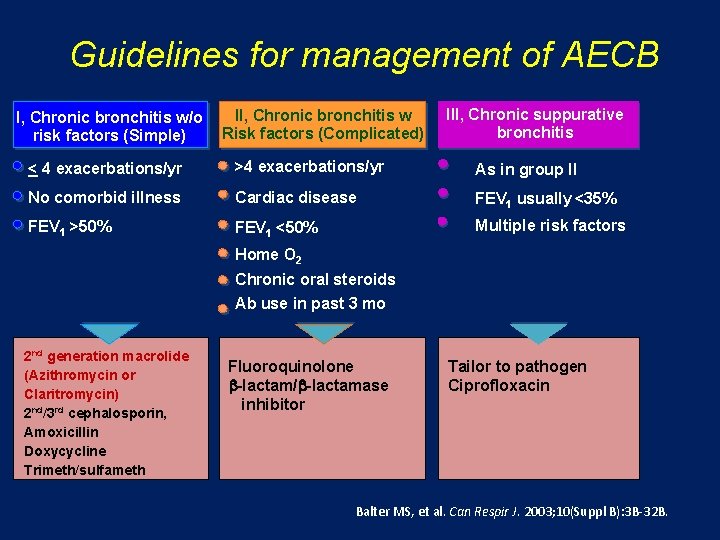
Guidelines for management of AECB I, Chronic bronchitis w/o risk factors (Simple) II, Chronic bronchitis w Risk factors (Complicated) III, Chronic suppurative bronchitis < 4 exacerbations/yr >4 exacerbations/yr As in group II No comorbid illness Cardiac disease FEV 1 usually <35% FEV 1 >50% FEV 1 <50% Multiple risk factors Home O 2 Chronic oral steroids Ab use in past 3 mo H. influenzae H. Spp M. catarrhalis S. pneumoniae Group I plus Klebsiella spp + Other gram-negatives Increased b-lactam resistance Group II plus P. Aeruginosa & Multi-resistant Enterobacteriaceae Balter MS, et al. Can Respir J. 2003; 10(Suppl B): 3 B-32 B.

Guidelines for management of AECB I, Chronic bronchitis w/o risk factors (Simple) II, Chronic bronchitis w Risk factors (Complicated) III, Chronic suppurative bronchitis < 4 exacerbations/yr >4 exacerbations/yr As in group II No comorbid illness Cardiac disease FEV 1 usually <35% FEV 1 >50% FEV 1 <50% Multiple risk factors Home O 2 Chronic oral steroids Ab use in past 3 mo 2 nd generation macrolide (Azithromycin or Claritromycin) 2 nd/3 rd cephalosporin, Amoxicillin Doxycycline Trimeth/sulfameth Fluoroquinolone b-lactam/b-lactamase inhibitor Tailor to pathogen Ciprofloxacin Balter MS, et al. Can Respir J. 2003; 10(Suppl B): 3 B-32 B.

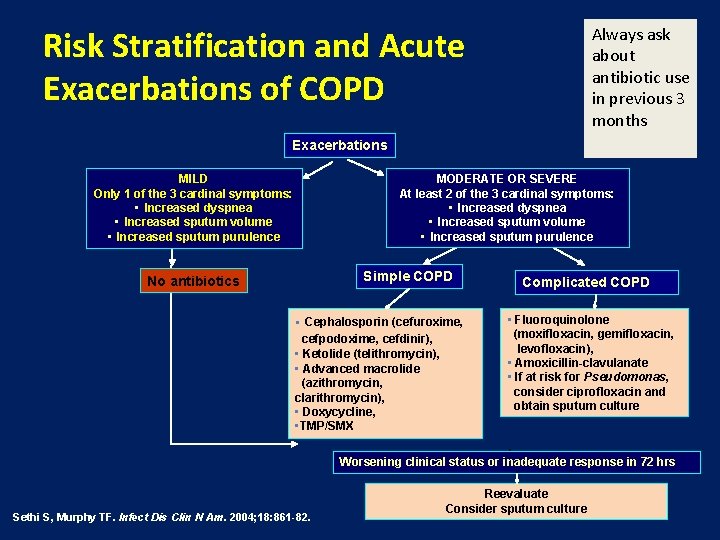
Always ask about antibiotic use in previous 3 months Risk Stratification and Acute Exacerbations of COPD Exacerbations MILD Only 1 of the 3 cardinal symptoms: • Increased dyspnea • Increased sputum volume • Increased sputum purulence MODERATE OR SEVERE At least 2 of the 3 cardinal symptoms: • Increased dyspnea • Increased sputum volume • Increased sputum purulence Simple COPD No antibiotics • Cephalosporin (cefuroxime, cefpodoxime, cefdinir), • Ketolide (telithromycin), • Advanced macrolide (azithromycin, clarithromycin), • Doxycycline, • TMP/SMX Complicated COPD • Fluoroquinolone (moxifloxacin, gemifloxacin, levofloxacin), • Amoxicillin-clavulanate • If at risk for Pseudomonas, consider ciprofloxacin and obtain sputum culture Worsening clinical status or inadequate response in 72 hrs Sethi S, Murphy TF. Infect Dis Clin N Am. 2004; 18: 861 -82. Reevaluate Consider sputum culture

Pathogenesis of Exacerbations Impaired host defenses: Hrespiratory virus Hnew strains of bacteria Henvironmental irritants Acute cycle Antibio tics Smoking/Irritants Chronic bacterial colonization Damaged respiratory epithelium Acute on chronic inflammation (bacterial + host mediated inflammatory factors) Chronic cycle Chronic inflammation Progressive loss of lung function and deteriorating quality of life (bacterial + host mediated inflammatory factors)

Azithromycin (gol azalides) • Azithromycin inhibits bacterial protein synthesis by binding to the 50 S ribosomal subunit of susceptible bacterial species 1– 3 – The ribosome is the site of protein synthesis in bacteria and other cells – This action interferes with the translocation of the growing peptide chain from one side of the ribosome to the other • A key point of differentiation between azithromycin and other macrolides is that it demonstrates a marked post-antibiotic effect (PAE)4 – Generally, antibiotics having PAE allows for less frequent dosing than those with minimal PAE while remaining effective • Azithromycin is bactericidal: it prevents bacteria from growing by interfering with their protein synthesis 2, 4 1. Murray PR. Medical Microbiology; 2. Retsema J, et al. Antimicrob Agents Chemother 1987; 31: 1939– 47; 3. Pfizer Egypt, Product Document, Zithromax®; 4. Lode H, et al. J Antimicrob Chemother 1996; 37(Suppl. C): 1– 8 21

Azithromycin • Azithromycin has a comprehensive spectrum of activity for its indications in adults and children 1– 3 • Azithromycin shows significant activity against Gram-positive and Gram-negative pathogens 1 (Typical and Atypical) • The antibacterial spectrum includes most of the frequent causes of community-acquired infections, including: 1 – – Haemophilus influenzae Streptococcus pneumoniae Moraxella catarrhalis Streptococcus pyogenes • The distinctive pharmacokinetic profile results in high and sustained concentration in a wide range of cells and tissues 2 1. Murray PR. Medical Microbiology; 2. Retsema J, et al. Antimicrob Agents Chemother 1987; 31: 1939– 47; 3. Pfizer Egypt, Product Document, Zithromax®; 4. Lode H, et al. J Antimicrob Chemother 1996; 37(Suppl. C): 1– 8 22

Beneficial Effects of Macrolide Activities as Antibacterial and Immunomodulatory Soichiro Kanoh, and Bruce K. Rubin Clin. Microbiol. Rev. 2010; 23: 590 -615

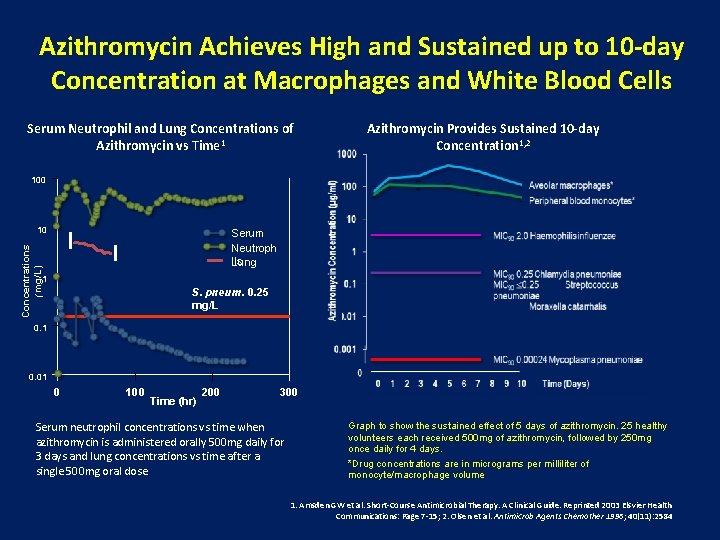
Azithromycin Achieves High and Sustained up to 10 -day Concentration at Macrophages and White Blood Cells Serum Neutrophil and Lung Concentrations of Azithromycin vs Time 1 Azithromycin Provides Sustained 10 -day Concentration 1, 2 100 10 Concentrations (mg/L) Serum Neutroph ils Lung 1 S. pneum. 0. 25 mg/L 0. 1 0. 01 0 100 Time (hr) 200 300 Serum neutrophil concentrations vs time when azithromycin is administered orally 500 mg daily for 3 days and lung concentrations vs time after a single 500 mg oral dose. Graph to show the sustained effect of 5 days of azithromycin. 25 healthy volunteers each received 500 mg of azithromycin, followed by 250 mg once daily for 4 days. *Drug concentrations are in micrograms per milliliter of monocyte/macrophage volume 1. Amsden GW et al. Short-Course Antimicrobial Therapy. A Clinical Guide. Reprinted 2003 Elsvier Health Communications: Page 7 -15; 2. Olsen et al. Antimicrob Agents Chemother 1996; 40(11): 2584

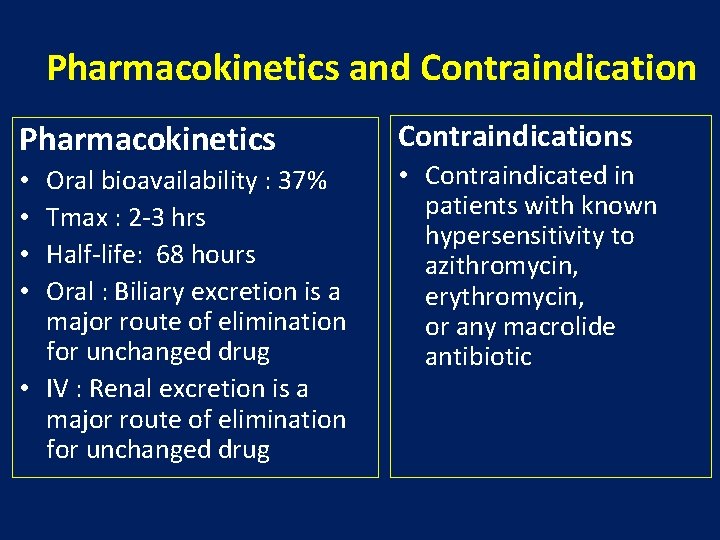
Pharmacokinetics and Contraindication Pharmacokinetics Oral bioavailability : 37% Tmax : 2 -3 hrs Half-life: 68 hours Oral : Biliary excretion is a major route of elimination for unchanged drug • IV : Renal excretion is a major route of elimination for unchanged drug • • Contraindications • Contraindicated in patients with known hypersensitivity to azithromycin, erythromycin, or any macrolide antibiotic

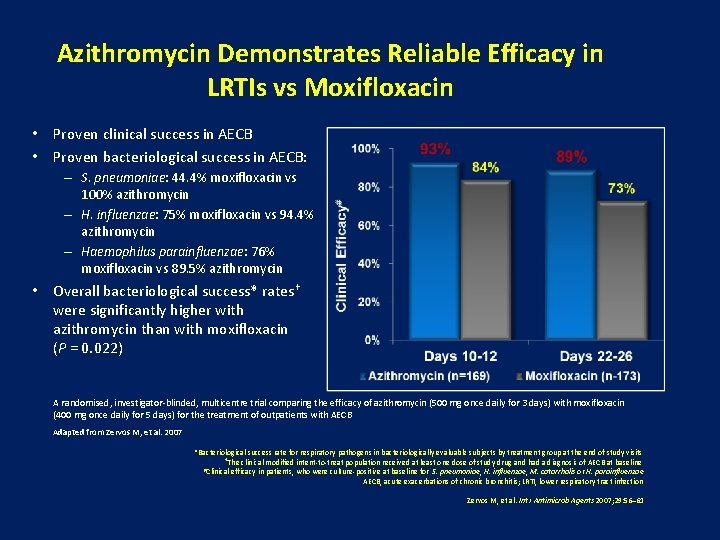
Azithromycin Demonstrates Reliable Efficacy in LRTIs vs Moxifloxacin • Proven clinical success in AECB • Proven bacteriological success in AECB: – S. pneumoniae: 44. 4% moxifloxacin vs 100% azithromycin – H. influenzae: 75% moxifloxacin vs 94. 4% azithromycin – Haemophilus parainfluenzae: 76% moxifloxacin vs 89. 5% azithromycin • Overall bacteriological success* rates† were significantly higher with azithromycin than with moxifloxacin (P = 0. 022) A randomised, investigator-blinded, multicentre trial comparing the efficacy of azithromycin (500 mg once daily for 3 days) with moxifloxacin (400 mg once daily for 5 days) for the treatment of outpatients with AECB Adapted from Zervos M, et al. 2007 *Bacteriological success rate for respiratory pathogens in bacteriologically evaluable subjects by treatment group at the end of study visits †The clinical modified intent-to-treat population received at least one dose of study drug and had a diagnosis of AECB at baseline #Clinical efficacy in patients, who were culture-positive at baseline for S. pneumoniae, H. influenzae, M. catarrhalis or H. parainfluenzae AECB, acute exacerbations of chronic bronchitis; LRTI, lower respiratory tract infection Zervos M, et al. Int J Antimicrob Agents 2007; 29: 56– 61

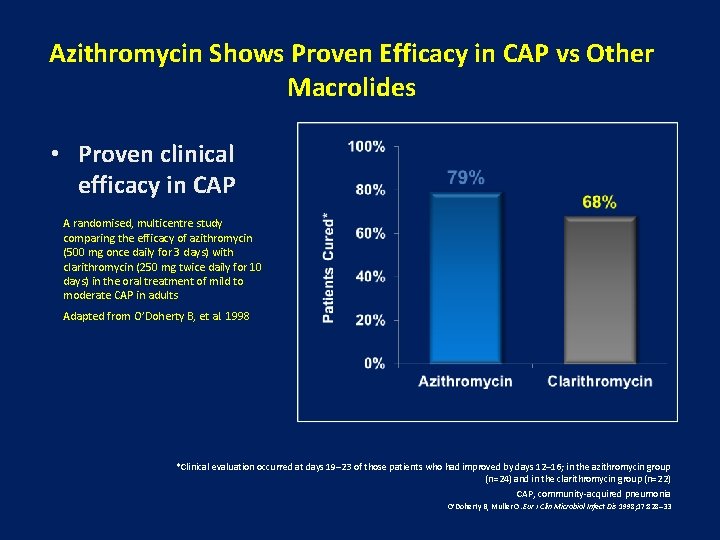
Azithromycin Shows Proven Efficacy in CAP vs Other Macrolides • Proven clinical efficacy in CAP A randomised, multicentre study comparing the efficacy of azithromycin (500 mg once daily for 3 days) with clarithromycin (250 mg twice daily for 10 days) in the oral treatment of mild to moderate CAP in adults Adapted from O’Doherty B, et al. 1998 *Clinical evaluation occurred at days 19– 23 of those patients who had improved by days 12– 16; in the azithromycin group (n=24) and in the clarithromycin group (n=22) CAP, community-acquired pneumonia O’Doherty B, Muller O. Eur J Clin Microbiol Infect Dis 1998; 17: 828– 33

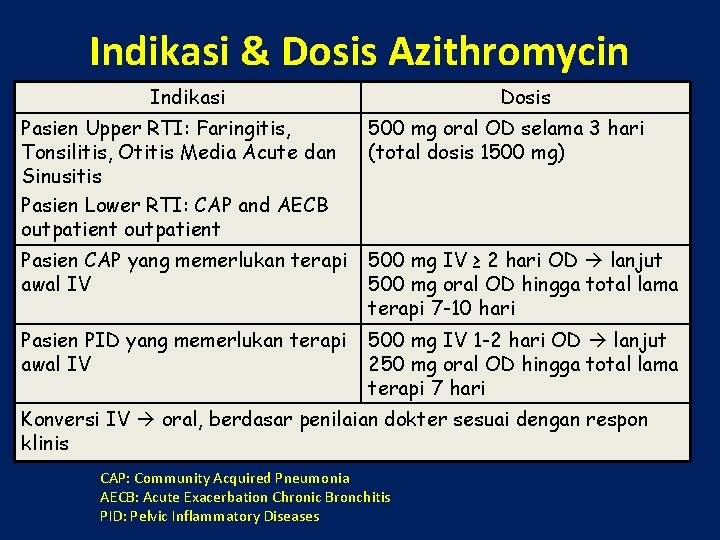
Indikasi & Dosis Azithromycin Indikasi Dosis Pasien Upper RTI: Faringitis, Tonsilitis, Otitis Media Acute dan Sinusitis Pasien Lower RTI: CAP and AECB outpatient 500 mg oral OD selama 3 hari (total dosis 1500 mg) Pasien CAP yang memerlukan terapi awal IV 500 mg IV ≥ 2 hari OD lanjut 500 mg oral OD hingga total lama terapi 7 -10 hari Pasien PID yang memerlukan terapi awal IV 500 mg IV 1 -2 hari OD lanjut 250 mg oral OD hingga total lama terapi 7 hari Konversi IV oral, berdasar penilaian dokter sesuai dengan respon klinis CAP: Community Acquired Pneumonia AECB: Acute Exacerbation Chronic Bronchitis PID: Pelvic Inflammatory Diseases

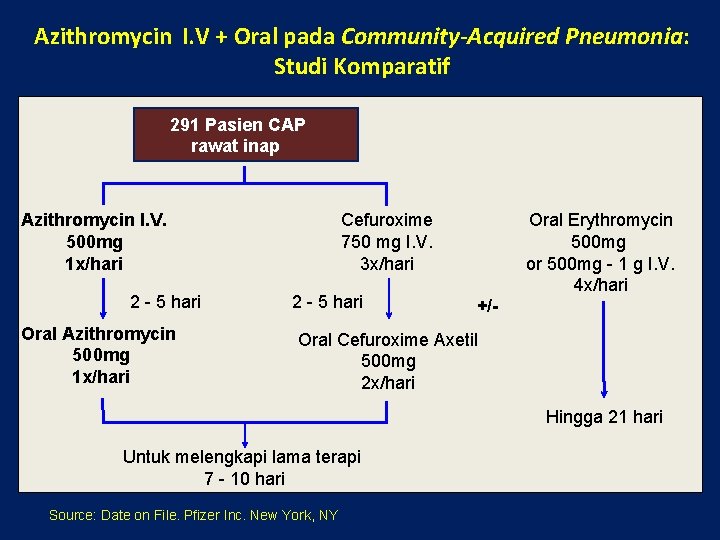
Azithromycin I. V + Oral pada Community-Acquired Pneumonia: Studi Komparatif 291 Pasien CAP rawat inap Azithromycin I. V. 500 mg 1 x/hari 2 - 5 hari Oral Azithromycin 500 mg 1 x/hari Cefuroxime 750 mg I. V. 3 x/hari 2 - 5 hari Oral Erythromycin 500 mg or 500 mg - 1 g I. V. 4 x/hari +/- Oral Cefuroxime Axetil 500 mg 2 x/hari Hingga 21 hari Untuk melengkapi lama terapi 7 - 10 hari Source: Date on File. Pfizer Inc. New York, NY

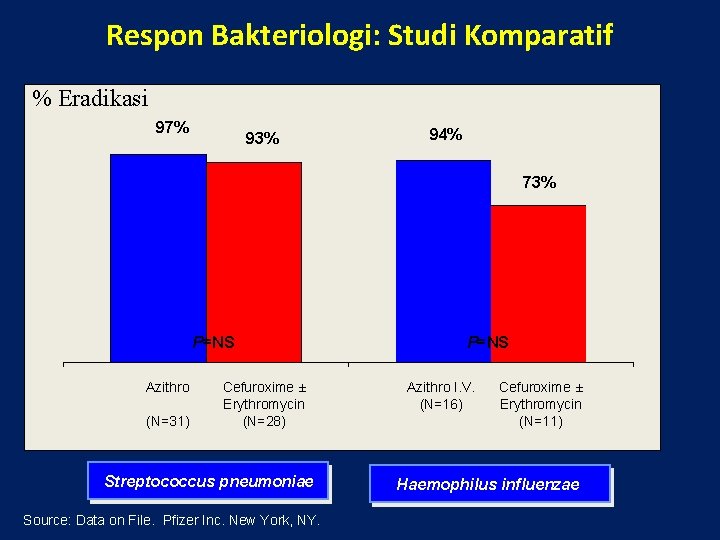
Respon Bakteriologi: Studi Komparatif % Eradikasi 97% 93% 94% 73% P=NS Azithro (N=31) Cefuroxime ± Erythromycin (N=28) Streptococcus pneumoniae Source: Data on File. Pfizer Inc. New York, NY. P=NS Azithro I. V. (N=16) Cefuroxime ± Erythromycin (N=11) Haemophilus influenzae

Keberhasilan Klinis* 10 - 14 Hari Paska Terapi (studi hari ke 17 - 24): Studi Komparatif % Keberhasilan klinis 10 - 14 hari 78% 74% P=NS Azithro® (N=137) Cefuroxime ± Erythromycin (N=131) Total rata-rata lama Terapi pada Evaluable Patients (I. V. & Oral) (Hari) • Azithromycin : 8, 6 hari Cured Plus Improved • Cefuroxime + Erythromycin : 10, 3 hari Source: Data on File. Pfizer Inc. New York, NY.

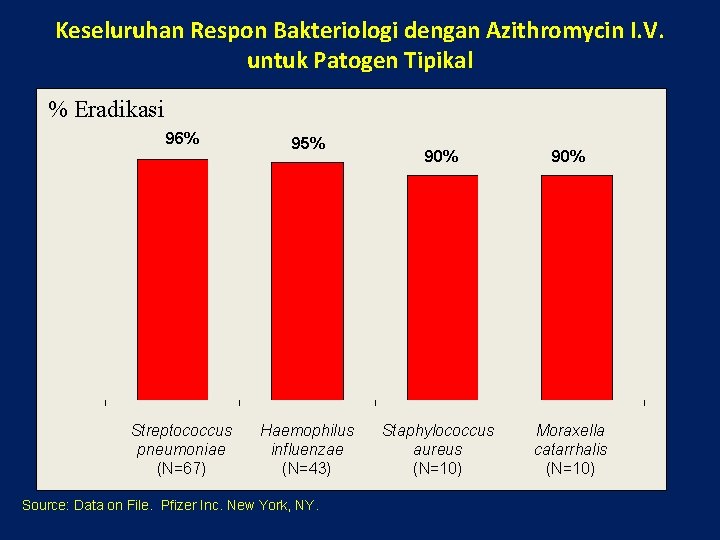
Keseluruhan Respon Bakteriologi dengan Azithromycin I. V. untuk Patogen Tipikal % Eradikasi 96% 95% Streptococcus pneumoniae (N=67) Haemophilus influenzae (N=43) Source: Data on File. Pfizer Inc. New York, NY. 90% Staphylococcus aureus (N=10) Moraxella catarrhalis (N=10)

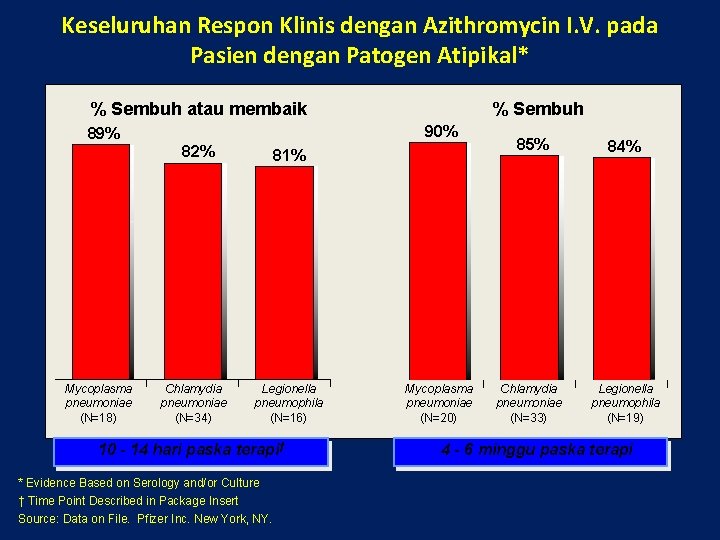
Keseluruhan Respon Klinis dengan Azithromycin I. V. pada Pasien dengan Patogen Atipikal* % Sembuh atau membaik 90% 89% 82% Mycoplasma pneumoniae (N=18) % Sembuh Chlamydia pneumoniae (N=34) 81% Legionella pneumophila (N=16) 10 - 14 hari paska terapi† * Evidence Based on Serology and/or Culture † Time Point Described in Package Insert Source: Data on File. Pfizer Inc. New York, NY. Mycoplasma pneumoniae (N=20) 85% Chlamydia pneumoniae (N=33) 84% Legionella pneumophila (N=19) 4 - 6 minggu paska terapi

Efek Samping Terkait Terapi & Penghentian Terapi: Studi Community -Acquired Pneumonia Komparatif & Non-Komparatif Event Azithro® I. V. (N=414) n (%) Gastrointestinal Diare 18 (4. 3) Mual 16 (3. 9) Nyeri perut Muntah Lokasi infus 11 (2. 7) 6 (1. 4) Nyeri Infeksi/radang 27 (6. 5) 13 (3. 1) u Only 1. 2% Discontinued I. V and Only 2. 4% Discontinued I. V. /Oral Therapy with Azitro Because of Clinical or Laboratory Side Effects Source: Data on File. Pfizer Inc. New York, NY.

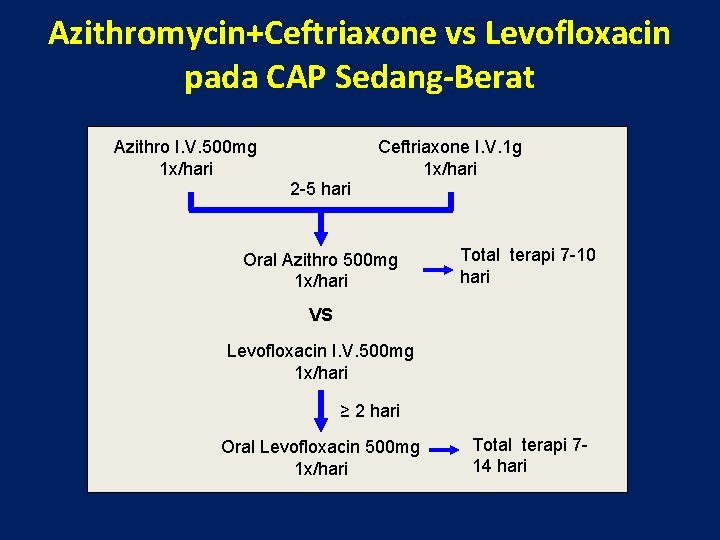
Azithromycin+Ceftriaxone vs Levofloxacin pada CAP Sedang-Berat Azithro I. V. 500 mg 1 x/hari Ceftriaxone I. V. 1 g 1 x/hari 2 -5 hari Oral Azithro 500 mg 1 x/hari Total terapi 7 -10 hari VS Levofloxacin I. V. 500 mg 1 x/hari ≥ 2 hari Oral Levofloxacin 500 mg 1 x/hari Total terapi 714 hari

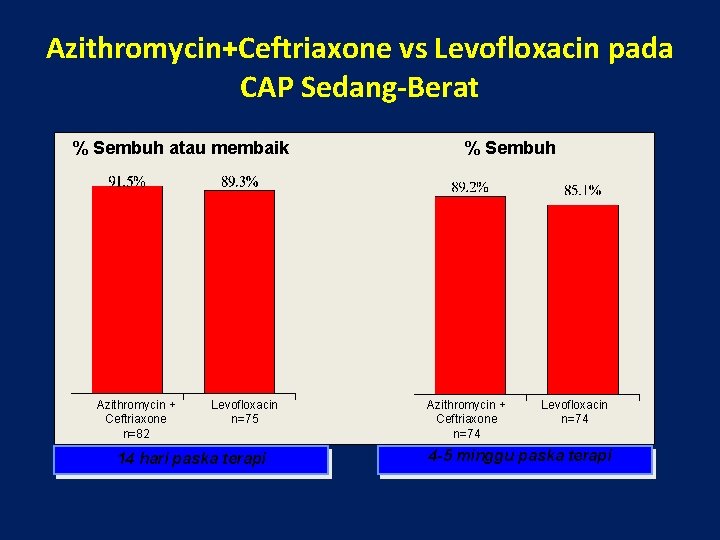
Azithromycin+Ceftriaxone vs Levofloxacin pada CAP Sedang-Berat % Sembuh atau membaik Azithromycin + Ceftriaxone n=82 Levofloxacin n=75 14 hari paska terapi % Sembuh Azithromycin + Ceftriaxone n=74 Levofloxacin n=74 4 -5 minggu paska terapi

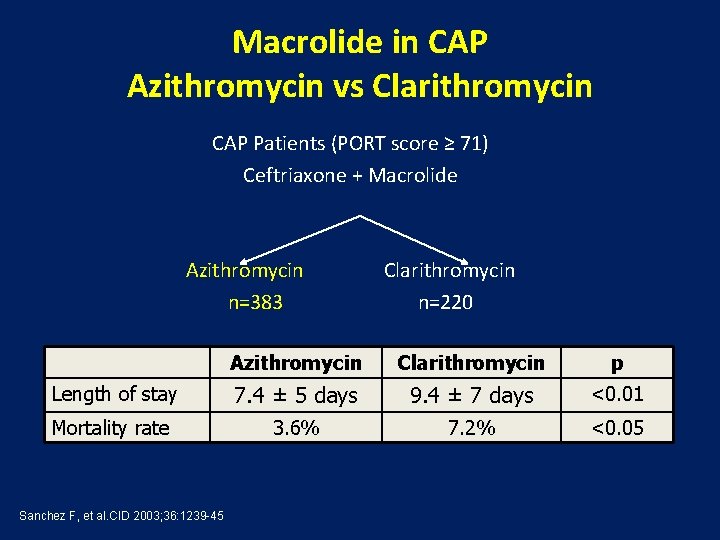
Macrolide in CAP Azithromycin vs Clarithromycin CAP Patients (PORT score ≥ 71) Ceftriaxone + Macrolide Azithromycin Clarithromycin n=383 n=220 Azithromycin Clarithromycin p Length of stay 7. 4 ± 5 days 9. 4 ± 7 days <0. 01 Mortality rate 3. 6% 7. 2% <0. 05 Sanchez F, et al. CID 2003; 36: 1239 -45

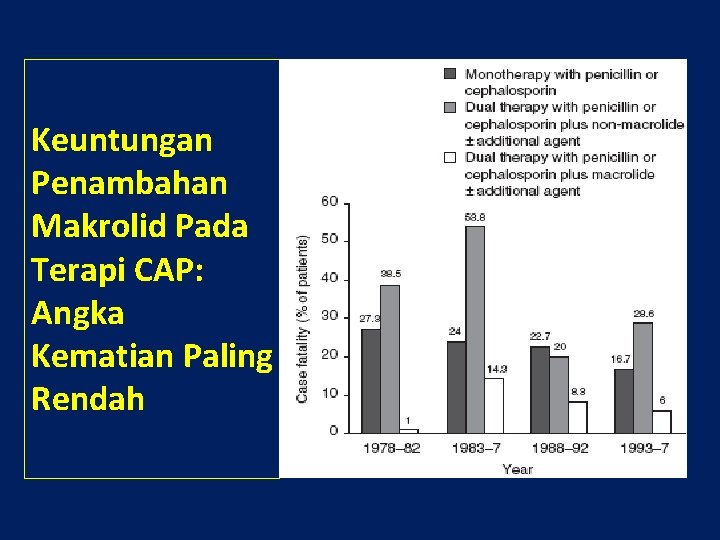
Keuntungan Penambahan Makrolid Pada Terapi CAP: Angka Kematian Paling Rendah

Advantages of Azithromycin for Acute Bacterial Lower Respiratory Tract Infection Out Patient Once daily dose Short duration therapy (3 or 5 days) Bactericidal activity (Typical and Atypical) AZITHROMYCIN High concentrations lung MICs Concentration up to 10 days Well tolerated

“Yes, Azithromycin Is Recommended in Guidelines of Respiratory Tract Infections” • Recommended as a first-line choice by reputable adult and paediatric regional and international guidelines including: – IDSA/ATS (Infectious Diseases of America/American Thoracic Society) consensus guidelines on the management of community-acquired pneumonia in adults (2007)1 – IDSA the management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America (2011) 2 – IDSA clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America (2012)3 – GCC (Gulf Cooperation Council) practice guidelines for the management of community-acquired pneumonia (2007)4 – Medical Associates clinical practice guideline for the management of acute ear infection in children 2 months to 12 years (2012)5

Take Home Messages • LRTIs are most common infections requiring antibiotic tx in outpatient or inpatient setting • Abuse of antibiotics is frequent for these indications – Strict criteria are required for treatment • Atypical bacteria have a roles in etiology of LRTI CAP • Macrolides are effective and safe agents for LRTIs – Azithromycin with properities is suitable for all these indications • Long-high life, high tissue penetration, immunomodulatory properities, confidence compliance and safety.

Thank You 42
 Dr. prayudi santoso, sp. pd-kp, mkes
Dr. prayudi santoso, sp. pd-kp, mkes Cvu curriculum
Cvu curriculum Objective for cv
Objective for cv Ivotopis
Ivotopis Minuta de acuerdos
Minuta de acuerdos Struktur curriculum vitae
Struktur curriculum vitae British curriculum vitae
British curriculum vitae Science experts network curriculum vitae
Science experts network curriculum vitae Caracteristicas externas de curriculum vitae
Caracteristicas externas de curriculum vitae Curriculum vitae forma
Curriculum vitae forma Curriculum vitae caracteristicas internas y externas
Curriculum vitae caracteristicas internas y externas Curriculum vitae esempi
Curriculum vitae esempi Contents of curriculum vitae
Contents of curriculum vitae Components of a curriculum vitae
Components of a curriculum vitae Sommaire dans un cv
Sommaire dans un cv Curriculum vitae de jesus de nazaret
Curriculum vitae de jesus de nazaret Curriculum vitae de carlos slim
Curriculum vitae de carlos slim Curriculum vitae esquema
Curriculum vitae esquema Caracteristicas externas e internas del mapa conceptual
Caracteristicas externas e internas del mapa conceptual Elementos de un curriculum vitae
Elementos de un curriculum vitae Curriculum vitae importancia
Curriculum vitae importancia Job application communication skills
Job application communication skills Vad betyder curriculum vitae
Vad betyder curriculum vitae Curriculum vitae výslovnost
Curriculum vitae výslovnost Freddy santoso
Freddy santoso Myopic
Myopic Jahja santoso
Jahja santoso Adhi setyo santoso
Adhi setyo santoso Stj budi santoso
Stj budi santoso Arbour vitae uteri
Arbour vitae uteri Eco vitae
Eco vitae Arbor vitae
Arbor vitae Endopelvic fascia
Endopelvic fascia Sheep brain superior view
Sheep brain superior view Non scholae sed vitae discimus
Non scholae sed vitae discimus Evangelium vitae 33
Evangelium vitae 33 Cv pisanje
Cv pisanje Curricuum vitae
Curricuum vitae Primary taste cortex
Primary taste cortex Bank niemowlaka rydygiera
Bank niemowlaka rydygiera What is vita
What is vita Finis vitae sed non amoris.
Finis vitae sed non amoris. Anamnesis vitae meaning
Anamnesis vitae meaning Integer vitae scelerisque purus
Integer vitae scelerisque purus