Curriculum Vitae Dr Prayudi Santoso Sp PDKP M








![ILLUMINATE: Indacaterol/glycopyrronium vs. fluticasone/salmeterol in COPD Pre-dose FEV 1 [L] 1. 65 ∆ = ILLUMINATE: Indacaterol/glycopyrronium vs. fluticasone/salmeterol in COPD Pre-dose FEV 1 [L] 1. 65 ∆ =](https://slidetodoc.com/presentation_image_h2/b03ff55a9624917daea2fd3f0b57dcae/image-30.jpg)
![Dosing frequency and adherence in COPD Days covered [%] 55 076 COPD patients 1999 Dosing frequency and adherence in COPD Days covered [%] 55 076 COPD patients 1999](https://slidetodoc.com/presentation_image_h2/b03ff55a9624917daea2fd3f0b57dcae/image-32.jpg)



- Slides: 35

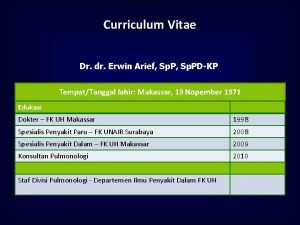
Curriculum Vitae Dr. Prayudi Santoso, Sp. PD-KP, M. Kes, FCCP, FINASIM E-mail: prayudimartha@yahoo. com Education : MD Internal Med Pulmonology Consultant MSc Medical School, Padjadjaran University, Bandung, Indonesia Collegiums of Internal Medicine, Indonesia Medical School, Padjadjaran University, Bandung Indonesia Occupation : Staf of Respirology Division & Critical Care Internal Medicine, Faculty of Medicine Padjadjaran university/Hasan Sadikin General Hospital, Indonesia Coordinator of MDR-TB Team Hasan Sadikin General Hospital Bandung, Indonesia Organization : Society of Internal Medicine West Java, - Indonesia Society of Respirologi Indonesia (PERPARI) Fellow American College of Chest Physcian (ACCP) Member European Respiratory Society (ERS)

Dual Bronchodilator in COPD from Clinical Data to Patient's Benefit in Real Life Prayudi Santoso Internal Medicine Department, Division of Pulmonary and Critical Care, Hasan Sadikin Hospital, University of Padjadjaran Medical School Bandung Indonesia 2016 prayudimartha@yahoo. com

Global Initiative for COPD Burden of COPD • COPD is a leading cause of morbidity and mortality worldwide • The burden of COPD is projected to increase in coming decades due to continued exposure to COPD risk factors and the aging of the world’s population • COPD is associated with significant economic burden http: //www. goldcopd. org

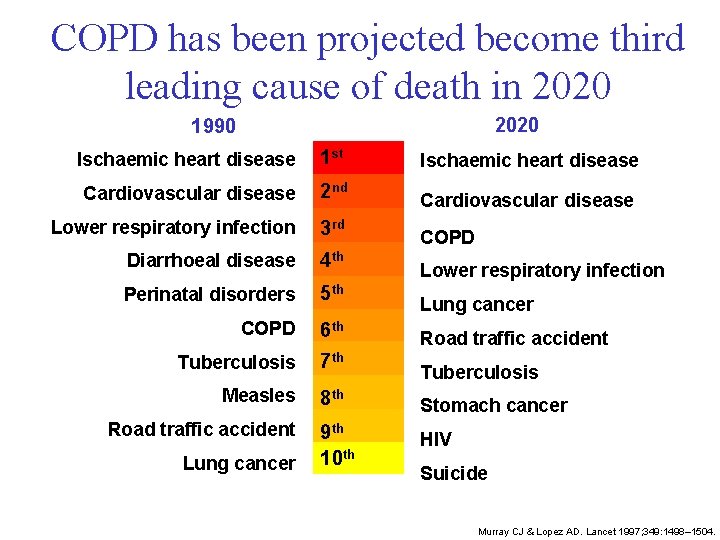
COPD has been projected become third leading cause of death in 2020 1990 Ischaemic heart disease 1 st Ischaemic heart disease Cardiovascular disease 2 nd Cardiovascular disease Lower respiratory infection 3 rd COPD Diarrhoeal disease 4 th Perinatal disorders 5 th COPD 6 th Tuberculosis 7 th Measles 8 th Stomach cancer 9 th 10 th HIV Road traffic accident Lung cancer Lower respiratory infection Lung cancer Road traffic accident Tuberculosis Suicide Murray CJ & Lopez AD. Lancet 1997; 349: 1498– 1504.

Bronchodilation is the foundation of pharmacological COPD treatment Reduction of airflow limitation and hyperinflation • Symptom relief • Improvement of physical performance www. goldcopd. org/. . . /GOLD 2015_summary. pdf

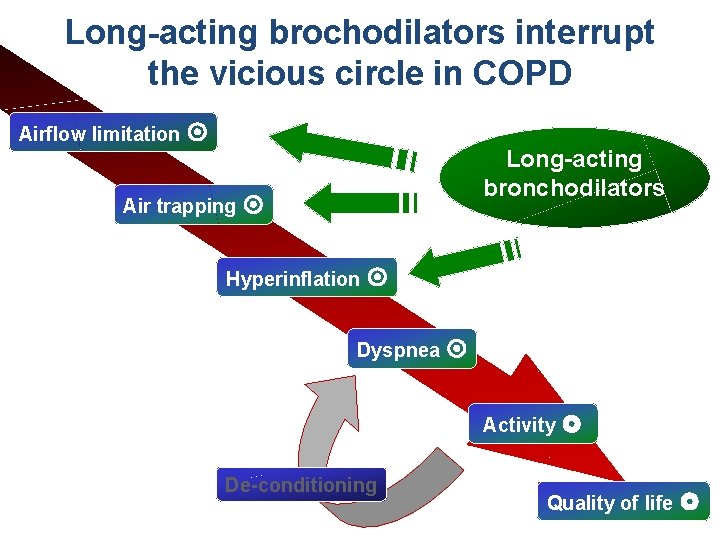
Long-acting brochodilators interrupt Vicious circle in COPD the vicious circle in COPD Flow limitation Airflow limitation Long-acting bronchodilators Air trapping Hyperinflation Dyspnea Activity De-conditioning Quality of life

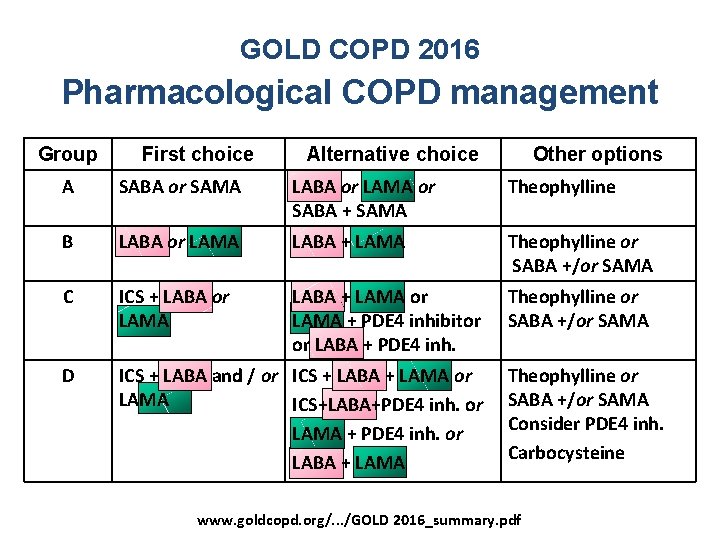
GOLD COPD 2016 Pharmacological COPD management Group First choice Alternative choice Other options A SABA or SAMA LABA or LAMA or SABA + SAMA Theophylline B LABA or LAMA LABA + LAMA Theophylline or SABA +/or SAMA C ICS + LABA or LAMA LABA + LAMA or LAMA + PDE 4 inhibitor or LABA + PDE 4 inh. Theophylline or SABA +/or SAMA D ICS + LABA and / or ICS + LABA + LAMA or LAMA ICS+LABA+PDE 4 inh. or LAMA + PDE 4 inh. or LABA + LAMA Theophylline or SABA +/or SAMA Consider PDE 4 inh. Carbocysteine www. goldcopd. org/. . . /GOLD 2016_summary. pdf

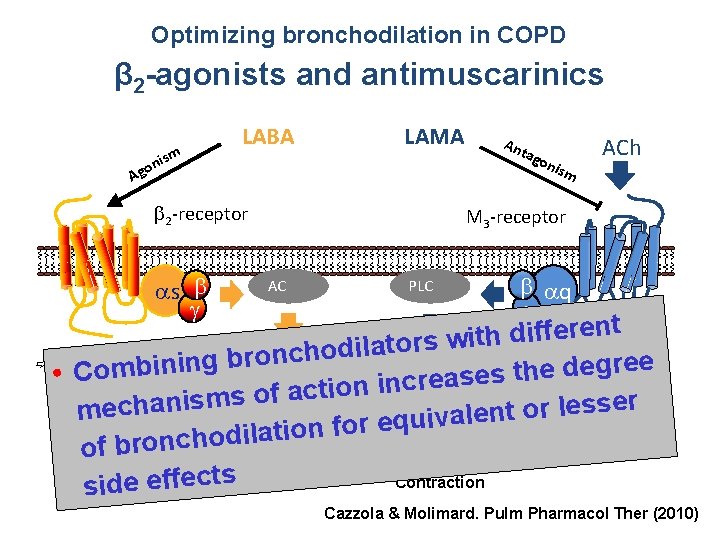
Optimizing bronchodilation in COPD β 2 -agonists and antimuscarinics LABA ism n o g LAMA A 2 -receptor An tag oni s m ACh M 3 -receptor h s AC PLC q t n e r e f f i d h t i s 2+ w r o t a l i Ca c. AMP d o h c ron Antagonism ofgree b β 2 stimulation and g n i e b d m e o h t • C s acetylcholine via M 3 e PKA-mediated Inhibition s a e r c n i n o i t c inhibits vagal tone of MLCK directly reducesms of a s r i e n s a s h e l ec contraction r smooth o PKA mmuscle MLCK t n e al v i u q e r o f n o ati l i d o h c n o r b of Contraction side effects Relaxation Cazzola & Molimard. Pulm Pharmacol Ther (2010)

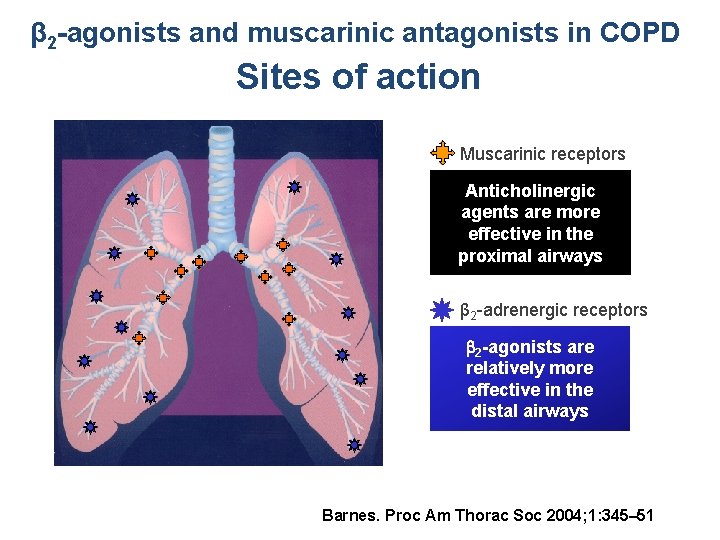
β 2 -agonists and muscarinic antagonists in COPD Sites of action Muscarinic receptors Anticholinergic agents are more effective in the proximal airways β 2 -adrenergic receptors 2 -agonists are relatively more effective in the distal airways Barnes. Proc Am Thorac Soc 2004; 1: 345– 51

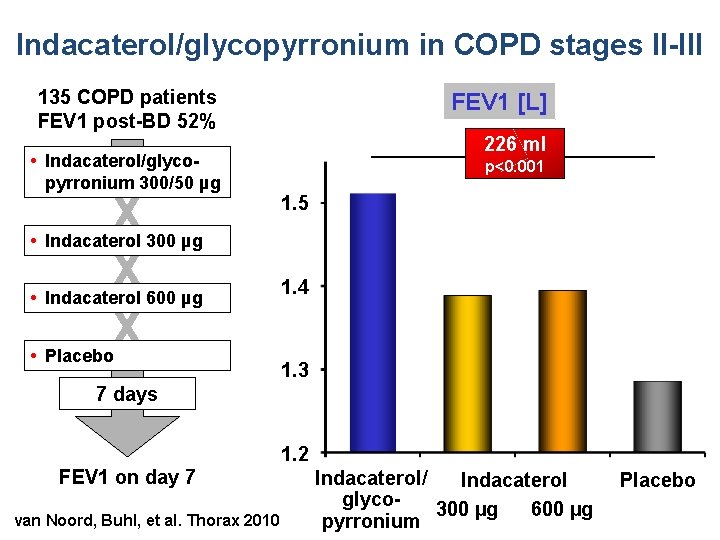
Indacaterol/glycopyrronium in COPD stages II-III 135 COPD patients FEV 1 post-BD 52% • Indacaterol/glycopyrronium 300/50 µg FEV 1 [L] X X X 226 ml p<0. 001 1. 5 • Indacaterol 300 µg • Indacaterol 600 µg • Placebo 1. 4 1. 3 7 days FEV 1 on day 7 van Noord, Buhl, et al. Thorax 2010 1. 2 Indacaterol/ Indacaterol glyco 300 µg 600 µg pyrronium Placebo

Fixed Dose Combination indacaterol/glycopyrronium The IGNITE Program 11 studies with more than 10, 000 patients across 52 countries

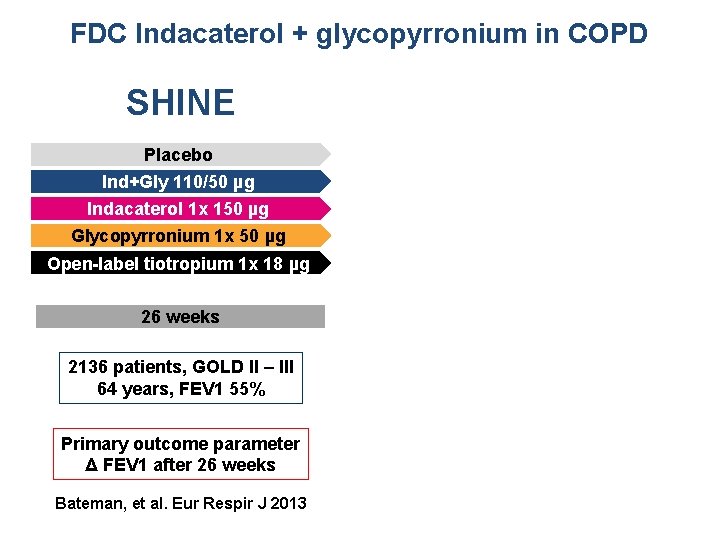
FDC Indacaterol + glycopyrronium in COPD SHINE SPARK Placebo Ind+Gly 110/50 µg QVA 149 1 x 110/50 µg Indacaterol 1 x 150 µg Glycopyrronium 1 x 50 µg Open-label tiotropium 1 x 18 µg 26 weeks 64 weeks 2136 patients, GOLD II – III 64 years, FEV 1 55% 2206 patients, GOLD III – IV 63 years, FEV 1 37% Primary outcome parameter Δ FEV 1 after 26 weeks Primary outcome parameter Δ exacerbations in 64 weeks Bateman, et al. Eur Respir J 2013 Wedzicha, et al. Lancet Respir Med 1(3): 199 -209, 2013

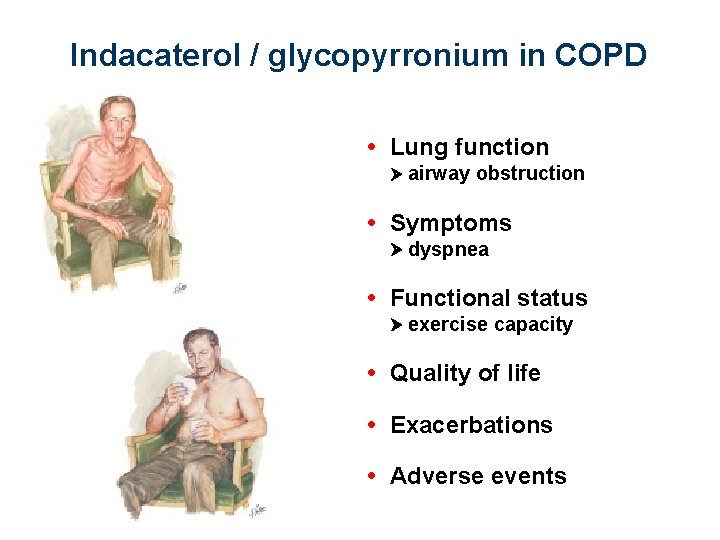
Indacaterol + glycopyrronium in COPD • Lung function airway obstruction

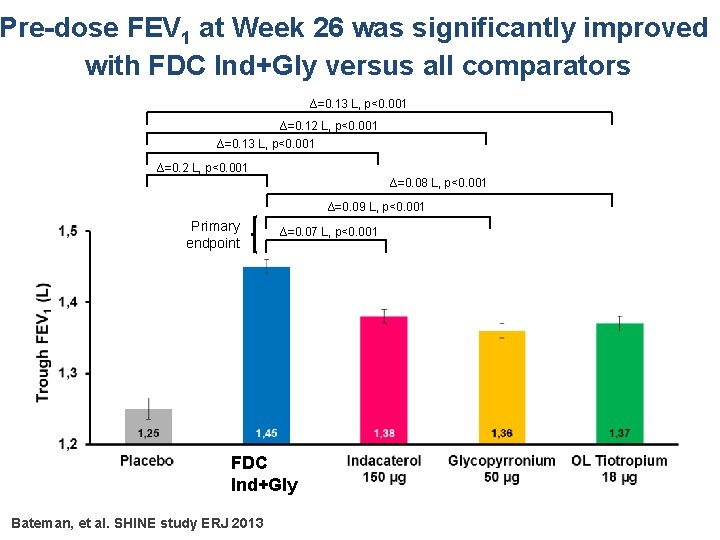
Pre-dose FEV 1 at Week 26 was significantly improved with FDC Ind+Gly versus all comparators ∆=0. 13 L, p<0. 001 ∆=0. 12 L, p<0. 001 ∆=0. 13 L, p<0. 001 ∆=0. 2 L, p<0. 001 ∆=0. 08 L, p<0. 001 ∆=0. 09 L, p<0. 001 Primary endpoint ∆=0. 07 L, p<0. 001 FDC Ind+Gly Bateman, et al. SHINE study ERJ 2013

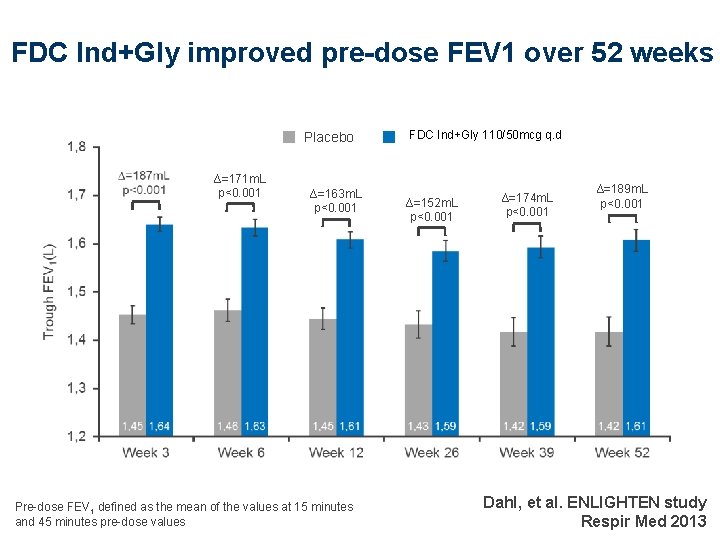
FDC Ind+Gly improved pre-dose FEV 1 over 52 weeks Placebo ∆=171 m. L p<0. 001 ∆=163 m. L p<0. 001 Pre-dose FEV 1 defined as the mean of the values at 15 minutes and 45 minutes pre-dose values FDC Ind+Gly 110/50 mcg QVA 149 110/50 μg q. d ∆=152 m. L p<0. 001 ∆=174 m. L p<0. 001 ∆=189 m. L p<0. 001 Dahl, et al. ENLIGHTEN study Respir Med 2013

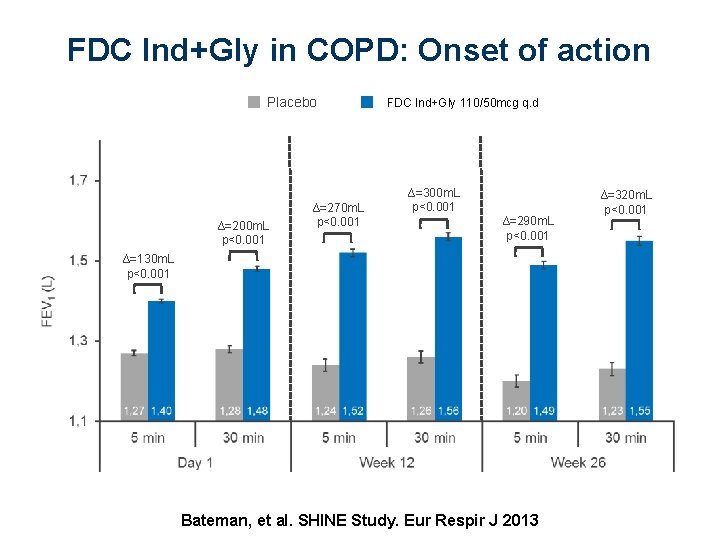
FDC Ind+Gly in COPD: Onset of action Placebo ∆=200 m. L p<0. 001 ∆=270 m. L p<0. 001 FDC Ind+Gly 110/50 mcg QVA 149 110/50 μg q. d ∆=300 m. L p<0. 001 ∆=290 m. L p<0. 001 ∆=130 m. L p<0. 001 Bateman, et al. SHINE Study. Eur Respir J 2013 ∆=320 m. L p<0. 001

Indacaterol / glycopyrronium in COPD • Lung function airway obstruction • Symptoms dyspnea

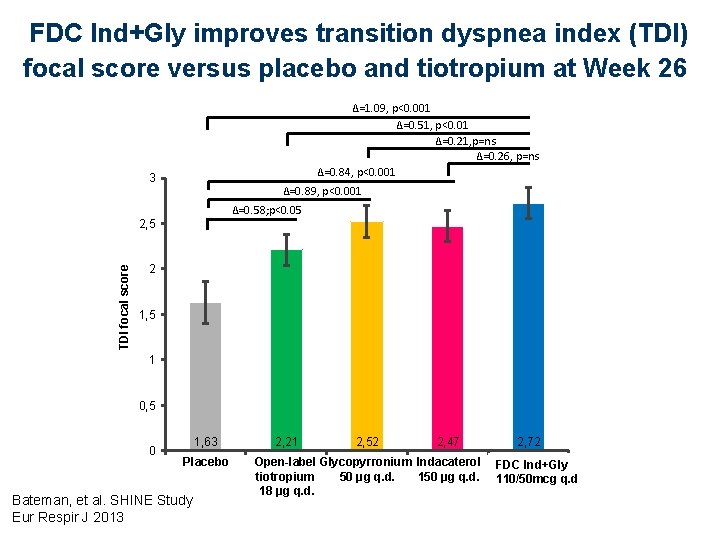
FDC Ind+Gly improves transition dyspnea index (TDI) focal score versus placebo and tiotropium at Week 26 ∆=1. 09, p<0. 001 ∆=0. 51, p<0. 01 ∆=0. 21, p=ns ∆=0. 26, p=ns ∆=0. 84, p<0. 001 3 ∆=0. 89, p<0. 001 ∆=0. 58; p<0. 05 TDI focal score 2, 5 2 1, 5 1 0, 5 0 1, 63 Placebo Bateman, et al. SHINE Study Eur Respir J 2013 2, 21 2, 52 2, 47 2, 72 Open-label Glycopyrronium Indacaterol FDC QVA 149 Ind+Gly tiotropium 50 μg q. d. 110/50 mcg q. d 18 μg q. d.

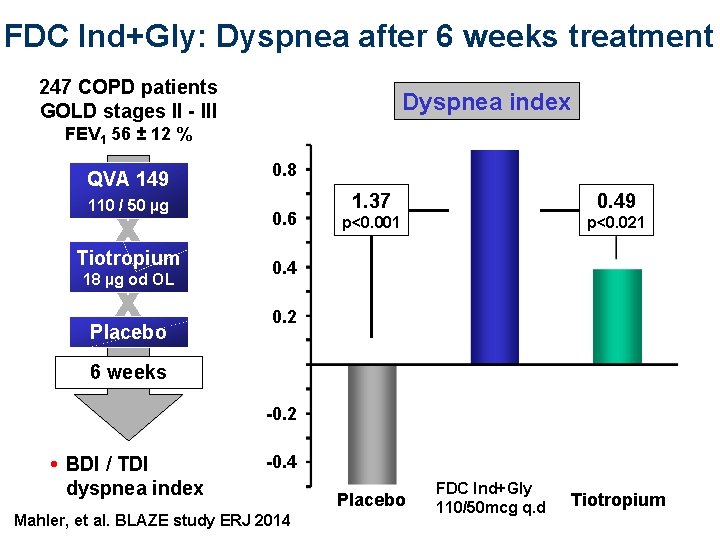
FDC Ind+Gly: Dyspnea after 6 weeks treatment 247 COPD patients GOLD stages II - III Dyspnea index FEV 1 56 ± 12 % QVA 149 110 / 50 µg X X Tiotropium 18 µg od OL Placebo 0. 8 0. 6 1. 37 0. 49 p<0. 001 p<0. 021 0. 4 0. 2 6 weeks -0. 2 • BDI / TDI dyspnea index -0. 4 Mahler, et al. BLAZE study ERJ 2014 Placebo FDC Ind+Gly QVA 149 110/50 mcg q. d Tiotropium

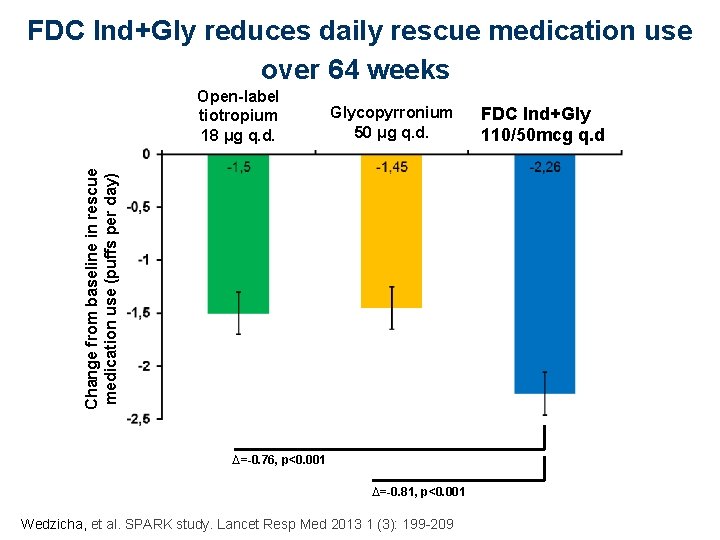
FDC Ind+Gly reduces daily rescue medication use over 64 weeks Glycopyrronium 50 μg q. d. Change from baseline in rescue medication use (puffs per day) Open-label tiotropium 18 μg q. d. ∆=-0. 76, p<0. 001 ∆=-0. 81, p<0. 001 Wedzicha, et al. SPARK study. Lancet Resp Med 2013 1 (3): 199 -209 FDCQVA 149 Ind+Gly 110/50 μg q. d. 110/50 mcg q. d

Indacaterol / glycopyrronium in COPD • Lung function airway obstruction • Symptoms dyspnea • Functional status exercise capacity • Quality of life

FDC Ind+Gly improves SGRQ total score versus placebo and open-label tiotropium at Week 26 ∆=– 3. 01, p<0. 01 42 SGRQ total score Worse ∆=– 1. 92, p= 0. 065 ∆=– 1. 83, p=0. 078 ∆=– 0. 88, p=ns ∆=– 2. 13, p=0. 01 ∆=– 1. 18; p=ns ∆=– 1. 09; p=ns 40 38 36 Better 34 40, 02 Placebo 39, 14 38, 19 38, 1 37, 01 Open-label Glycopyrronium Indacaterol FDC QVA 149 Ind+Gly tiotropium 50 μg q. d. 110/50 mcg q. d 18 μg q. d. Lower scores indicate better health-related quality of life SGRQ = St. George’s Respiratory Questionnaire Bateman, et al. SHINE study Eur Respir J 2013

Indacaterol / glycopyrronium in COPD • Lung function airway obstruction • Symptoms dyspnea • Functional status exercise capacity • Quality of life • Exacerbations

FDC Ind+Gly in COPD: Exacerbations FDC Ind+Gly 110/50 mcg q. d COPD exazcrbations (yearly rate) 0, 84 (0, 75 -0, 95)* Rate reductions (95% CI; p-value): *p=0. 0052, †p=0. 0072, ‡p=0. 096, §p=0. 038, ¶p=0. 036, **p=0. 18, ††p=0. 0017, ‡‡p=0. 0012 Wedzicha, et al. Lancet Respiratory Medicine 1(3): 199 -209, 2013

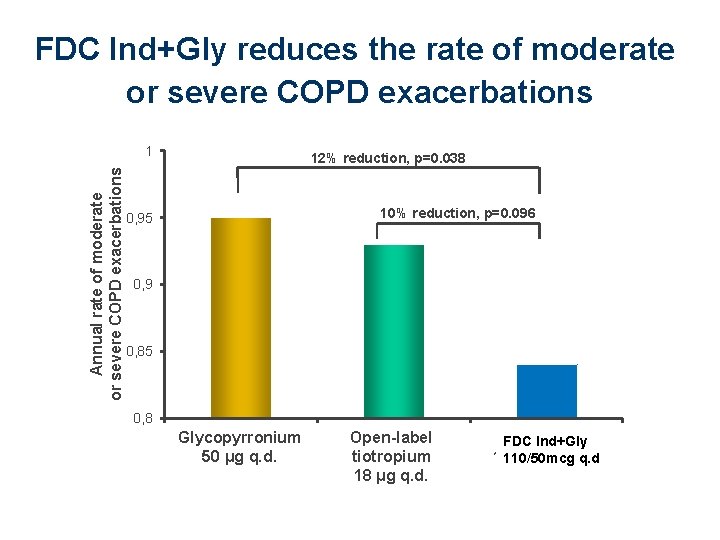
FDC Ind+Gly reduces the rate of moderate or severe COPD exacerbations Annual rate of moderate or severe COPD exacerbations 1 12% reduction, p=0. 038 10% reduction, p=0. 096 0, 95 0, 9 0, 85 0, 8 Glycopyrronium 50 μg Glycopyrronium q. d. 50 μg Tiotropium 18 μg q. d. QVA 149 150/50 μg q. d. Open-label QVA 149 FDC Ind+Gly tiotropium 110/50 μg q. d. 110/50 mcg q. d 18 μg q. d.

Indacaterol / glycopyrronium in COPD • Lung function airway obstruction • Symptoms dyspnea • Functional status exercise capacity • Quality of life • Exacerbations • Adverse events

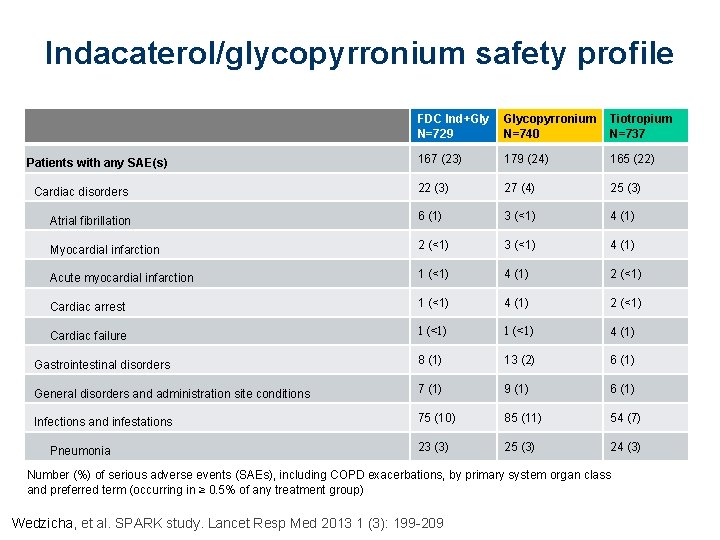
Indacaterol/glycopyrronium safety profile FDC Ind+Gly N=729 Glycopyrronium N=740 Tiotropium N=737 167 (23) 179 (24) 165 (22) 22 (3) 27 (4) 25 (3) Atrial fibrillation 6 (1) 3 (<1) 4 (1) Myocardial infarction 2 (<1) 3 (<1) 4 (1) Acute myocardial infarction 1 (<1) 4 (1) 2 (<1) Cardiac arrest 1 (<1) 4 (1) 2 (<1) Cardiac failure 1 (<1) 4 (1) Gastrointestinal disorders 8 (1) 13 (2) 6 (1) General disorders and administration site conditions 7 (1) 9 (1) 6 (1) Infections and infestations 75 (10) 85 (11) 54 (7) 23 (3) 25 (3) 24 (3) Patients with any SAE(s) Cardiac disorders Pneumonia Number (%) of serious adverse events (SAEs), including COPD exacerbations, by primary system organ class and preferred term (occurring in ≥ 0. 5% of any treatment group) Wedzicha, et al. SPARK study. Lancet Resp Med 2013 1 (3): 199 -209

COPD management Symptoms Frequent exacerbations A M A L & A B LA plus ICS t oflumilas R : e iv t a n r e Alt

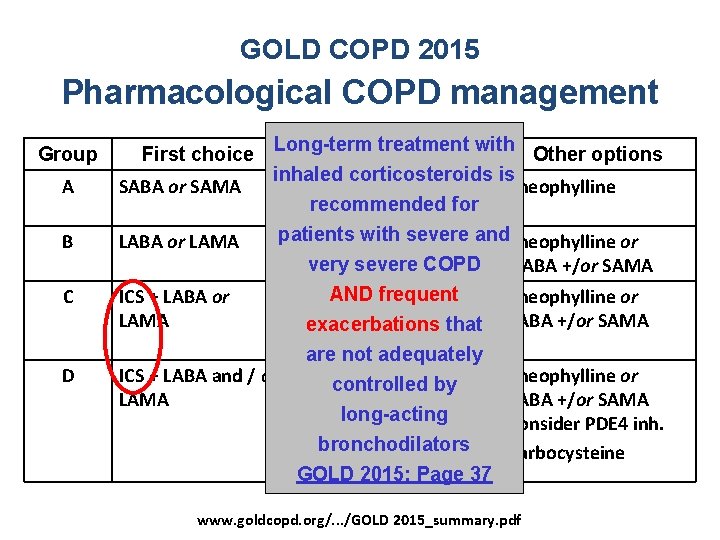
GOLD COPD 2015 Pharmacological COPD management Group First choice A SABA or SAMA B LABA or LAMA C D Long-term treatment with Alternative choice Other options inhaled corticosteroids is LABA or LAMA or Theophylline recommended for SABA + SAMA patients with severe and. Theophylline or LABA + LAMA very severe COPD frequent ICS + LABA or LABAAND + LAMA or LAMA + PDE 4 inhibitor exacerbations that orare LABA inh. not+ PDE 4 adequately ICS + LABA and / or ICS +controlled LABA + LAMA byor LAMA ICS+LABA+PDE 4 inh. or long-acting LAMA + PDE 4 inh. or bronchodilators LABA + LAMA GOLD 2015: Page 37 SABA +/or SAMA Theophylline or SABA +/or SAMA Consider PDE 4 inh. Carbocysteine www. goldcopd. org/. . . /GOLD 2015_summary. pdf
![ILLUMINATE Indacaterolglycopyrronium vs fluticasonesalmeterol in COPD Predose FEV 1 L 1 65 ILLUMINATE: Indacaterol/glycopyrronium vs. fluticasone/salmeterol in COPD Pre-dose FEV 1 [L] 1. 65 ∆ =](https://slidetodoc.com/presentation_image_h2/b03ff55a9624917daea2fd3f0b57dcae/image-30.jpg)
ILLUMINATE: Indacaterol/glycopyrronium vs. fluticasone/salmeterol in COPD Pre-dose FEV 1 [L] 1. 65 ∆ = 100 ml, p <0. 001 Dyspnea [TDI score] 2. 5 1. 60 2. 0 1. 55 1. 50 1. 45 1. 6 FDC Ind+Gly FP/Salm QVA 149 500/50 µg 110/50 mcg q. d Week 26, TDI: Transitional Dyspnea Index 0. 5 ∆ = 0. 76, p = 0003 1. 6 2. 36 Ind+Gly FP/Salm FDC QVA 149 500/50 µg 110/50 mcg 100/50 µg q. d Vogelmeier, et al. Lancet Respir Med 2013

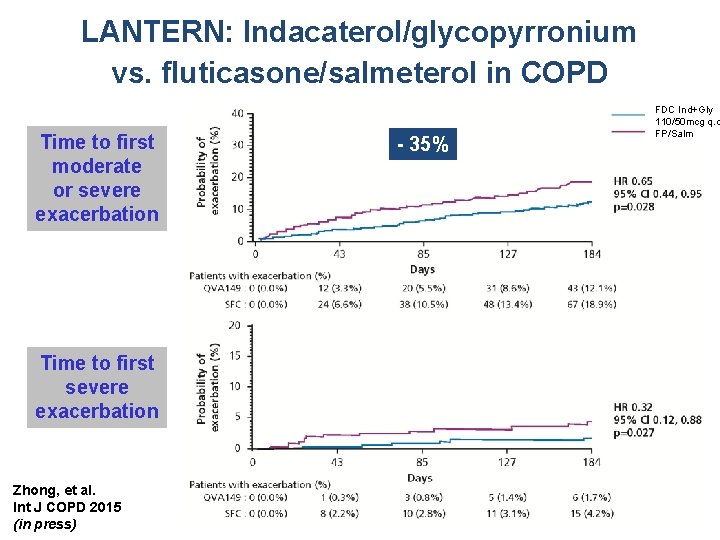
LANTERN: Indacaterol/glycopyrronium vs. fluticasone/salmeterol in COPD Time to first moderate or severe exacerbation Time to first severe exacerbation Zhong, et al. Int J COPD 2015 (in press) - 35% FDC Ind+Gly 110/50 mcg q. d FP/Salm
![Dosing frequency and adherence in COPD Days covered 55 076 COPD patients 1999 Dosing frequency and adherence in COPD Days covered [%] 55 076 COPD patients 1999](https://slidetodoc.com/presentation_image_h2/b03ff55a9624917daea2fd3f0b57dcae/image-32.jpg)
Dosing frequency and adherence in COPD Days covered [%] 55 076 COPD patients 1999 - 2006 40 • Dosing frequency of 1 st COPD drug claim after diagnosis 30 12 months 20 10 Proportion of days covered Toy, et al. Respir Med 2010 [%] QID TID BID QD 4 x 3 x 2 x 1 x

SUMMARY • Fixed dose combination indacaterol and glycopyrronium with different and complementary mechanisms of action • Improvements in lung function - rapid onset of action • Improvements in dyspnea and quality of life • Reduction of symptoms, on-demand medication, and exacerbations • • Improving adherence Side effects

Ultibro: fixed dose combination indacaterol 110 mcg and 2 glycopyrronium 50 mcg 34

35 | Presentation Title | Presenter Name | Date | Subject | Business Use Only
 Prayudi santoso
Prayudi santoso Curriculum vitae esquema
Curriculum vitae esquema Caracteristicas externas de memorandum
Caracteristicas externas de memorandum Cual es la sexta regla del manual de mooney
Cual es la sexta regla del manual de mooney Letra n n
Letra n n Special skills job application
Special skills job application Harvardmodellen
Harvardmodellen Curriculum vitae výslovnost
Curriculum vitae výslovnost Cvu curriculum
Cvu curriculum Cv reference
Cv reference Yivotopis
Yivotopis Minuta de acuerdos
Minuta de acuerdos Struktur curriculum vitae
Struktur curriculum vitae British curriculum vitae
British curriculum vitae Encv
Encv Características externas de un cuadro sinóptico
Características externas de un cuadro sinóptico Curriculum vitae forma
Curriculum vitae forma Mapa conceptual caracteristicas internas y externas
Mapa conceptual caracteristicas internas y externas Curriculum vitae esempi
Curriculum vitae esempi Curriculum vitaem
Curriculum vitaem Components of a curriculum vitae
Components of a curriculum vitae Sommaire dans un cv
Sommaire dans un cv Curriculum vitae de jesus de nazaret
Curriculum vitae de jesus de nazaret Curriculum vitae de carlos slim
Curriculum vitae de carlos slim Stj budi santoso
Stj budi santoso Refeeding syndrome electrolyte abnormalities
Refeeding syndrome electrolyte abnormalities Myopic
Myopic Jahja santoso
Jahja santoso Adhi setyo santoso
Adhi setyo santoso Fundacja evangelium vitae
Fundacja evangelium vitae What iscurriculum vitae
What iscurriculum vitae Inis vitae sed non amoris
Inis vitae sed non amoris Anamnesis vitae
Anamnesis vitae Integer vitae scelerisque purus
Integer vitae scelerisque purus Lignum vitae bearings
Lignum vitae bearings Regula vitae
Regula vitae