Control of Oxides of Nitrogen from Stationary Sources



![Solution • b) If this inital rate holds for 0. 03 seconds then [NO]=0. Solution • b) If this inital rate holds for 0. 03 seconds then [NO]=0.](https://slidetodoc.com/presentation_image_h2/d73eedaf4e2646377fe35b06648c21c6/image-19.jpg)







- Slides: 44

Control of Oxides of Nitrogen from Stationary Sources

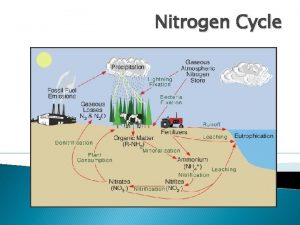
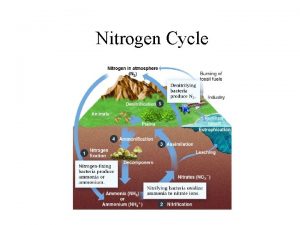
Oxides of Nitrogen • The gaseous oxides of nitrogen include: – N 2 O: nitrous oxide – NO: nitric oxide (free radical) – N 2 O 3: nitrogen trioxide – NO 2: Nitrogen dioxide (free radical) – N 2 O 5: nitrogen pentoxide An unstable form NO 3 also exists. Only N 2 O, NO, and NO 2 present in the atmosphere in significant concentrations

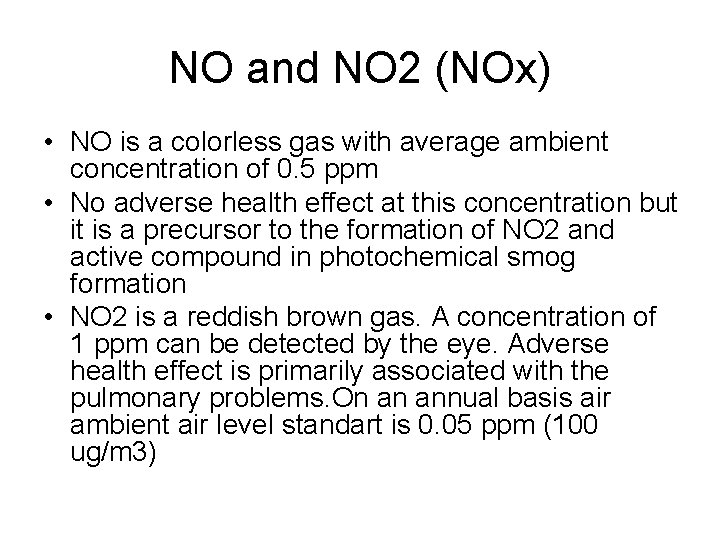
NO and NO 2 (NOx) • NO is a colorless gas with average ambient concentration of 0. 5 ppm • No adverse health effect at this concentration but it is a precursor to the formation of NO 2 and active compound in photochemical smog formation • NO 2 is a reddish brown gas. A concentration of 1 ppm can be detected by the eye. Adverse health effect is primarily associated with the pulmonary problems. On an annual basis air ambient air level standart is 0. 05 ppm (100 ug/m 3)

Sources and Concentrations of NOx • Over 90% of all man-made nitrogen oxides entering the atmosphere are from combustion sources • For the US, 50% is from mobile sources • For Turkey ambient NOx concentration is around xx • At an emission source the concentration of oxided of nitrogen is much higher than ambient values • For example the NOx concentration in the flue gas from the steam boiler of a power plant may reach 500 to 1000 ppm • From such combustion processes the NOx is the exhaust stack gas would be 90% or more NO and the rest NO 2

NOx Control • 1. Control over the reaction that produces the pollutant • 2. Remove NOx after it is formed

NOx Formation Basic Chemistry, Thermodynamics and Kinetics of the Formation Reactions • There are two sources of N that contribute to the formation of oxides of nitrogen – 1. Fuel N (Coal and fuel oil composition, note that no N in natural gas)(Fuel NOx) – 2. Air N (78% of air is N)(Thermal NOx)

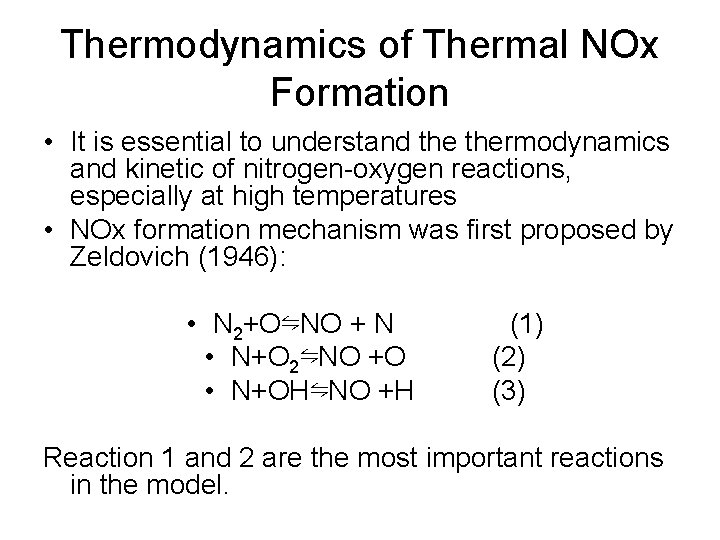
Thermodynamics of Thermal NOx Formation • It is essential to understand thermodynamics and kinetic of nitrogen-oxygen reactions, especially at high temperatures • NOx formation mechanism was first proposed by Zeldovich (1946): • N 2+O⇋NO + N • N+O 2⇋NO +O • N+OH⇋NO +H (1) (2) (3) Reaction 1 and 2 are the most important reactions in the model.

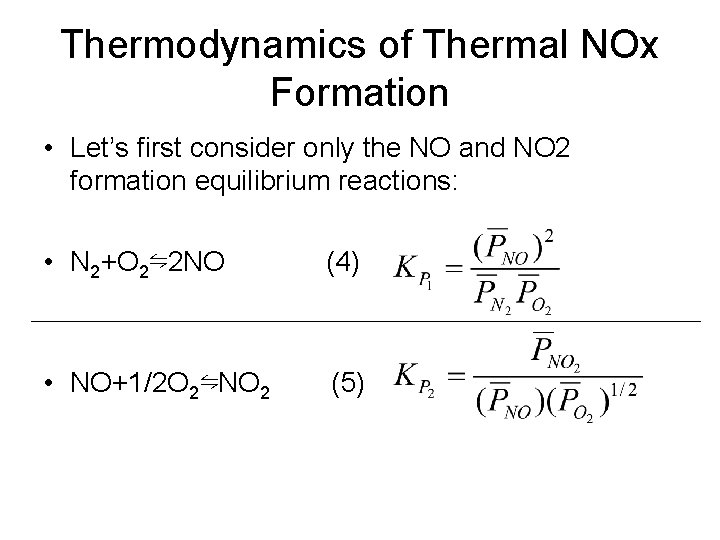
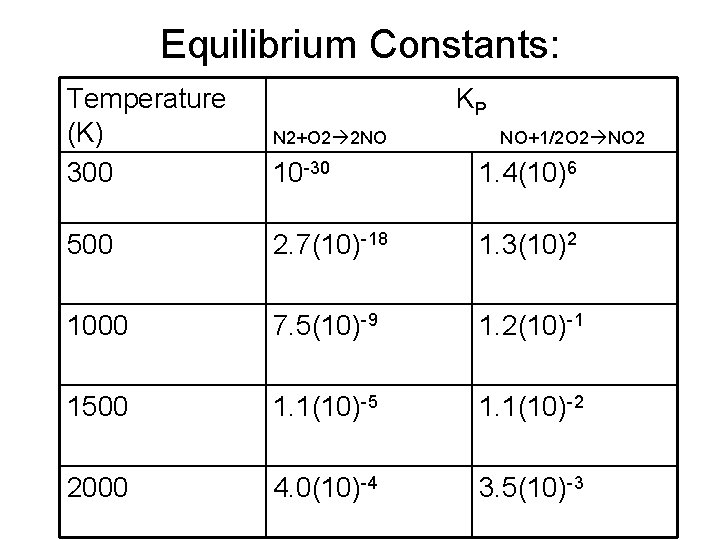
Thermodynamics of Thermal NOx Formation • Let’s first consider only the NO and NO 2 formation equilibrium reactions: • N 2+O 2⇋2 NO (4) • NO+1/2 O 2⇋NO 2 (5)

Equilibrium Constants: Temperature (K) 300 KP N 2+O 2 2 NO 10 -30 1. 4(10)6 500 2. 7(10)-18 1. 3(10)2 1000 7. 5(10)-9 1. 2(10)-1 1500 1. 1(10)-5 1. 1(10)-2 2000 4. 0(10)-4 3. 5(10)-3 NO+1/2 O 2 NO 2

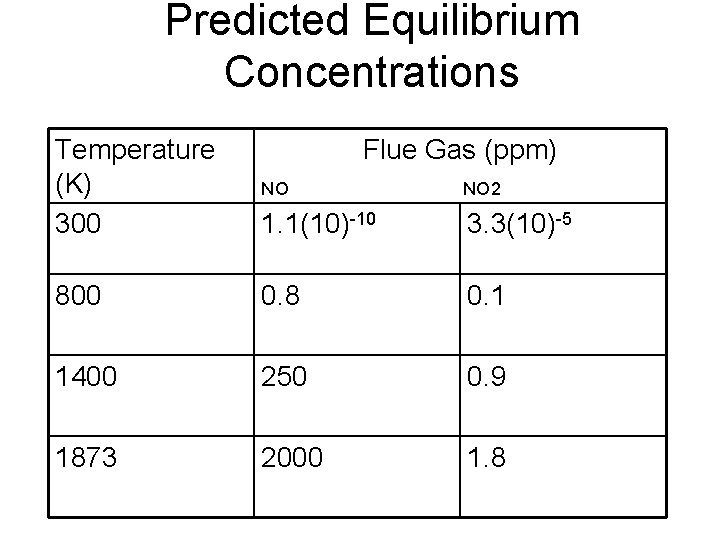
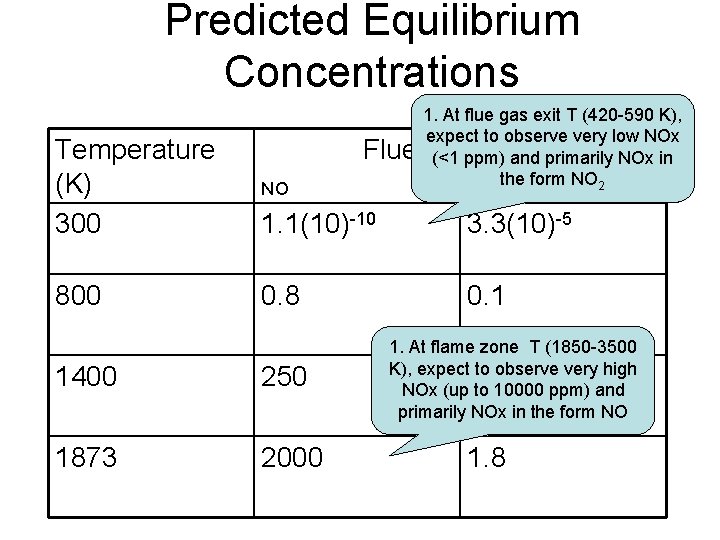
Predicted Equilibrium Concentrations Temperature (K) 300 Flue Gas (ppm) NO NO 2 1. 1(10)-10 3. 3(10)-5 800 0. 8 0. 1 1400 250 0. 9 1873 2000 1. 8

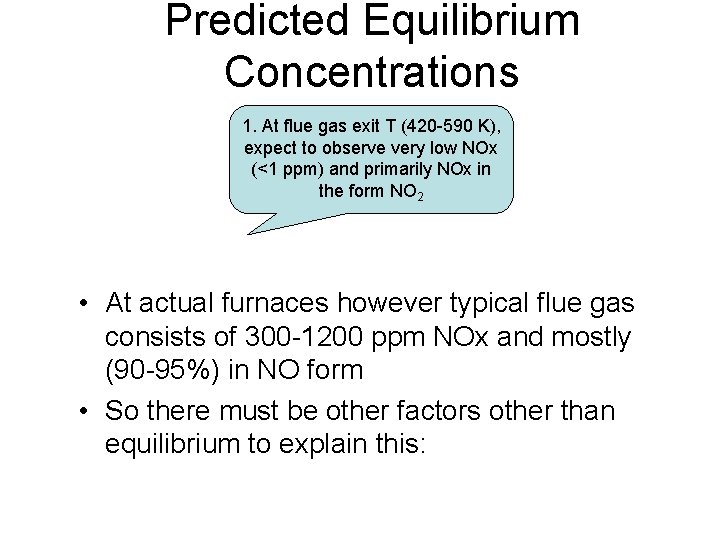
Predicted Equilibrium Concentrations Flue 1. At flue gas exit T (420 -590 K), expect to observe very low NOx Gas (ppm) (<1 ppm) and primarily NOx in the form NO 2 Temperature (K) 300 NO 1. 1(10)-10 3. 3(10)-5 800 0. 8 0. 1 1400 250 1873 2000 1. At flame zone T (1850 -3500 K), expect to observe very high 0. 9 NOx (up to 10000 ppm) and primarily NOx in the form NO 1. 8

Predicted Equilibrium Concentrations 1. At flue gas exit T (420 -590 K), expect to observe very low NOx (<1 ppm) and primarily NOx in the form NO 2 • At actual furnaces however typical flue gas consists of 300 -1200 ppm NOx and mostly (90 -95%) in NO form • So there must be other factors other than equilibrium to explain this:

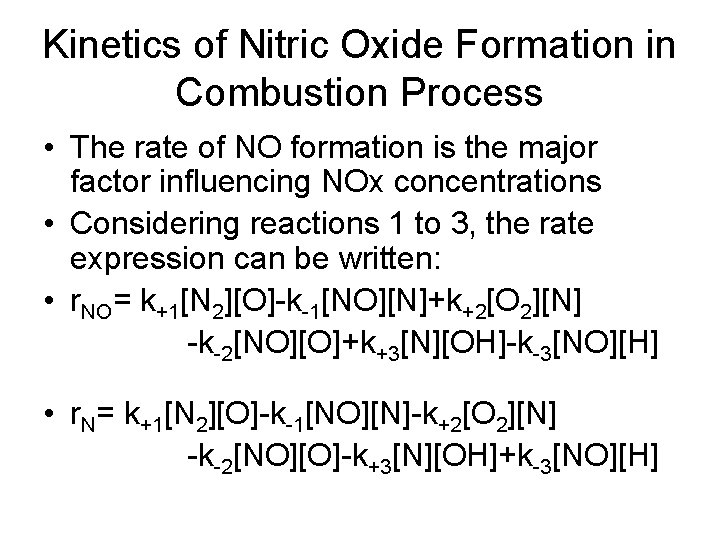
Kinetics of Nitric Oxide Formation in Combustion Process • The rate of NO formation is the major factor influencing NOx concentrations • Considering reactions 1 to 3, the rate expression can be written: • r. NO= k+1[N 2][O]-k-1[NO][N]+k+2[O 2][N] -k-2[NO][O]+k+3[N][OH]-k-3[NO][H] • r. N= k+1[N 2][O]-k-1[NO][N]-k+2[O 2][N] -k-2[NO][O]-k+3[N][OH]+k-3[NO][H]

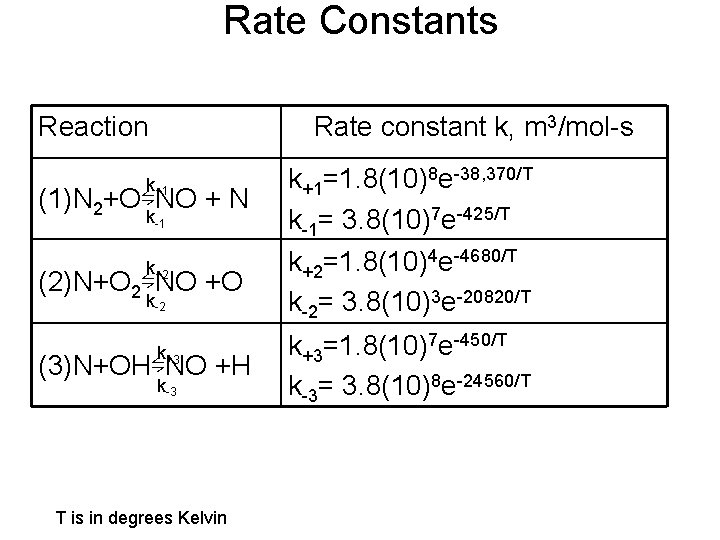
Rate Constants Reaction Rate constant k, m 3/mol-s k+1 (1)N 2+O⇋NO + N k-1 k+2 (2)N+O 2⇋NO +O k-2 k+3 (3)N+OH⇋NO +H k-3 T is in degrees Kelvin k+1=1. 8(10)8 e-38, 370/T k-1= 3. 8(10)7 e-425/T k+2=1. 8(10)4 e-4680/T k-2= 3. 8(10)3 e-20820/T k+3=1. 8(10)7 e-450/T k-3= 3. 8(10)8 e-24560/T

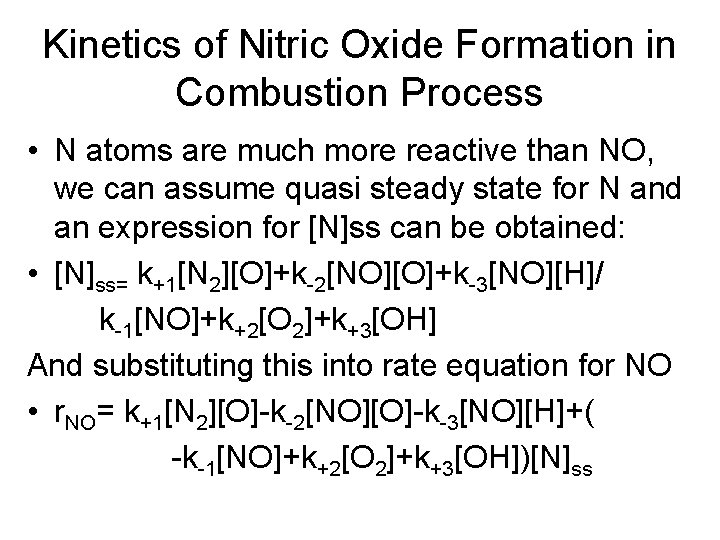
Kinetics of Nitric Oxide Formation in Combustion Process • N atoms are much more reactive than NO, we can assume quasi steady state for N and an expression for [N]ss can be obtained: • [N]ss= k+1[N 2][O]+k-2[NO][O]+k-3[NO][H]/ k-1[NO]+k+2[O 2]+k+3[OH] And substituting this into rate equation for NO • r. NO= k+1[N 2][O]-k-2[NO][O]-k-3[NO][H]+( -k-1[NO]+k+2[O 2]+k+3[OH])[N]ss

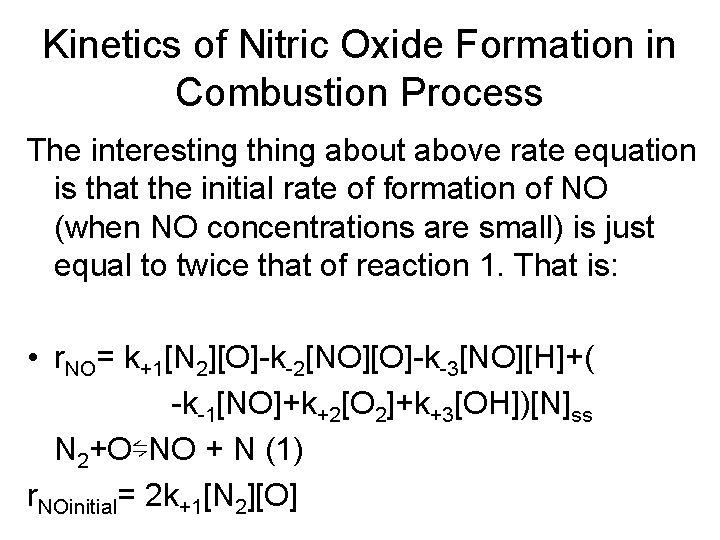
Kinetics of Nitric Oxide Formation in Combustion Process The interesting thing about above rate equation is that the initial rate of formation of NO (when NO concentrations are small) is just equal to twice that of reaction 1. That is: • r. NO= k+1[N 2][O]-k-2[NO][O]-k-3[NO][H]+( -k-1[NO]+k+2[O 2]+k+3[OH])[N]ss N 2+O⇋NO + N (1) r. NOinitial= 2 k+1[N 2][O]

Example 16. 2 • Given a HC flame at 1870 C where the mole fractions of N 2 gas and O atoms are 0. 75 and 9. 5(10)-4 respectively, • a) calculate the initial rate of NO formation (in mole(m 3 -s) • b) if this rate holds constant for 0. 03 seconds, calculate the concentration in ppm of NO in the gases leaving the flame zone

Solution • a) at T = 1870 C = 2143 K, k+1=1. 8(10)8 e-38, 370/2143=3. 015 m 3/mol-s Assuming P= 1 atm, the molar density of the gases: [N 2]=0. 75 x 5. 686=4. 26 mol/m 3 [O]=9. 5(19)-4 x 5. 686=0. 0054 mol/m 3 r. NOinitial= 2 k+1[N 2][O]=2(3. 015)(4. 26)(0. 0054) =0. 1388 mol/m 3 -s
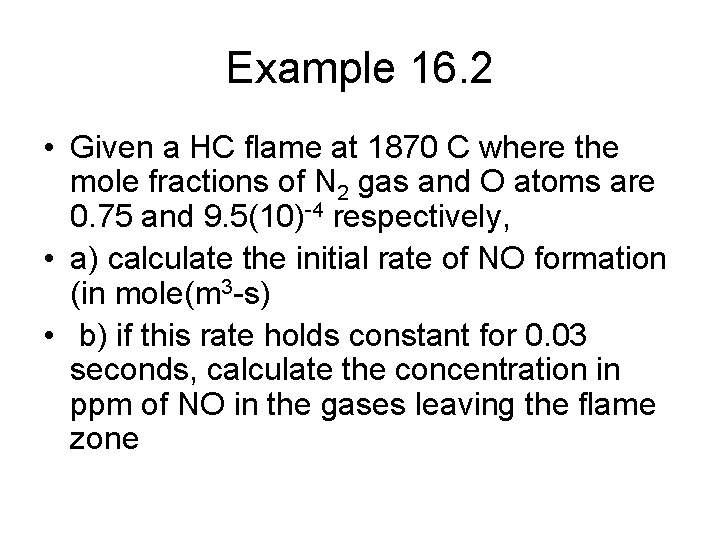
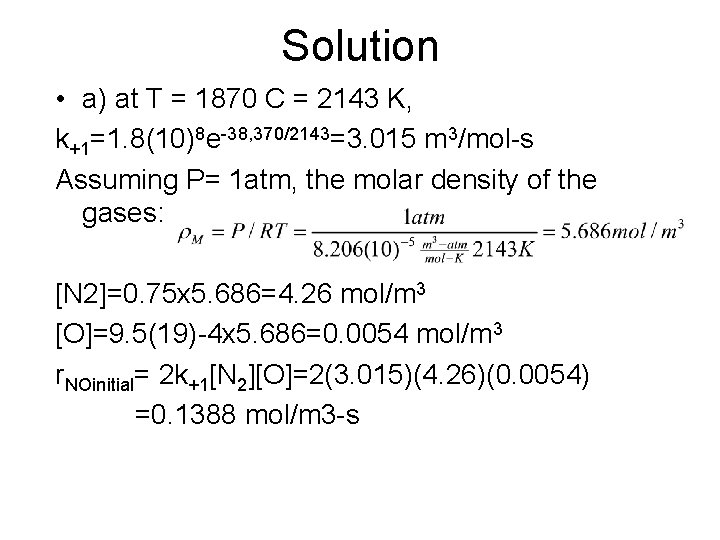
![Solution b If this inital rate holds for 0 03 seconds then NO0 Solution • b) If this inital rate holds for 0. 03 seconds then [NO]=0.](https://slidetodoc.com/presentation_image_h2/d73eedaf4e2646377fe35b06648c21c6/image-19.jpg)
Solution • b) If this inital rate holds for 0. 03 seconds then [NO]=0. 1388(0. 03)=4. 16(10)-3 mol/m 3

Research on NOx Formation • Experimental results in various studies showed that NO concentrations in the flame zone are significantly higher than could have been formed by the Zeldovich mechanism • This may be due to “prompt” NO formation • Prompt NO: NO formed in the first five milliseconds (40 -100 ppm)

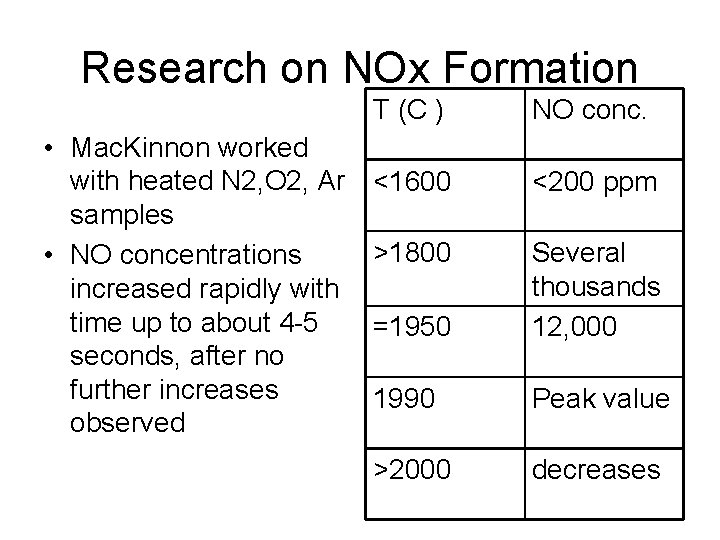
Research on NOx Formation • Mac. Kinnon worked with heated N 2, O 2, Ar samples • NO concentrations increased rapidly with time up to about 4 -5 seconds, after no further increases observed T (C ) NO conc. <1600 <200 ppm >1800 =1950 Several thousands 12, 000 1990 Peak value >2000 decreases

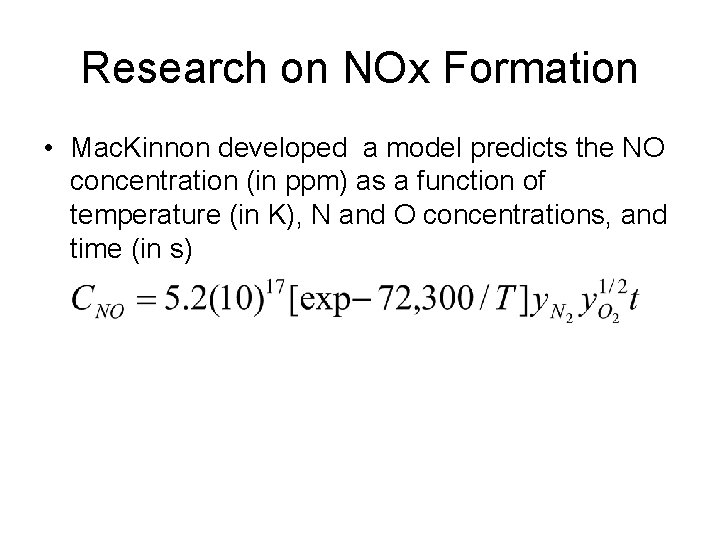
Research on NOx Formation • Mac. Kinnon developed a model predicts the NO concentration (in ppm) as a function of temperature (in K), N and O concentrations, and time (in s)

NOx Formation from Fuel Nitrogen • When a fuel contains organically bound N, the contribution of the fuel bound N to the total NOx production is significant

Example 16. 3

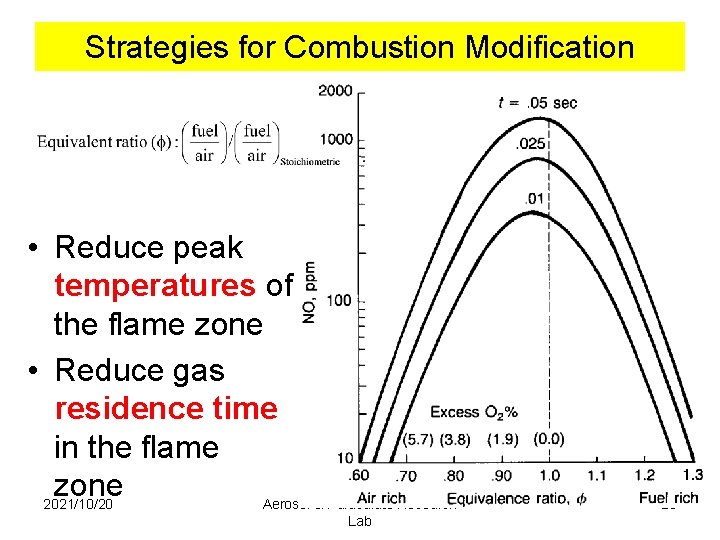
Strategies for Combustion Modification • Reduce peak temperatures of the flame zone • Reduce gas residence time in the flame zone 2021/10/20 Aerosol & Particulate Research Lab 25

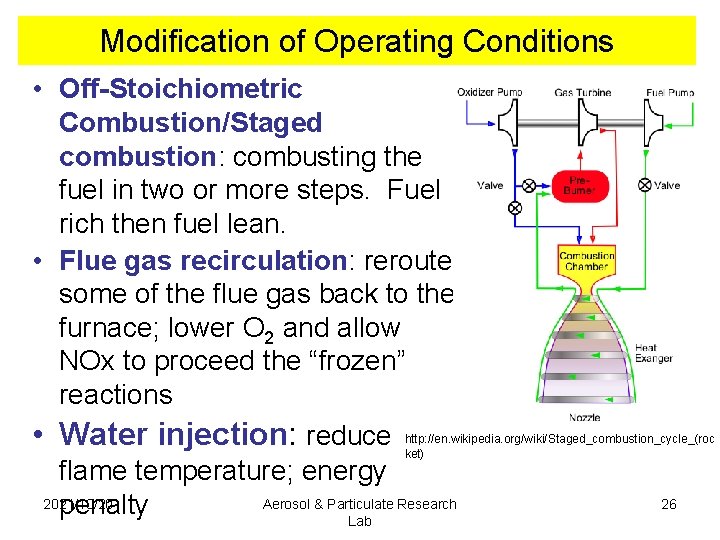
Modification of Operating Conditions • Off-Stoichiometric Combustion/Staged combustion: combusting the fuel in two or more steps. Fuel rich then fuel lean. • Flue gas recirculation: reroute some of the flue gas back to the furnace; lower O 2 and allow NOx to proceed the “frozen” reactions • Water injection: reduce http: //en. wikipedia. org/wiki/Staged_combustion_cycle_(roc ket) flame temperature; energy 2021/10/20 Aerosol & Particulate Research 26 penalty Lab

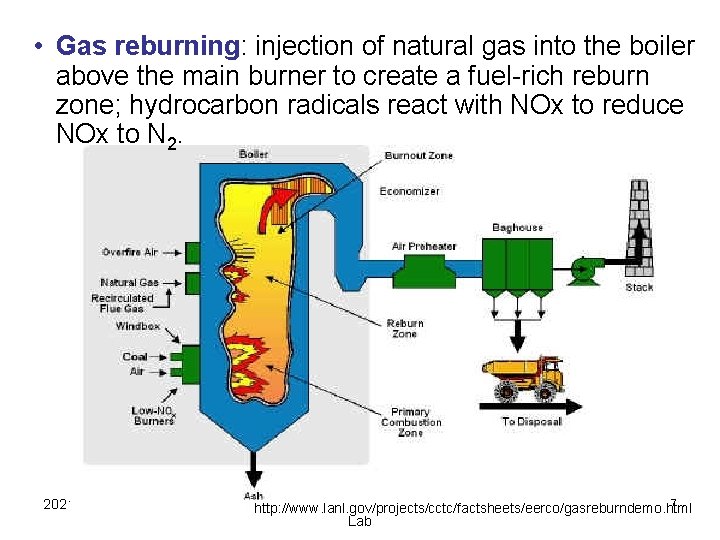
• Gas reburning: injection of natural gas into the boiler above the main burner to create a fuel-rich reburn zone; hydrocarbon radicals react with NOx to reduce NOx to N 2. 2021/10/20 Aerosol & Particulate Research 27 http: //www. lanl. gov/projects/cctc/factsheets/eerco/gasreburndemo. html Lab

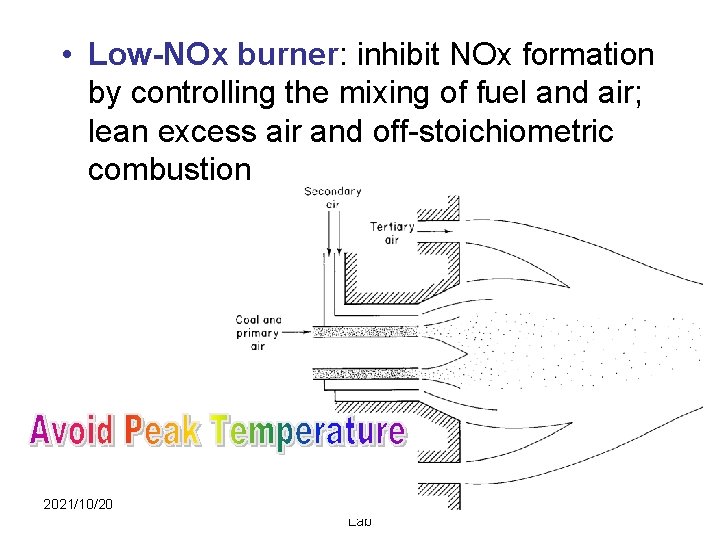
• Low-NOx burner: inhibit NOx formation by controlling the mixing of fuel and air; lean excess air and off-stoichiometric combustion 2021/10/20 Aerosol & Particulate Research Lab 28

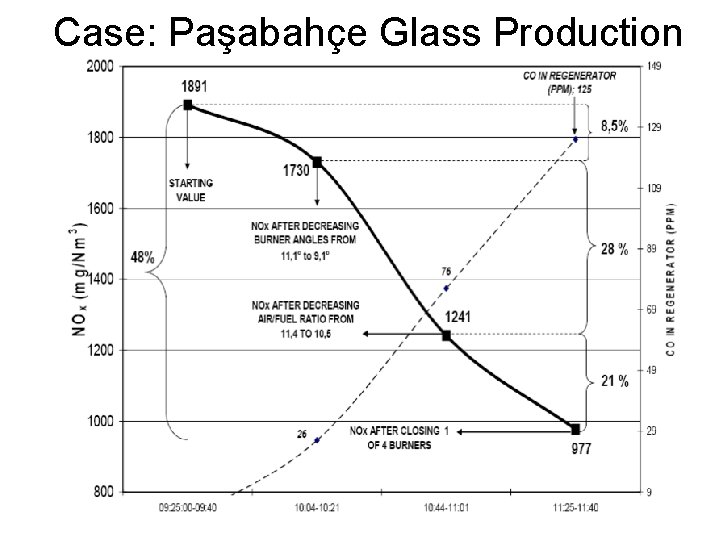
Case: Paşabahçe Glass Production


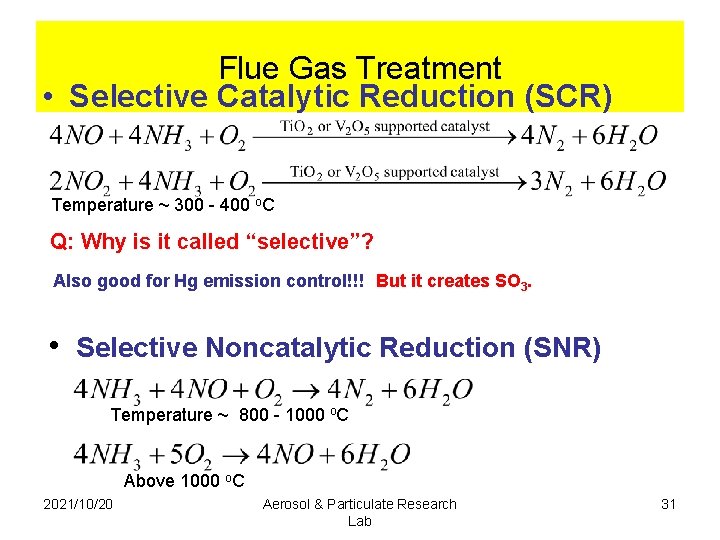
Flue Gas Treatment • Selective Catalytic Reduction (SCR) Temperature ~ 300 - 400 o. C Q: Why is it called “selective”? Also good for Hg emission control!!! But it creates SO 3. • Selective Noncatalytic Reduction (SNR) Temperature ~ 800 - 1000 o. C Above 1000 o. C 2021/10/20 Aerosol & Particulate Research Lab 31

SCR • • • Removal efficiency is over 90% Expenses from use of catalyst High operation and capital cost Large area requirement Requires temperature control for optiumum reduction

SNCR • No catalyst cost • High temperatures (850 -1100) • Low removal efficiency ( %30 -%66 less than SCR)

Other Control Methods • Absorption • Adsorption • Biological

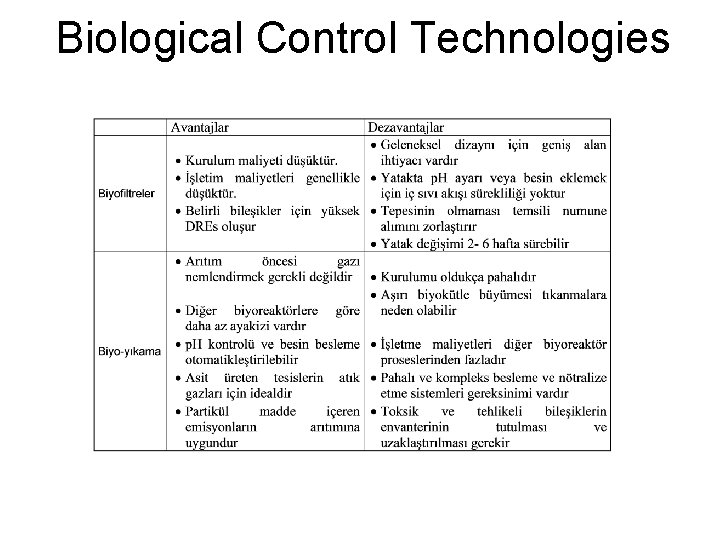
Biological Control Technologies Under aerobic conditions, nitrification and chemical oxidation leads to NOx oxidation to nitrate. İlk denemelerde, yüksek O 2 konsantrasyonlu aerobik şartlarda (>%17 Oksijen) NO giderimi toluenle muamele edilmiş silika pelet dolgulu bir biyofiltrede %97 mertebesinde başarılmıştır

Biological Control Technologies Anoksik koşullarda ise NOx denitrifikasyon prosesi yüksek bir verimle azot gazına indirgenmektedir. Toprak kompostu içeren laboratuar ölçekli bir biyofiltre çalışmasında NO 2 için %100’e yaklaşan bir giderim verimi elde edilmiştir. (Okuno et. al, 2000). Tüm bunlar Biyo. De. Nox teknolojilerinin NOx kontrolü için gelecekte de artan oranlarda kullanılacağını göstermektedir.

Biological Control Technologies

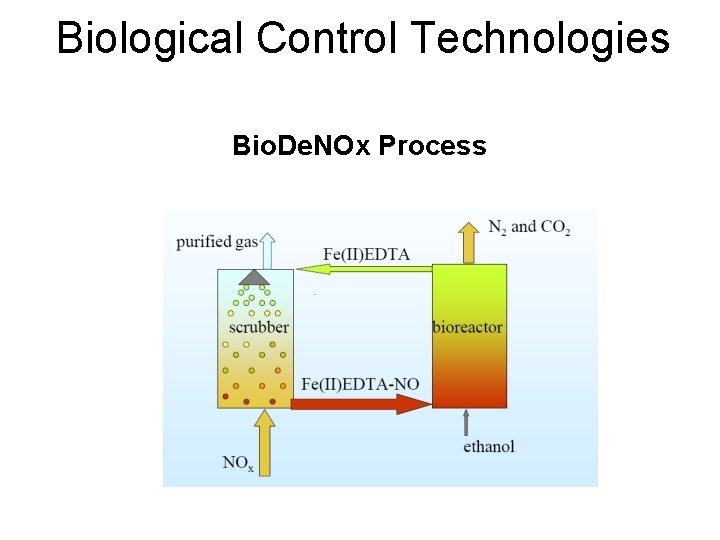
Biological Control Technologies Bio. De. NOx Process

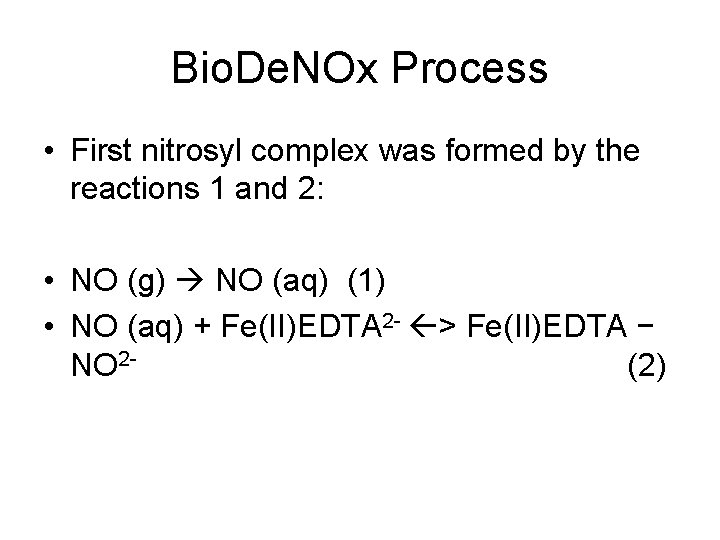
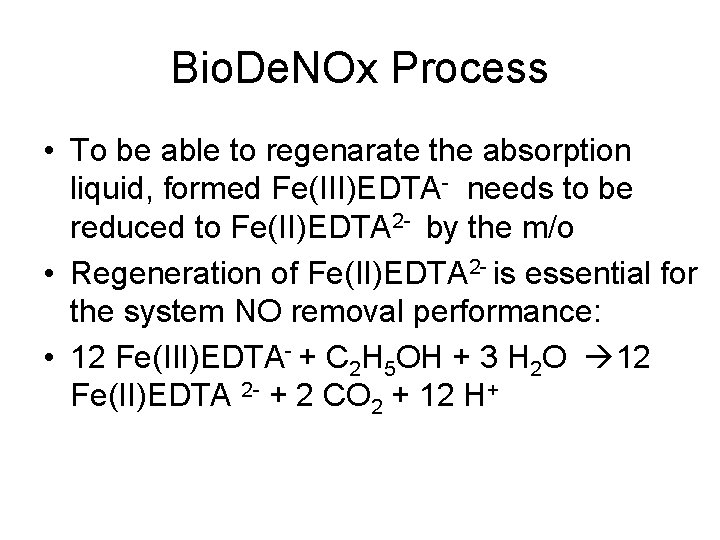
Bio. De. NOx Process • First nitrosyl complex was formed by the reactions 1 and 2: • NO (g) NO (aq) (1) • NO (aq) + Fe(II)EDTA 2 - > Fe(II)EDTA − NO 2(2)

Bio. De. NOx Process • To be able to regenarate the absorption liquid, formed Fe(III)EDTA- needs to be reduced to Fe(II)EDTA 2 - by the m/o • Regeneration of Fe(II)EDTA 2 - is essential for the system NO removal performance: • 12 Fe(III)EDTA- + C 2 H 5 OH + 3 H 2 O 12 Fe(II)EDTA 2 - + 2 CO 2 + 12 H+

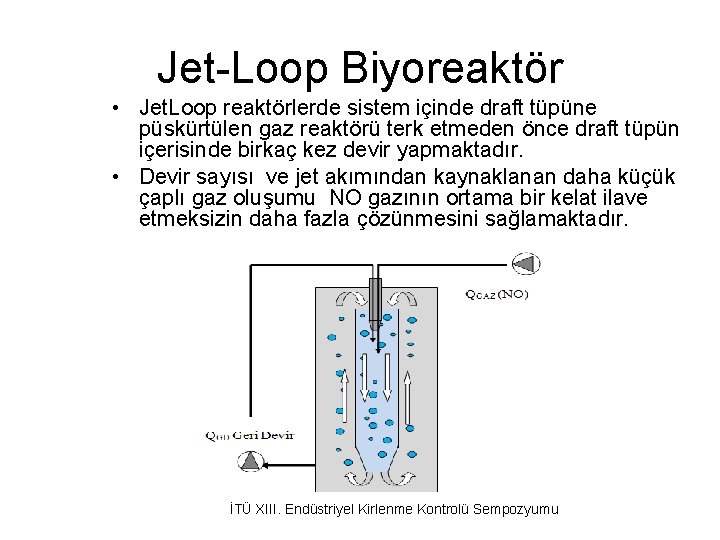
Jet-Loop Biyoreaktör • Jet. Loop reaktörlerde sistem içinde draft tüpüne püskürtülen gaz reaktörü terk etmeden önce draft tüpün içerisinde birkaç kez devir yapmaktadır. • Devir sayısı ve jet akımından kaynaklanan daha küçük çaplı gaz oluşumu NO gazının ortama bir kelat ilave etmeksizin daha fazla çözünmesini sağlamaktadır. İTÜ XIII. Endüstriyel Kirlenme Kontrolü Sempozyumu

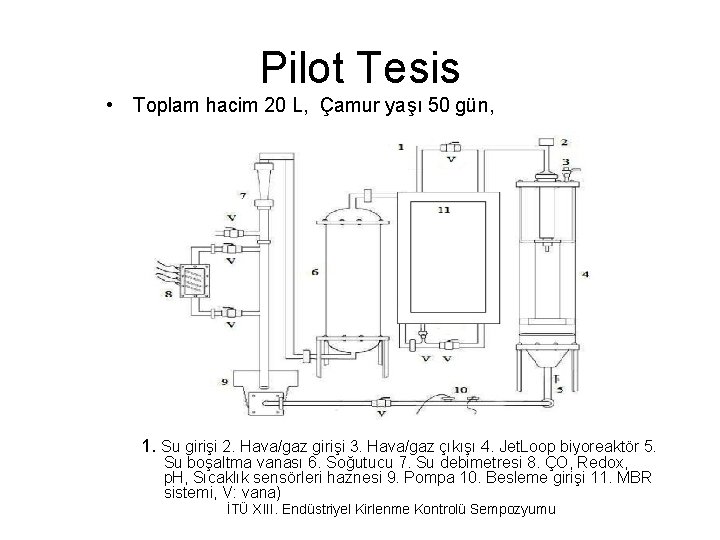
Pilot Tesis • Toplam hacim 20 L, Çamur yaşı 50 gün, 1. Su girişi 2. Hava/gaz girişi 3. Hava/gaz çıkışı 4. Jet. Loop biyoreaktör 5. Su boşaltma vanası 6. Soğutucu 7. Su debimetresi 8. ÇO, Redox, p. H, Sıcaklık sensörleri haznesi 9. Pompa 10. Besleme girişi 11. MBR sistemi, V: vana) İTÜ XIII. Endüstriyel Kirlenme Kontrolü Sempozyumu

Pilot Tesis İTÜ XIII. Endüstriyel Kirlenme Kontrolü Sempozyumu

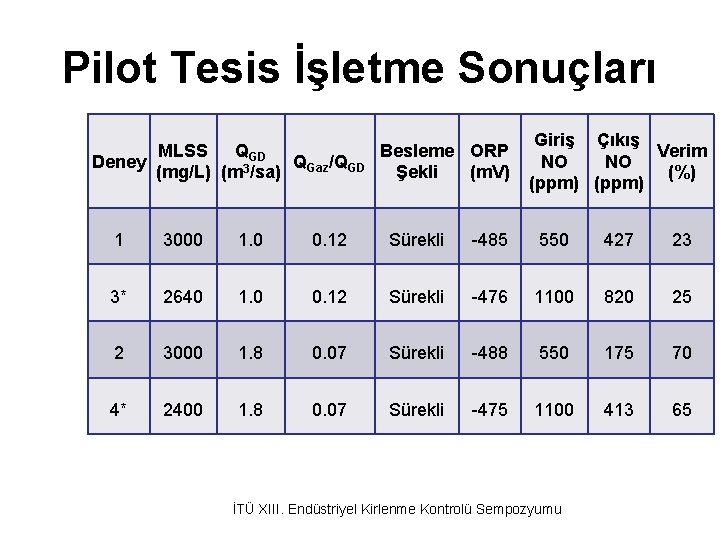
Pilot Tesis İşletme Sonuçları MLSS QGD Besleme ORP Deney Q /Q (mg/L) (m 3/sa) Gaz GD Şekli (m. V) Giriş Çıkış Verim NO NO (%) (ppm) 1 3000 1. 0 0. 12 Sürekli -485 550 427 23 3* 2640 1. 0 0. 12 Sürekli -476 1100 820 25 2 3000 1. 8 0. 07 Sürekli -488 550 175 70 4* 2400 1. 8 0. 07 Sürekli -475 1100 413 65 İTÜ XIII. Endüstriyel Kirlenme Kontrolü Sempozyumu
 Dp periodic table
Dp periodic table Diamond melting point
Diamond melting point Ari rokeach
Ari rokeach Natural nitrogen for plants
Natural nitrogen for plants Pyrimidine synthesis
Pyrimidine synthesis Sources of carbon and nitrogen in pyrimidine ring
Sources of carbon and nitrogen in pyrimidine ring Neutral oxides
Neutral oxides Acidic oxide
Acidic oxide Classify the following oxides
Classify the following oxides Oxides of group 13 elements
Oxides of group 13 elements Intermediate oxides in glass
Intermediate oxides in glass Print sources and web sources
Print sources and web sources Water resource
Water resource Auto power supply control from 4 different sources
Auto power supply control from 4 different sources Auto power supply control from 4 different sources
Auto power supply control from 4 different sources Stationary front symbol
Stationary front symbol How to find stationary points
How to find stationary points Fronts drawing
Fronts drawing Illustrate the four types of weather fronts
Illustrate the four types of weather fronts A stationary police officer directs radio waves
A stationary police officer directs radio waves Advantages of stationary armature
Advantages of stationary armature Difference between hauled and stationary container system
Difference between hauled and stationary container system Advantages and disadvantages of hauled container system
Advantages and disadvantages of hauled container system Input stationary
Input stationary Beehive shaped population pyramid
Beehive shaped population pyramid Expansive constrictive and stationary
Expansive constrictive and stationary Stationary velocity graph
Stationary velocity graph Mobile phase and stationary phase
Mobile phase and stationary phase Principles of high performance liquid chromatography
Principles of high performance liquid chromatography Principle gas chromatography
Principle gas chromatography How to find stationary points
How to find stationary points Input stationary
Input stationary Mobile phase and stationary phase
Mobile phase and stationary phase Stationary phase chromatography
Stationary phase chromatography Random process
Random process Leaching solid liquid extraction
Leaching solid liquid extraction Traveling guideline hair cutting
Traveling guideline hair cutting Stationary distribution
Stationary distribution Non stationary process
Non stationary process Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Stationary front
Stationary front Strict sense stationary
Strict sense stationary Joe balances a stationary coin
Joe balances a stationary coin Hplc detector types
Hplc detector types Free body diagram of a stationary boat
Free body diagram of a stationary boat