Congreso SEOM 2019 Precision Cancer Medicine Lillian L







- Slides: 32

Congreso SEOM 2019 Precision Cancer Medicine Lillian L. Siu, MD Princess Margaret Cancer Centre, Toronto, Canada BMO Chair in Precision Cancer Genomics 1

Financial Disclosures: • Consultant for: Merck (compensated), Pfizer (compensated), Celgene (compensated), Astra. Zeneca/Medimmune (compensated), Morphosys (compensated), Roche (compensated), Gene. Seeq (compensated), Loxo (compensated), Oncorus (compensated), Symphogen (compensated) • Speaker’s Bureau for: None • Grant/Research support from (Clinical Trials): Novartis, Bristol-Myers Squibb, Pfizer, Boerhinger-Ingelheim, Regeneron, Glaxo. Smith. Kline, Roche/Genentech, Karyopharm, Astra. Zeneca/Medimmune, Merck, Celgene, Astellas, Bayer, Abbvie, Amgen, Symphogen, Intensity Therapeutics • Stockholder in: Agios (spouse) • Employee of: None 2

Case: In-Frame BRAF Deletion Pancreatic Cancer 3

Case Presentation • 62 year old man • PMH: Prior back surgery, no family Hx, no diabetes, non smoker, nil alcohol • History: Abdominal pain radiating to back, 15 lb weight June 2018 – CT/MRI: – Tail: of Pancreas lesion 4. 6 cm, 3 x liver mets <=1. 1 cm, bone metastases, LAD, possible pleural disease – Enrolled OCTANE/COMPASS (profiling study) June 26, 2018 • FOLFIRINOX x 4 (July-Nov 2018) – disease progression 4

5

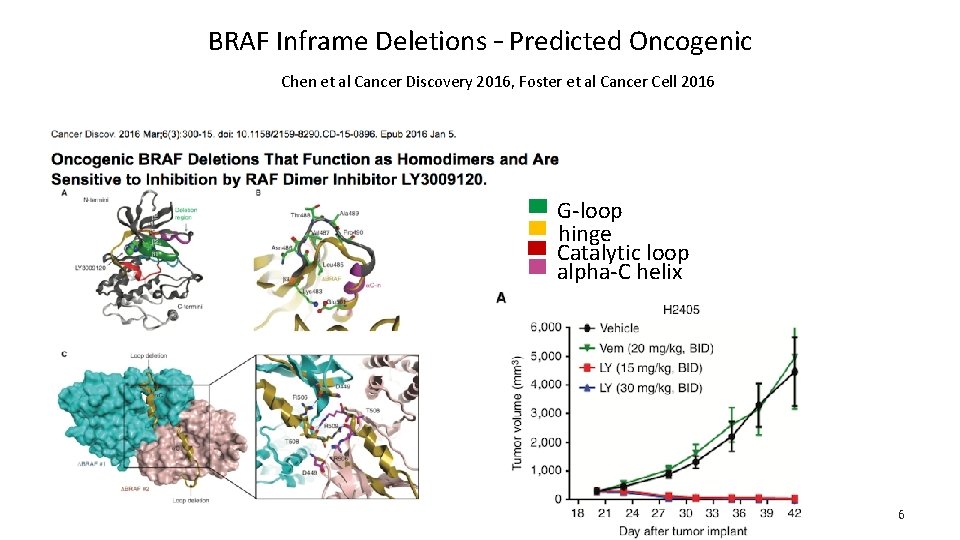
BRAF Inframe Deletions – Predicted Oncogenic Chen et al Cancer Discovery 2016, Foster et al Cancer Cell 2016 G-loop hinge Catalytic loop alpha-C helix 6

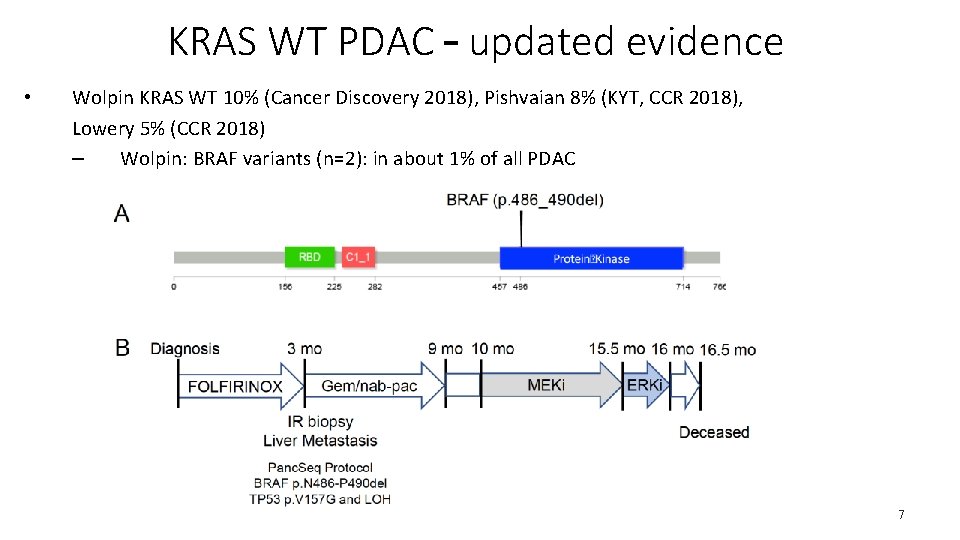
KRAS WT PDAC – updated evidence • Wolpin KRAS WT 10% (Cancer Discovery 2018), Pishvaian 8% (KYT, CCR 2018), Lowery 5% (CCR 2018) – Wolpin: BRAF variants (n=2): in about 1% of all PDAC 7

CAPTUR – CAnadian Profiling and Targeted Agent Utilization Trial Disease Site Committee: Precision Medicine Disease Type: Mixed solid tumors, multiple myeloma, B cell non-Hodgkin lymphoma, pediatric patients Study Chair(s): Lillian Siu and Daniel Renouf CCTG Senior Investigator: Janet Dancey CCTG Senior Biostatistician: Bingshu Chen Lead Group CCTG Trial Type (Intergroup, International, CCTG only)

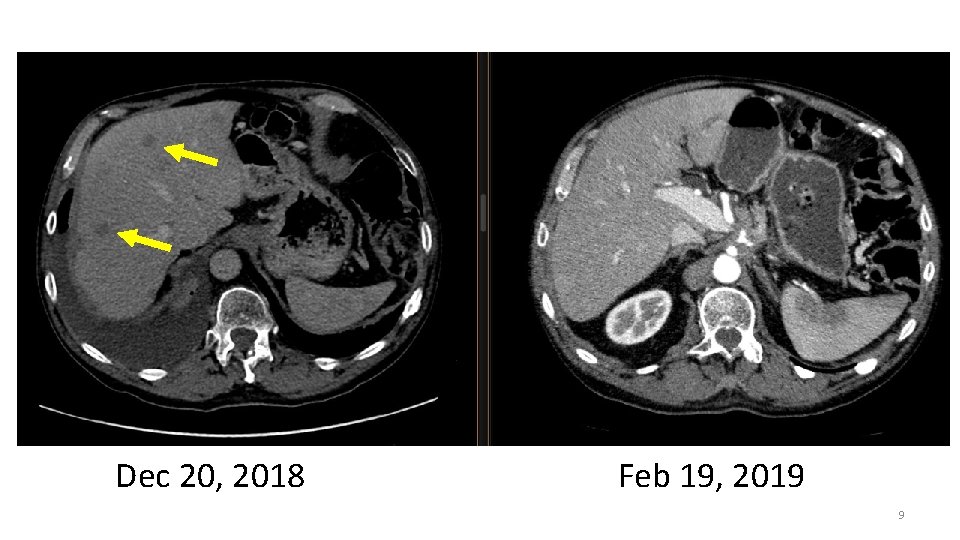
Dec 20, 2018 Feb 19, 2019 9

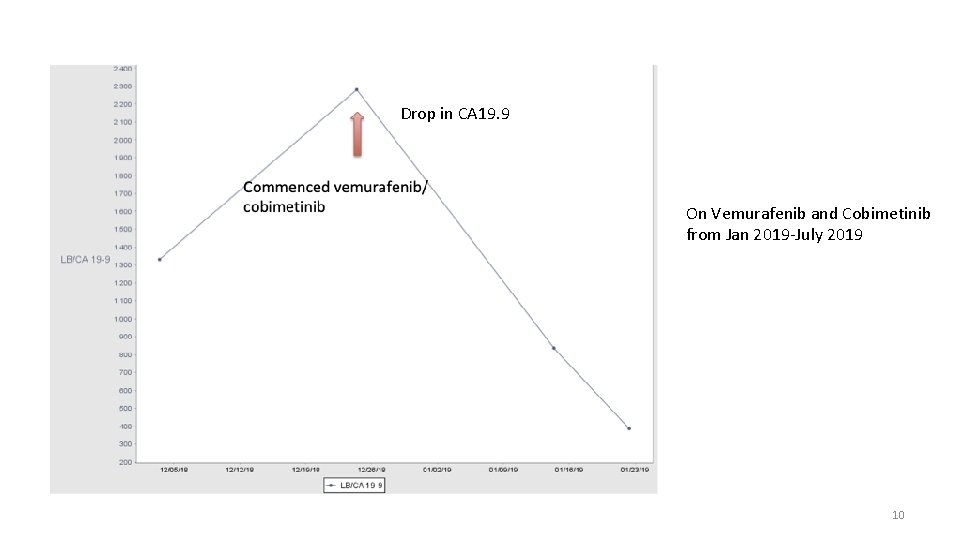
Drop in CA 19. 9 On Vemurafenib and Cobimetinib from Jan 2019 -July 2019 10

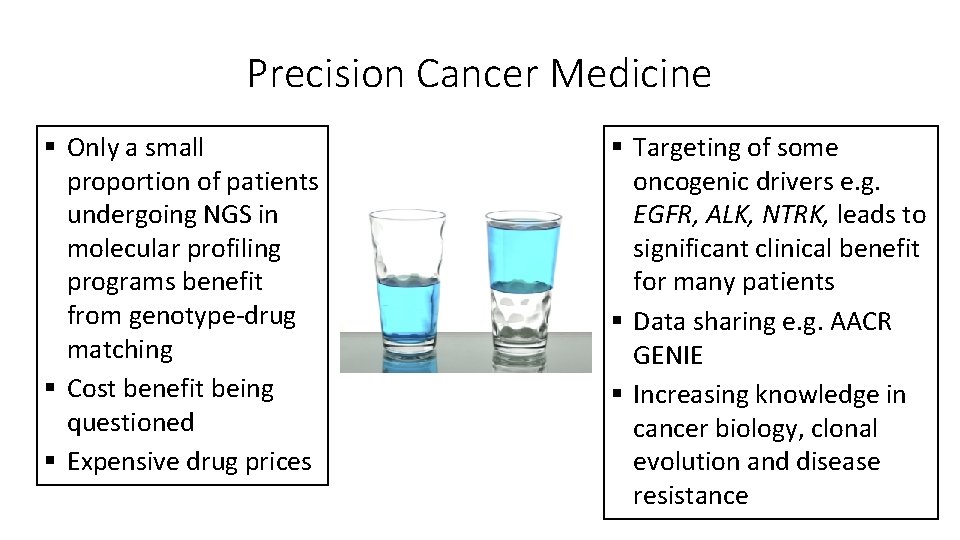
Precision Cancer Medicine § Only a small proportion of patients undergoing NGS in molecular profiling programs benefit from genotype-drug matching § Cost benefit being questioned § Expensive drug prices § Targeting of some oncogenic drivers e. g. EGFR, ALK, NTRK, leads to significant clinical benefit for many patients § Data sharing e. g. AACR GENIE § Increasing knowledge in cancer biology, clonal evolution and disease resistance

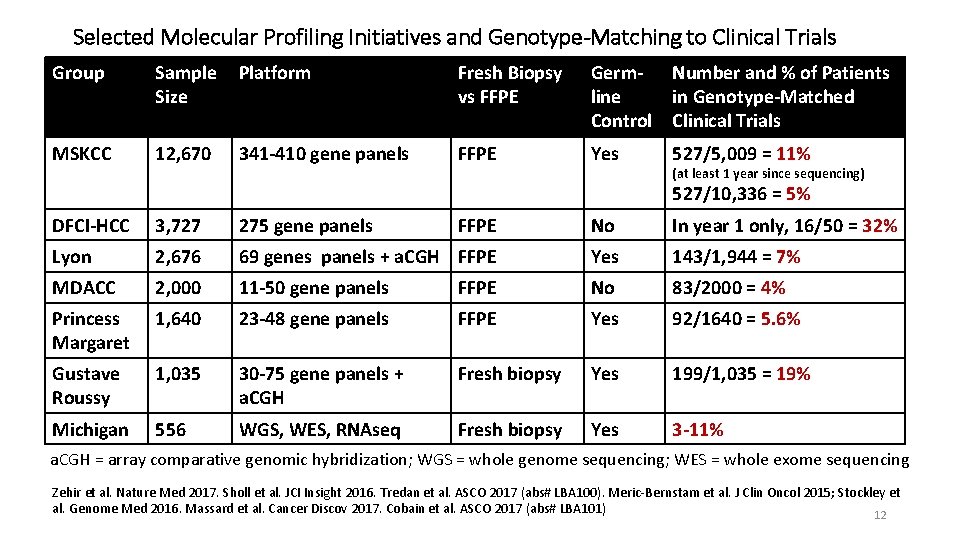
Selected Molecular Profiling Initiatives and Genotype-Matching to Clinical Trials Group Sample Size Platform Fresh Biopsy vs FFPE Germline Control Number and % of Patients in Genotype-Matched Clinical Trials MSKCC 12, 670 341 -410 gene panels FFPE Yes 527/5, 009 = 11% (at least 1 year since sequencing) 527/10, 336 = 5% DFCI-HCC 3, 727 275 gene panels FFPE No In year 1 only, 16/50 = 32% Lyon 2, 676 69 genes panels + a. CGH FFPE Yes 143/1, 944 = 7% MDACC 2, 000 11 -50 gene panels FFPE No 83/2000 = 4% Princess Margaret 1, 640 23 -48 gene panels FFPE Yes 92/1640 = 5. 6% Gustave Roussy 1, 035 30 -75 gene panels + a. CGH Fresh biopsy Yes 199/1, 035 = 19% Michigan 556 WGS, WES, RNAseq Fresh biopsy Yes 3 -11% a. CGH = array comparative genomic hybridization; WGS = whole genome sequencing; WES = whole exome sequencing Zehir et al. Nature Med 2017. Sholl et al. JCI Insight 2016. Tredan et al. ASCO 2017 (abs# LBA 100). Meric-Bernstam et al. J Clin Oncol 2015; Stockley et al. Genome Med 2016. Massard et al. Cancer Discov 2017. Cobain et al. ASCO 2017 (abs# LBA 101) 12

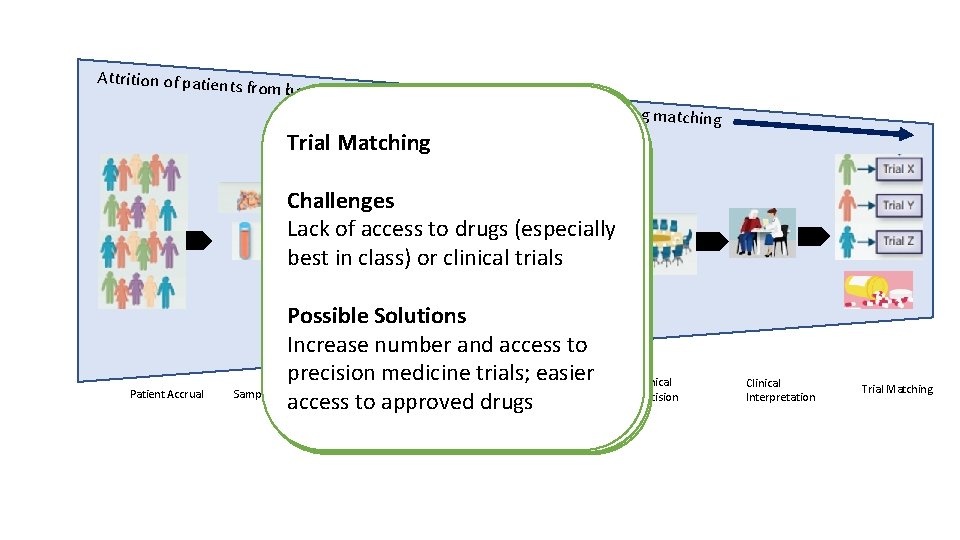
Attrition of patien ts from beginning of molecular profiling to genotype-drug Laboratory Operations Trial Matching Clinical Interpretation Variant Patient Accrual Sample Collection Clinical Decision Utility Patient Accrual Challenges Challenges Technical issues of NGS(especially and other Lack of access tosamples drugs Physician factors: busy clinics, Challenges with variant Patient factors: medical, logistics Inadequacy of for Lack of access to molecular tumor Low rate of actionable results assays best in class) or clinical trials lack of genomic understanding interpretation profiling board Possible Solutions Possible Solutions Appropriate patient selection, Possible. Solutions Possible Expansion of target identification Advances inknowledgebases, technology including Increase number andconsent access to Navigators and tools to help Integrated navigators, efficient and Improvement in sample collection Increase availability to MTB e. g. beyond genomics limits of detection and coverage precision medicine trials; easier physicians manage profiling artificial intelligence tools for Variant Laboratory IRB processes and processing, liquid biopsies Clinical virtual MTB Sample Collection Interpretation Operations depth access to approved drugs Utility results automation matching Clinical Decision Clinical Interpretation Trial Matching

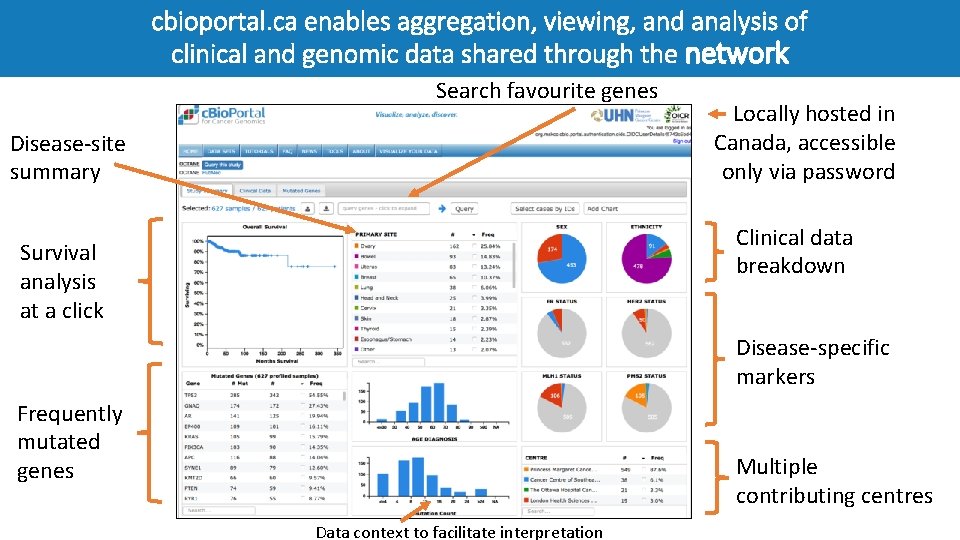
cbioportal. ca enables aggregation, viewing, and analysis of clinical and genomic data shared through the network Search favourite genes Disease-site summary Locally hosted in Canada, accessible only via password Clinical data breakdown Survival analysis at a click Disease-specific markers Frequently mutated genes Multiple contributing centres Data context to facilitate interpretation

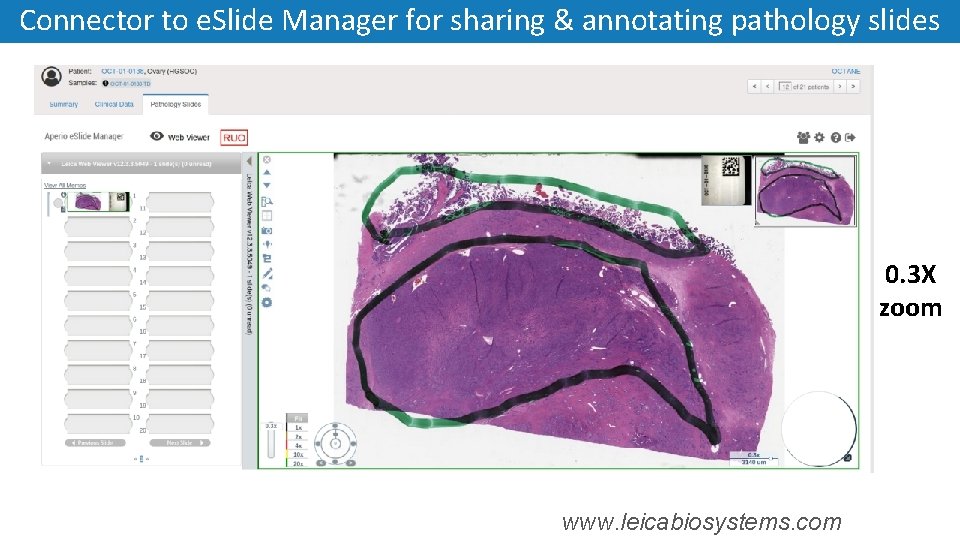
Connector to e. Slide Manager for sharing & annotating pathology slides 0. 3 X zoom www. leicabiosystems. com

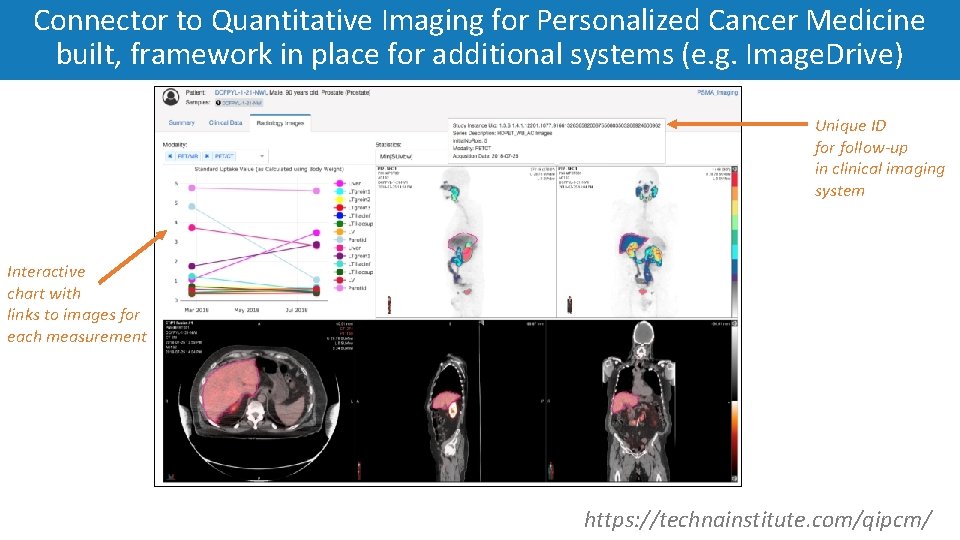
Connector to Quantitative Imaging for Personalized Cancer Medicine built, framework in place for additional systems (e. g. Image. Drive) Unique ID for follow-up in clinical imaging system Interactive chart with links to images for each measurement https: //technainstitute. com/qipcm/

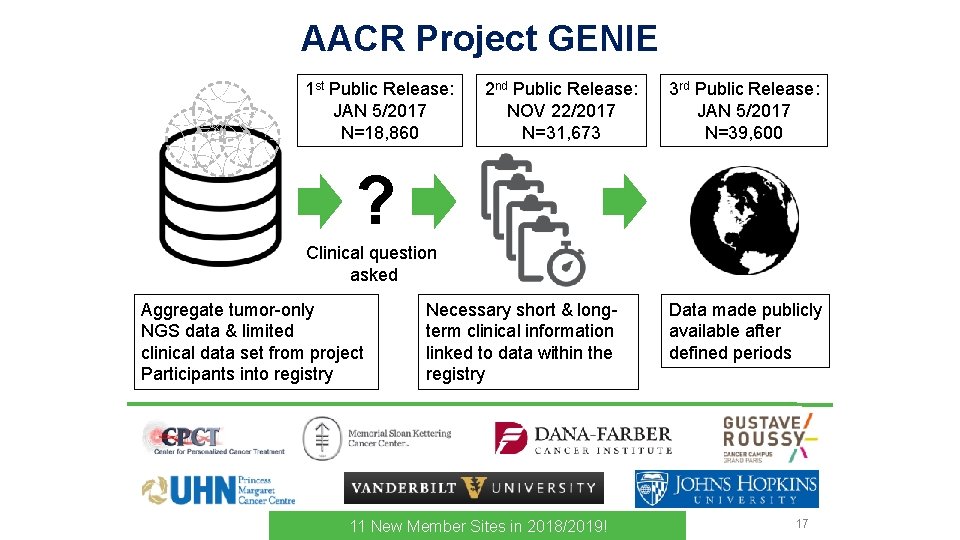
AACR Project GENIE 1 st Public Release: JAN 5/2017 N=18, 860 2 nd Public Release: NOV 22/2017 N=31, 673 3 rd Public Release: JAN 5/2017 N=39, 600 ? Clinical question asked Aggregate tumor-only NGS data & limited clinical data set from project Participants into registry Necessary short & longterm clinical information linked to data within the registry 11 New Member Sites in 2018/2019! Data made publicly available after defined periods 17

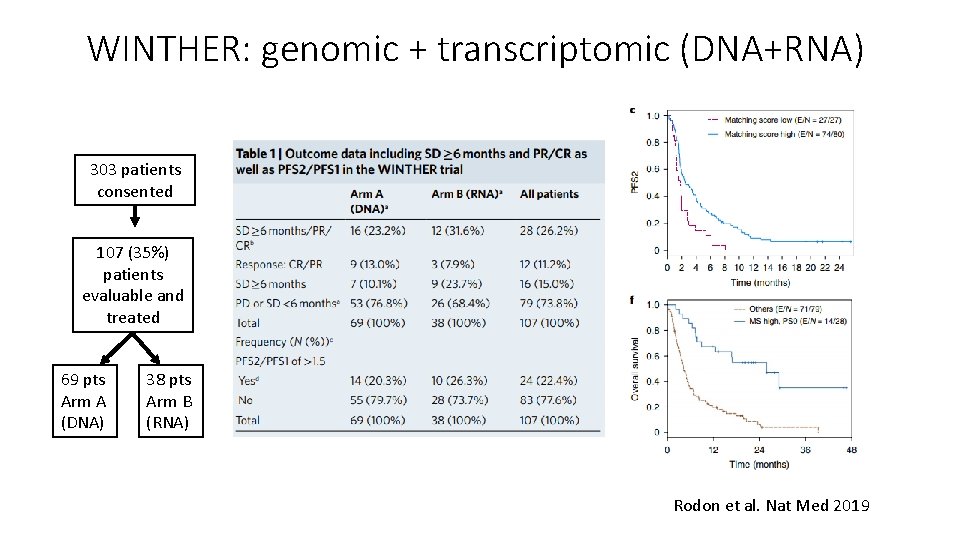
WINTHER: genomic + transcriptomic (DNA+RNA) 303 patients consented 107 (35%) patients evaluable and treated 69 pts Arm A (DNA) 38 pts Arm B (RNA) Rodon et al. Nat Med 2019

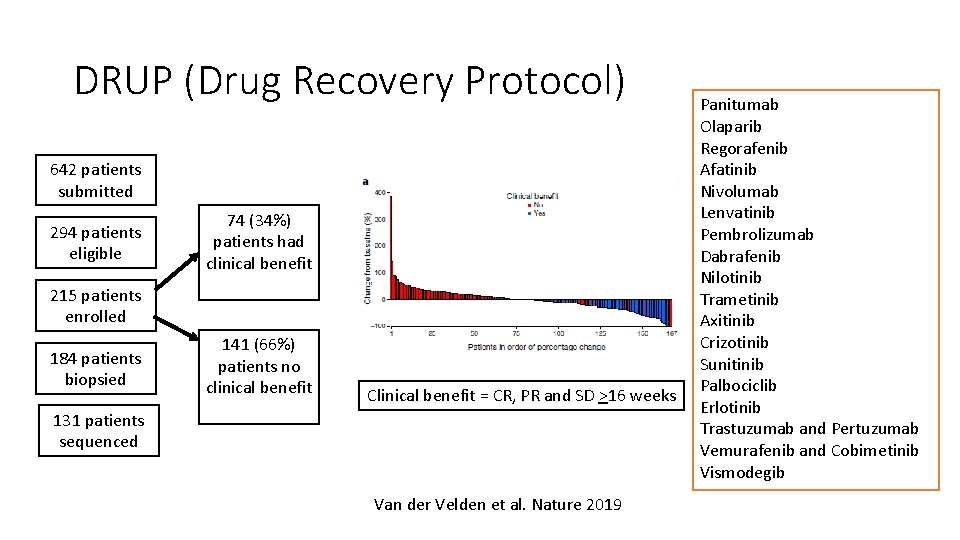
DRUP (Drug Recovery Protocol) 642 patients submitted 294 patients eligible 74 (34%) patients had clinical benefit 215 patients enrolled 184 patients biopsied 141 (66%) patients no clinical benefit Clinical benefit = CR, PR and SD >16 weeks 131 patients sequenced Van der Velden et al. Nature 2019 Panitumab Olaparib Regorafenib Afatinib Nivolumab Lenvatinib Pembrolizumab Dabrafenib Nilotinib Trametinib Axitinib Crizotinib Sunitinib Palbociclib Erlotinib Trastuzumab and Pertuzumab Vemurafenib and Cobimetinib Vismodegib

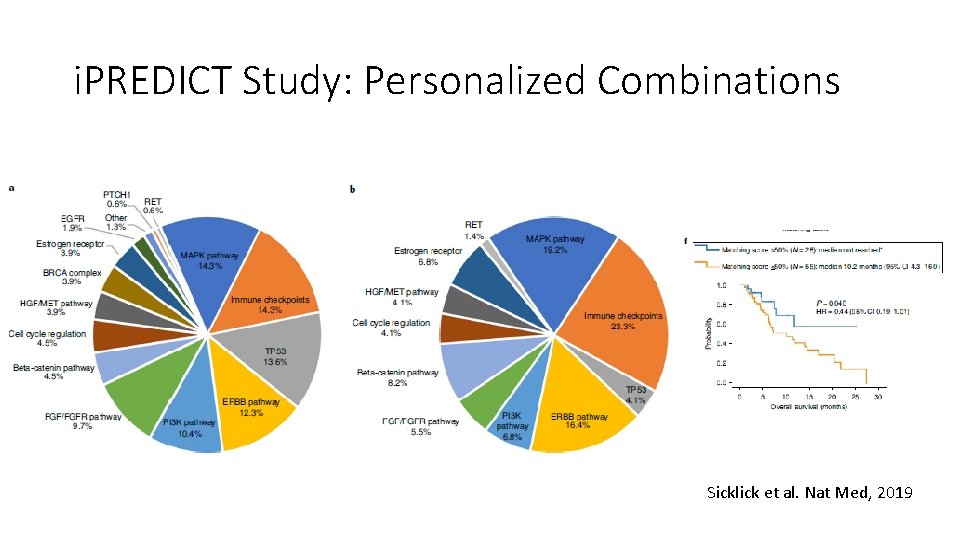
i. PREDICT Study: Personalized Combinations Sicklick et al. Nat Med, 2019

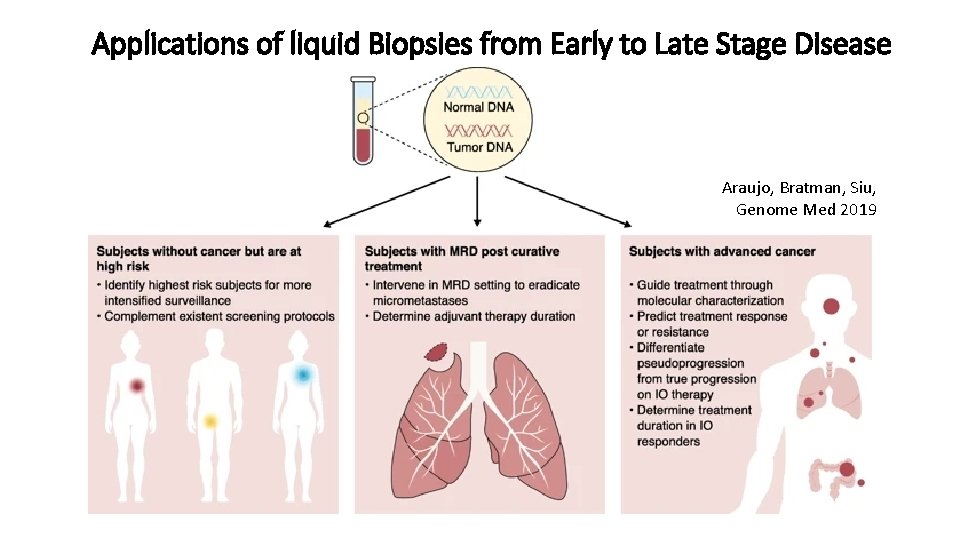
Applications of liquid Biopsies from Early to Late Stage Disease Araujo, Bratman, Siu, Genome Med 2019

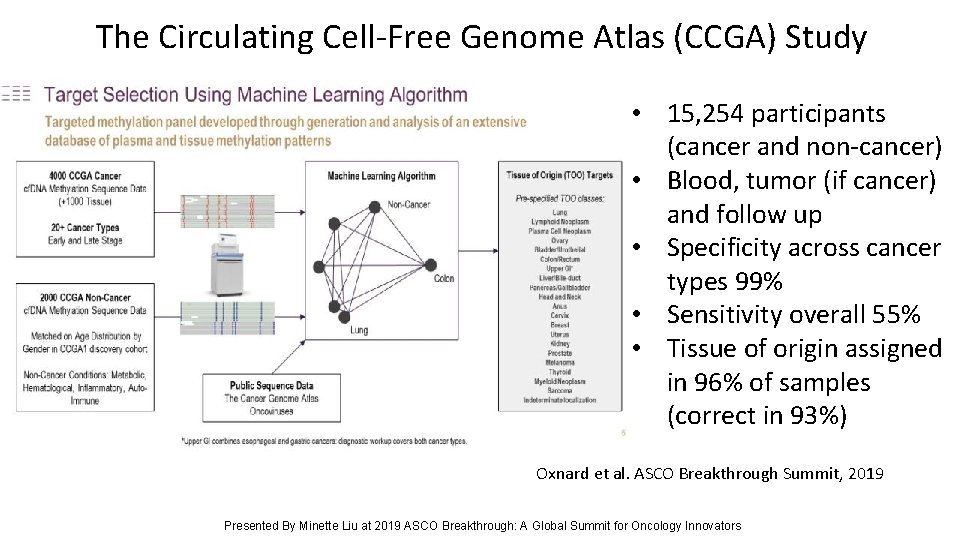
The Circulating Cell-Free Genome Atlas (CCGA) Study • 15, 254 participants (cancer and non-cancer) • Blood, tumor (if cancer) and follow up • Specificity across cancer types 99% • Sensitivity overall 55% • Tissue of origin assigned in 96% of samples (correct in 93%) Oxnard et al. ASCO Breakthrough Summit, 2019 Presented By Minette Liu at 2019 ASCO Breakthrough: A Global Summit for Oncology Innovators

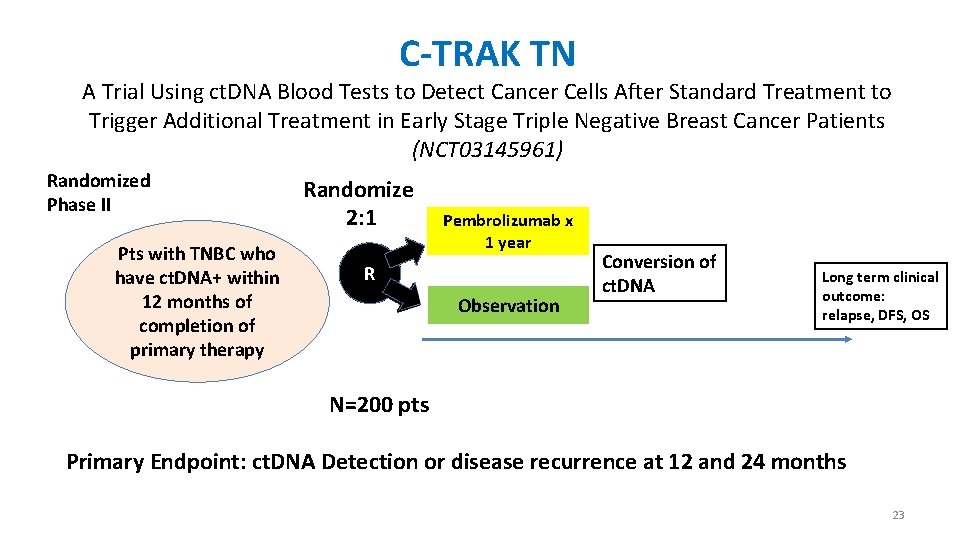
C-TRAK TN A Trial Using ct. DNA Blood Tests to Detect Cancer Cells After Standard Treatment to Trigger Additional Treatment in Early Stage Triple Negative Breast Cancer Patients (NCT 03145961) Randomized Phase II Pts with TNBC who have ct. DNA+ within 12 months of completion of primary therapy Randomize 2: 1 Pembrolizumab x 1 year R Observation Conversion of ct. DNA Long term clinical outcome: relapse, DFS, OS N=200 pts Primary Endpoint: ct. DNA Detection or disease recurrence at 12 and 24 months 23

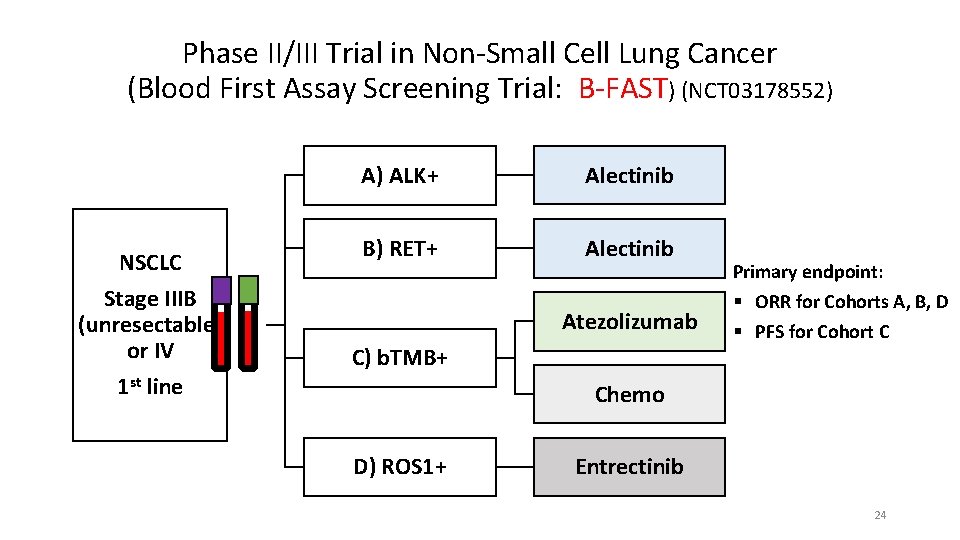
Phase II/III Trial in Non-Small Cell Lung Cancer (Blood First Assay Screening Trial: B-FAST) (NCT 03178552) NSCLC Stage IIIB (unresectable) or IV 1 st line A) ALK+ Alectinib B) RET+ Alectinib Atezolizumab C) b. TMB+ Primary endpoint: § ORR for Cohorts A, B, D § PFS for Cohort C Chemo D) ROS 1+ Entrectinib 24

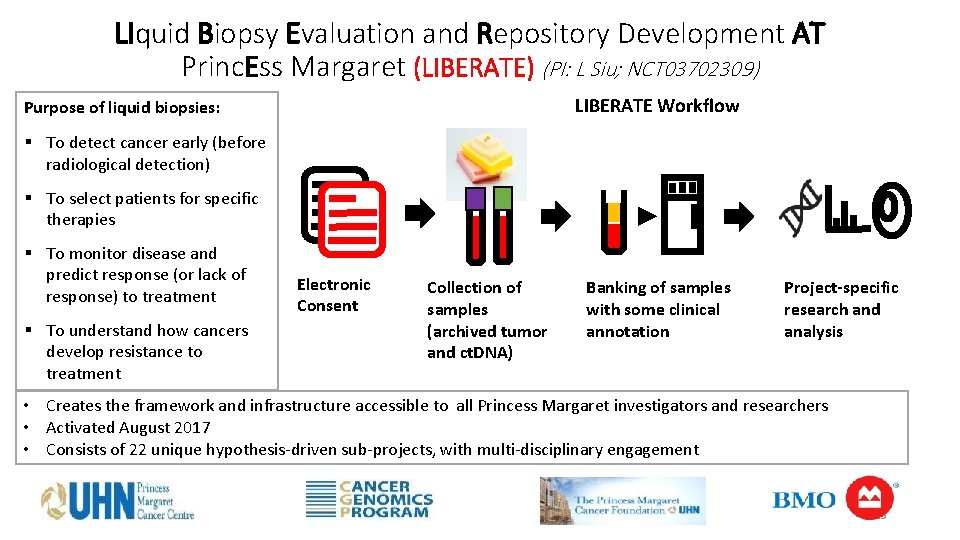
LIquid Biopsy Evaluation and Repository Development AT Princ. Ess Margaret (LIBERATE) (PI: L Siu; NCT 03702309) LIBERATE Workflow Purpose of liquid biopsies: § To detect cancer early (before radiological detection) § To select patients for specific therapies § To monitor disease and predict response (or lack of response) to treatment § To understand how cancers develop resistance to treatment Electronic Consent Collection of samples (archived tumor and ct. DNA) Banking of samples with some clinical annotation Project-specific research and analysis • Creates the framework and infrastructure accessible to all Princess Margaret investigators and researchers • Activated August 2017 • Consists of 22 unique hypothesis-driven sub-projects, with multi-disciplinary engagement 25

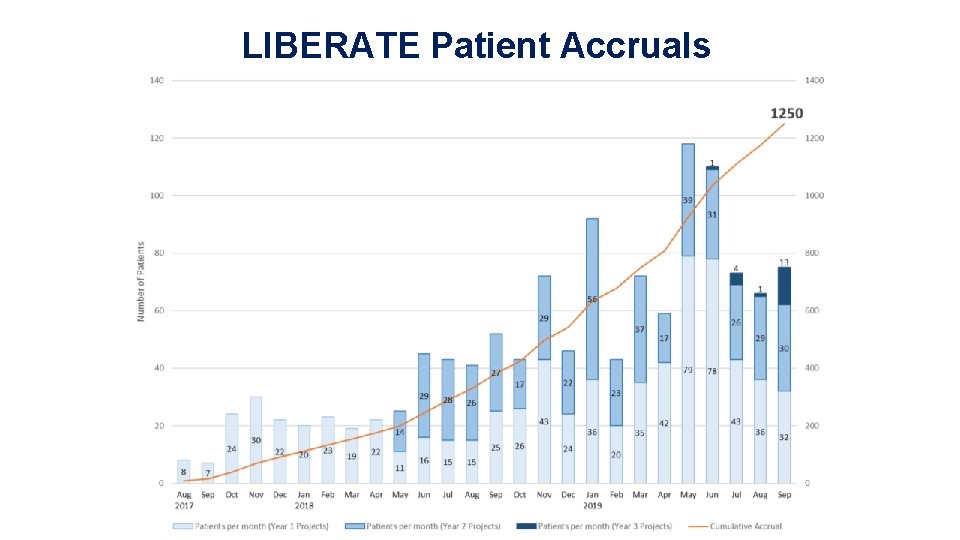
LIBERATE Patient Accruals 26

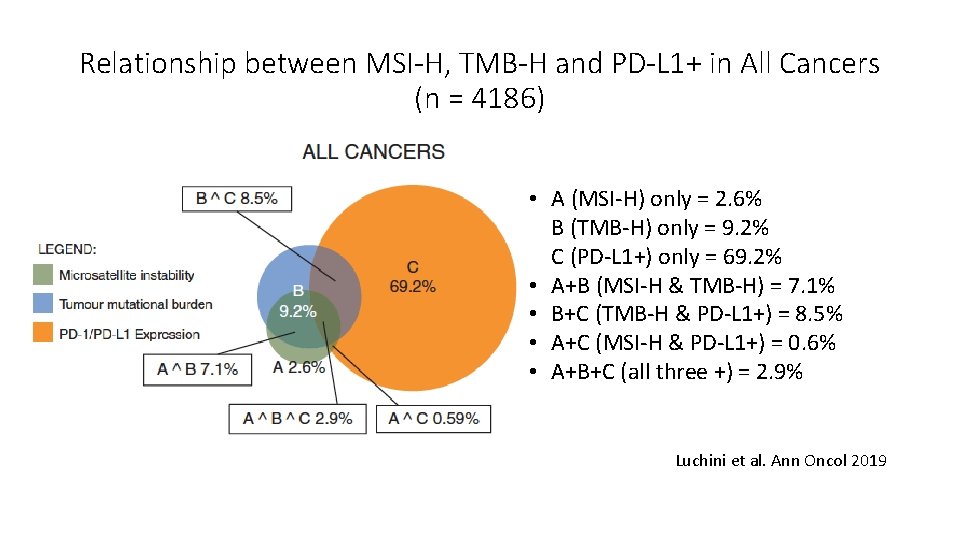
Relationship between MSI-H, TMB-H and PD-L 1+ in All Cancers (n = 4186) • A (MSI-H) only = 2. 6% B (TMB-H) only = 9. 2% C (PD-L 1+) only = 69. 2% • A+B (MSI-H & TMB-H) = 7. 1% • B+C (TMB-H & PD-L 1+) = 8. 5% • A+C (MSI-H & PD-L 1+) = 0. 6% • A+B+C (all three +) = 2. 9% Luchini et al. Ann Oncol 2019

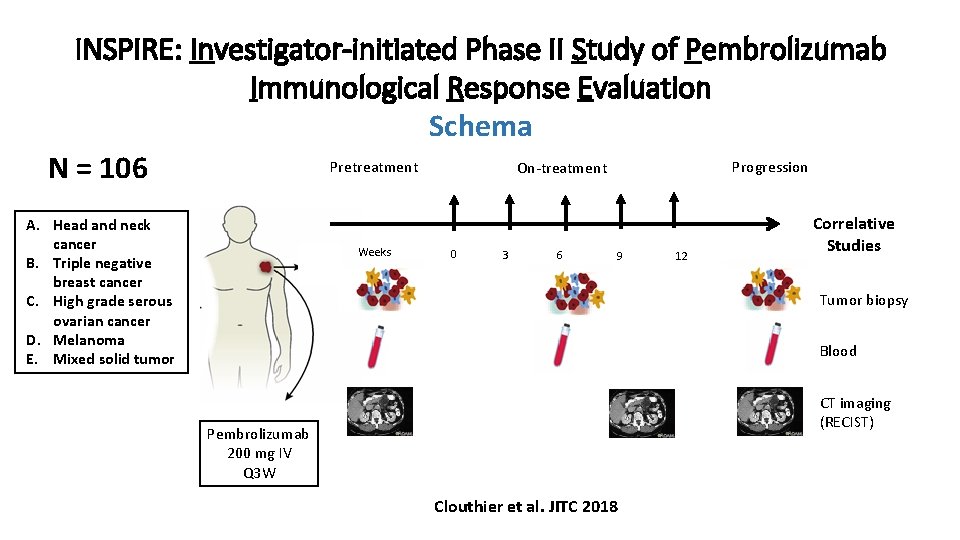
INSPIRE: Investigator-initiated Phase II Study of Pembrolizumab Immunological Response Evaluation Schema Pretreatment Progression On-treatment N = 106 A. Head and neck cancer B. Triple negative breast cancer C. High grade serous ovarian cancer D. Melanoma E. Mixed solid tumor Weeks 0 3 6 9 12 Correlative Studies Tumor biopsy Blood CT imaging (RECIST) Pembrolizumab 200 mg IV Q 3 W Clouthier et al. JITC 2018

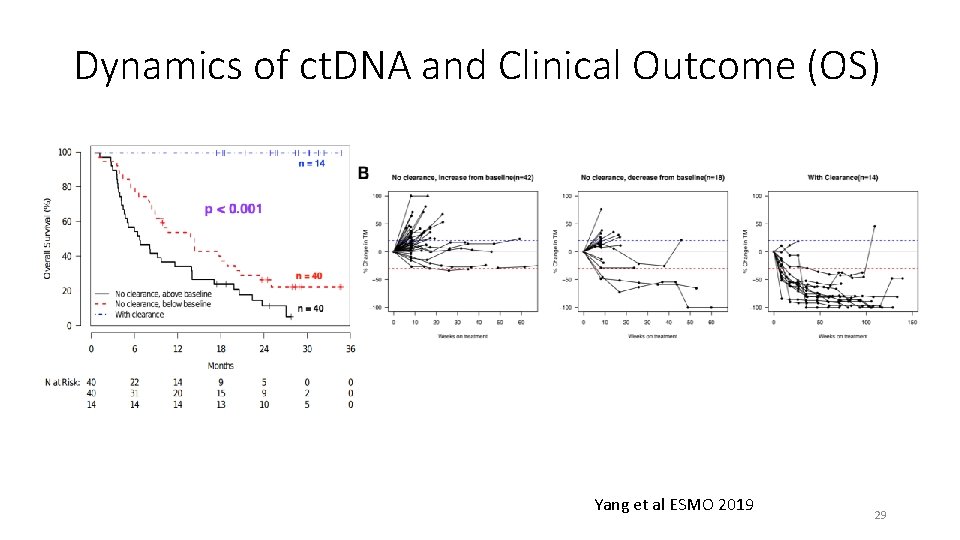
Dynamics of ct. DNA and Clinical Outcome (OS) Yang et al ESMO 2019 29

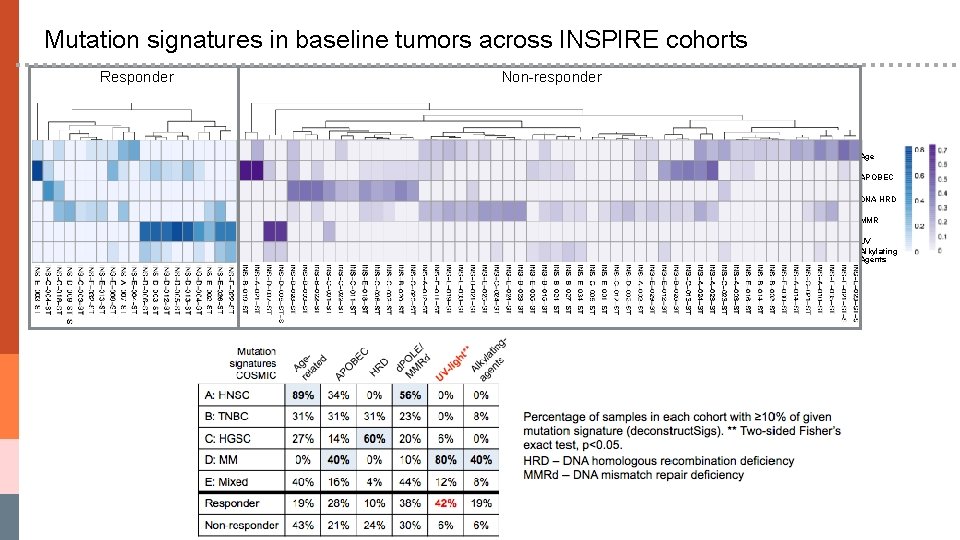
Mutation signatures in baseline tumors across INSPIRE cohorts Responder Non-responder Age APOBEC DNA HRD MMR UV Alkylating Agents

Conclusions • Ongoing efforts to maximize clinical utility of precision cancer medicine initiatives • Focus on target patient populations • Broadening technical scope to increase molecular matches – including immuno-oncology • Designing innovative clinical trials • Data sharing • Increasing the knowledge base • Dynamic monitoring using liquid biopsies – and ultimately conduct dynamic clinical trials • Linkage to real world evidence

Phase I Program Cancer Genomics Program Tumor Immunotherapy Program at Princess Margaret 32
 Hierro carboximaltosa
Hierro carboximaltosa Comorbilida
Comorbilida Semi precision attachment abutment
Semi precision attachment abutment Bcd gösterimi
Bcd gösterimi Non precision instruments are
Non precision instruments are Leicester precision medicine institute
Leicester precision medicine institute Precision medicine ecosystem
Precision medicine ecosystem Ethical issues in precision medicine
Ethical issues in precision medicine Leicester precision medicine institute
Leicester precision medicine institute Primer congreso
Primer congreso Características del congreso
Características del congreso Asopedia congreso
Asopedia congreso Rilco congreso 2021
Rilco congreso 2021 Crosiet
Crosiet Your privilege is showing lillian medville
Your privilege is showing lillian medville Lillian helsinki
Lillian helsinki Lillian hjorth
Lillian hjorth Contribution of frank and lillian gilbreth
Contribution of frank and lillian gilbreth Delta sigma theta pyramid preparation period
Delta sigma theta pyramid preparation period Lillian cohn
Lillian cohn Cchd lillian
Cchd lillian Lillian osei-boateng
Lillian osei-boateng Monique buys a $4 700 air conditioning
Monique buys a $4 700 air conditioning What eats a jaguarundi
What eats a jaguarundi Roll of thunder hear my cry strawberry
Roll of thunder hear my cry strawberry Frederick taylor and frank and lillian gilbreth
Frederick taylor and frank and lillian gilbreth Lillian siu
Lillian siu Precision planting graphite powder
Precision planting graphite powder Non precision vaporizer
Non precision vaporizer Sas numeric precision
Sas numeric precision Precision fluency shaping program
Precision fluency shaping program Precision planting definition
Precision planting definition Precision land management
Precision land management























































