Conference Series LLC Conferences Conference Series LLC is












- Slides: 34

Conference Series LLC Conferences Conference Series LLC is a pioneer and leading science event organizer, which publishes around 500 open access journals and conducts over 500 Medical, Clinical, Engineering, Life Sciences, Pharma scientific conferences all over the globe annually with the support of more than 1000 scientific associations and 30, 000 editorial board members and 3. 5 million followers to its credit. Conference Series LLC has organized 500 conferences, workshops and national symposiums across the major cities including San Francisco, Las Vegas, San Antonio, Omaha, Orlando, Raleigh, Santa Clara, Chicago, Philadelphia, Baltimore, United Kingdom, Valencia, Dubai, Beijing, Hyderabad, Bengaluru and Mumbai.

Development of HPLC-UV/MS-MS methods applied to the quality control of ursodeoxycholic acid in oral liquid pediatric formulations and raw material Pharm. Boscolo Oriana CIDEC – Tecnología Farmacéutica Facultad de Farmacia y Bioquímica Universidad de Buenos Aires


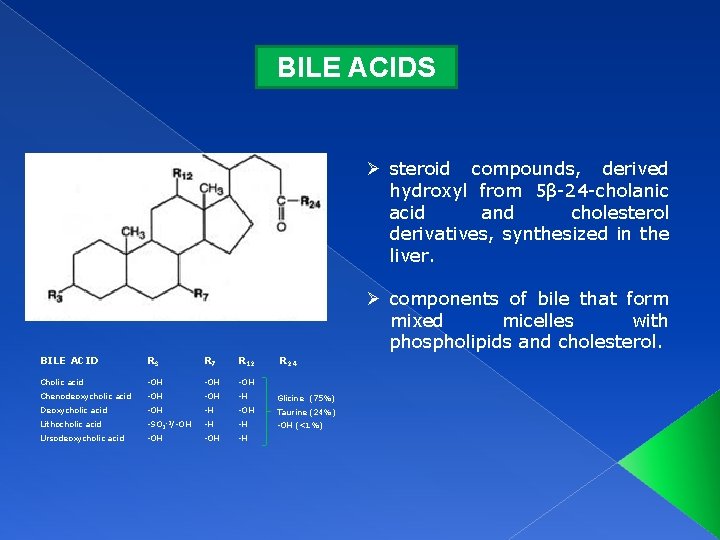
BILE ACIDS Ø steroid compounds, derived hydroxyl from 5β-24 -cholanic acid and cholesterol derivatives, synthesized in the liver. Ø components of bile that form mixed micelles with phospholipids and cholesterol. BILE ACID R 3 R 7 R 12 R 24 Cholic acid -OH -OH Chenodeoxycholic acid -OH -H Glicine (75%) Deoxycholic acid -OH -H -OH Taurine (24%) Lithocholic acid -SO 3 -2/-OH -H -H -OH (<1%) Ursodeoxycholic acid -OH -H

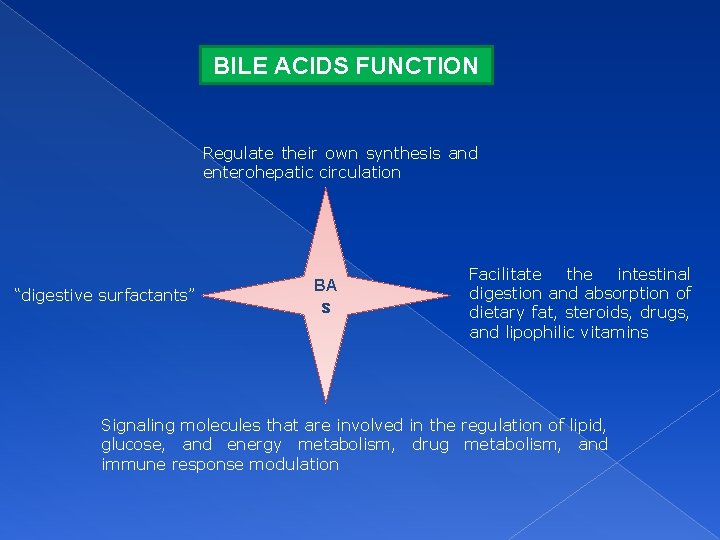
BILE ACIDS FUNCTION Regulate their own synthesis and enterohepatic circulation “digestive surfactants” BA s Facilitate the intestinal digestion and absorption of dietary fat, steroids, drugs, and lipophilic vitamins Signaling molecules that are involved in the regulation of lipid, glucose, and energy metabolism, drug metabolism, and immune response modulation

URSODEOXYCHOLIC ACID (UDCA) v Also known as ursodiol, is a secondary acid and the least hydrophobic of bile acids (BA). v Origin: “urso” = bear. v 7α CDCA 7β UDCA epimerase v Present in small amounts in the human beings: 1 -3 % BA

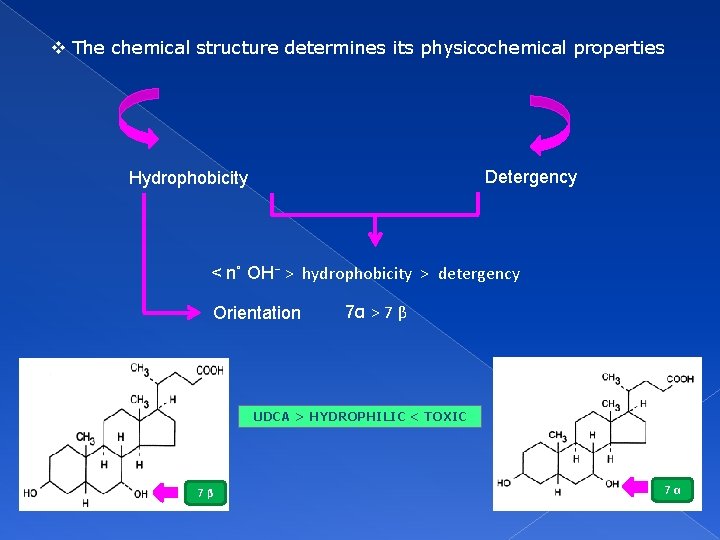
v The chemical structure determines its physicochemical properties Detergency Hydrophobicity < n˚ OH⁻ > hydrophobicity > detergency Orientation 7α > 7 β UDCA > HYDROPHILIC < TOXIC 7β 7α

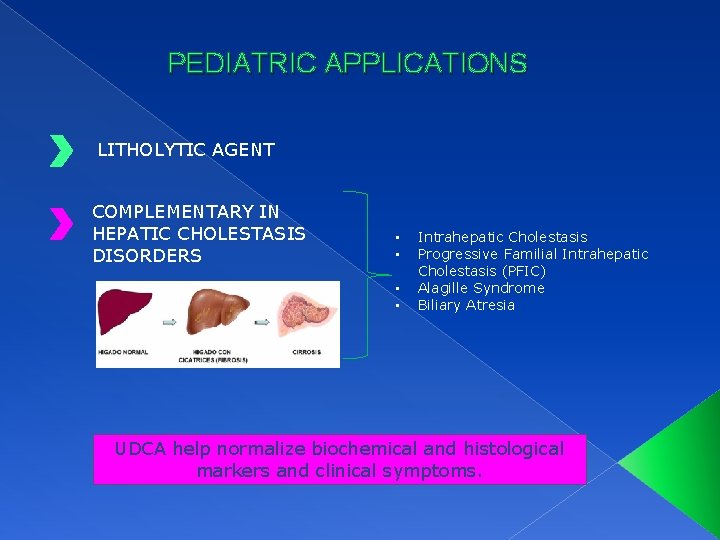
PEDIATRIC APPLICATIONS LITHOLYTIC AGENT COMPLEMENTARY IN HEPATIC CHOLESTASIS DISORDERS • • Intrahepatic Cholestasis Progressive Familial Intrahepatic Cholestasis (PFIC) Alagille Syndrome Biliary Atresia UDCA help normalize biochemical and histological markers and clinical symptoms.

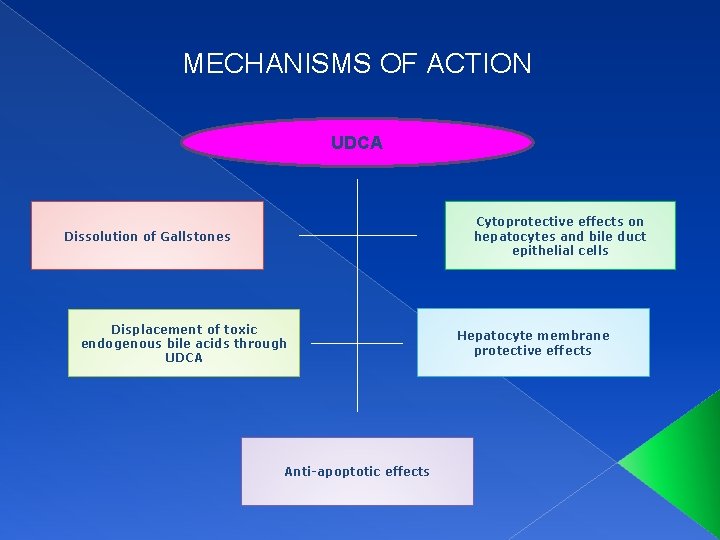
MECHANISMS OF ACTION UDCA Cytoprotective effects on hepatocytes and bile duct epithelial cells Dissolution of Gallstones Displacement of toxic endogenous bile acids through UDCA Anti-apoptotic effects Hepatocyte membrane protective effects

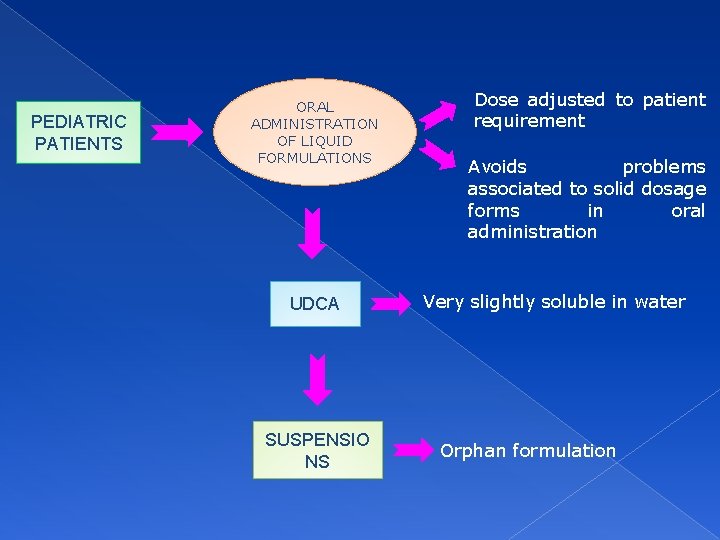
PEDIATRIC PATIENTS ORAL ADMINISTRATION OF LIQUID FORMULATIONS UDCA SUSPENSIO NS Dose adjusted to patient requirement Avoids problems associated to solid dosage forms in oral administration Very slightly soluble in water Orphan formulation

Development of an HPLC-UV analytical method for the UDCA determination in a pediatric suspension Suspension Compounds %P/V UDCA 2. 5 Glicerine 50 Vehicle q. s. 100 Sample Preparation 0. 5 g de suspension/25 m. L methanol Sonication 5‘ Centrifugation 10‘ 4000 rpm Vehicle Compounds %P/V Methylparaben 0. 08 Propylparaben 0. 02 Xanthan Gum 0. 2 Water q. s. 100 1/2 mobile phase (ACN : 0. 5 m. M H₃PO₄ p. H 3) (50: 50)

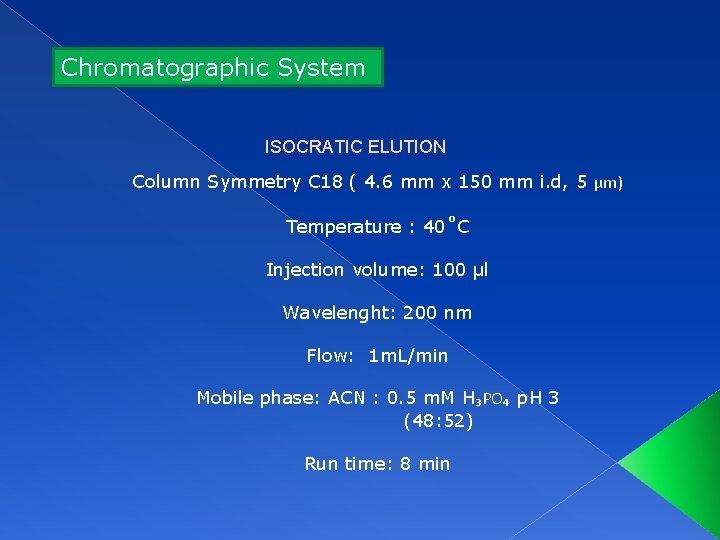
Chromatographic System ISOCRATIC ELUTION Column Symmetry C 18 ( 4. 6 mm x 150 mm i. d, 5 µm) Temperature : 40˚C Injection volume: 100 µl Wavelenght: 200 nm Flow: 1 m. L/min Mobile phase: ACN : 0. 5 m. M H₃PO₄ p. H 3 (48: 52) Run time: 8 min

Optimization and Validation Parameters SPECIFICITY LINEARITY LOD LOQ PRECISION ACCURACY ROBUSTNESS

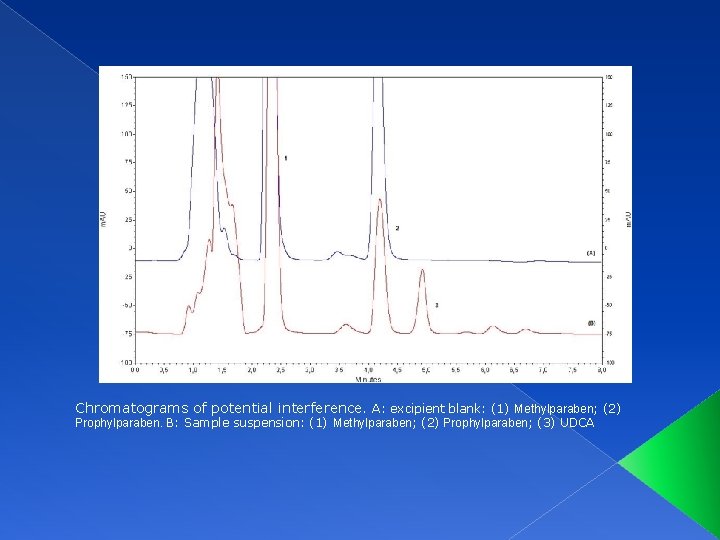
SPECIFICITY Specificity was evaluated with different excipients that could act as potential interferences. Glicerine Xanthan gum Methylparaben Prophylparaben

Chromatograms of potential interference. A: excipient blank: (1) Methylparaben; (2) Prophylparaben. B: Sample suspension: (1) Methylparaben; (2) Prophylparaben; (3) UDCA

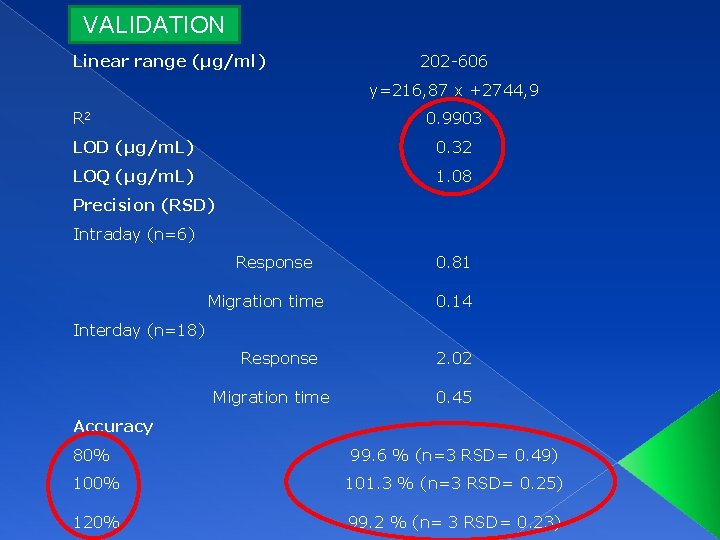
VALIDATION Linear range (µg/ml) 202 -606 y=216, 87 x +2744, 9 R 2 0. 9903 LOD (µg/m. L) 0. 32 LOQ (µg/m. L) 1. 08 Precision (RSD) Intraday (n=6) Response Migration time 0. 81 0. 14 Interday (n=18) Response Migration time 2. 02 0. 45 Accuracy 80% 99. 6 % (n=3 RSD= 0. 49) 100% 101. 3 % (n=3 RSD= 0. 25) 120% 99. 2 % (n= 3 RSD= 0. 23)

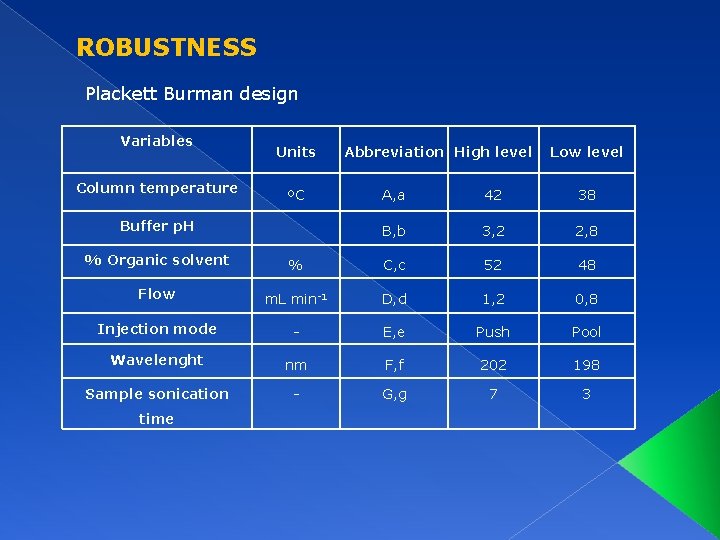
ROBUSTNESS Plackett Burman design Variables Column temperature Units ºC Buffer p. H Abbreviation High level Low level A, a 42 38 B, b 3, 2 2, 8 % Organic solvent % C, c 52 48 Flow m. L min-1 D, d 1, 2 0, 8 Injection mode - E, e Push Pool Wavelenght nm F, f 202 198 Sample sonication - G, g 7 3 time


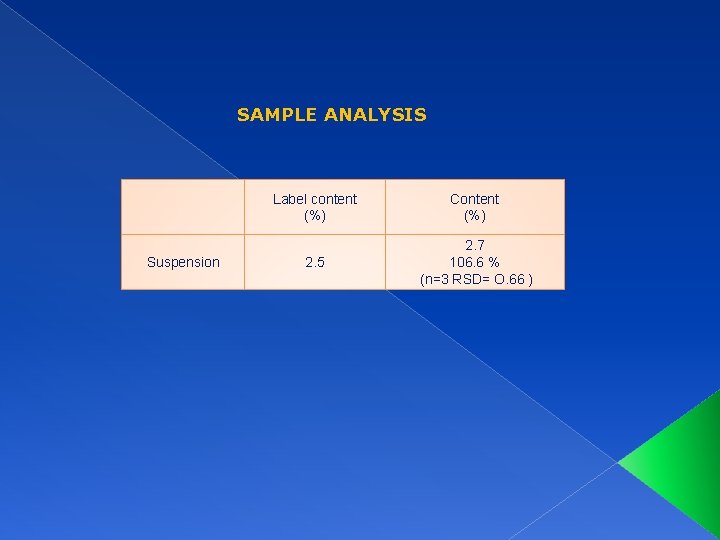
SAMPLE ANALYSIS Suspension Label content (%) Content (%) 2. 5 2. 7 106. 6 % (n=3 RSD= O. 66 )

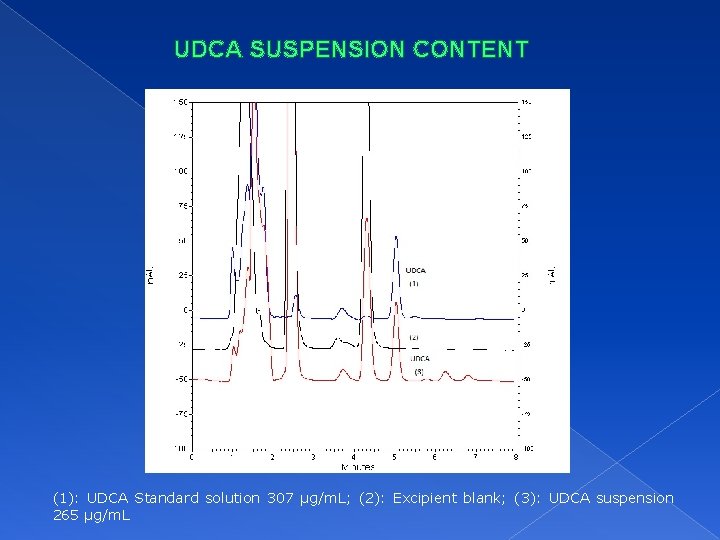
UDCA SUSPENSION CONTENT (1): UDCA Standard solution 307 µg/m. L; (2): Excipient blank; (3): UDCA suspension 265 µg/m. L

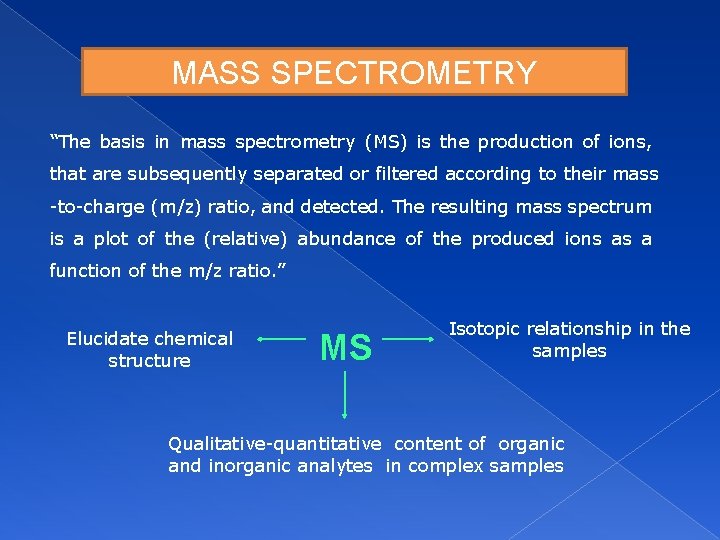
MASS SPECTROMETRY “The basis in mass spectrometry (MS) is the production of ions, that are subsequently separated or filtered according to their mass -to-charge (m/z) ratio, and detected. The resulting mass spectrum is a plot of the (relative) abundance of the produced ions as a function of the m/z ratio. ” Elucidate chemical structure MS Isotopic relationship in the samples Qualitative-quantitative content of organic and inorganic analytes in complex samples

INSTRUMENTATION Liquid Chromatography Ulimate 3000 (Thermo Fisher Scientific TSQ Quantum Access MAX (Thermo Fisher Scientific) Data System: Xcalibur 2. 1.

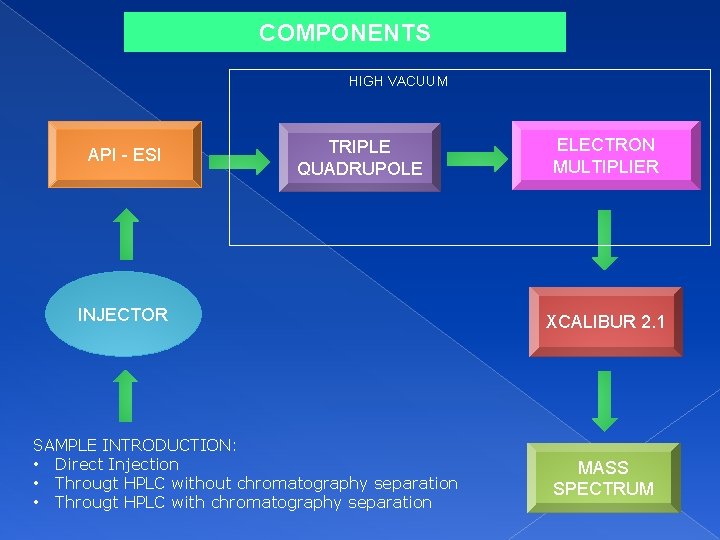
COMPONENTS HIGH VACUUM API - ESI TRIPLE QUADRUPOLE INJECTOR SAMPLE INTRODUCTION: • Direct Injection • Througt HPLC without chromatography separation • Througt HPLC with chromatography separation ELECTRON MULTIPLIER XCALIBUR 2. 1 MASS SPECTRUM

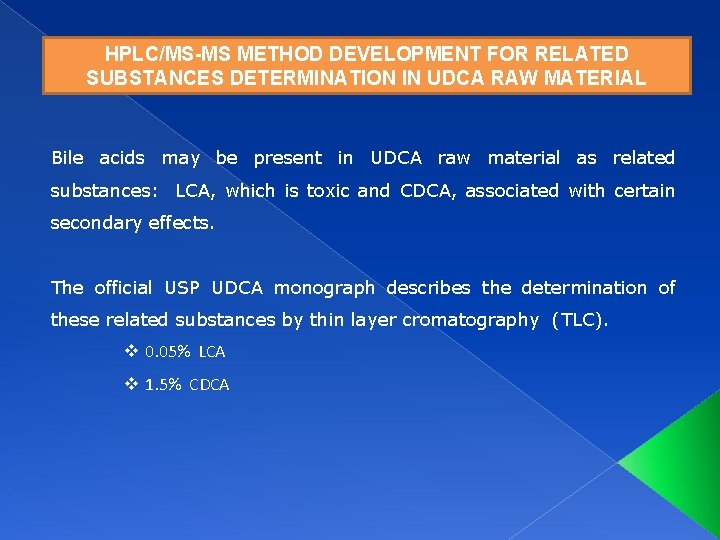
HPLC/MS-MS METHOD DEVELOPMENT FOR RELATED SUBSTANCES DETERMINATION IN UDCA RAW MATERIAL Bile acids may be present in UDCA raw material as related substances: LCA, which is toxic and CDCA, associated with certain secondary effects. The official USP UDCA monograph describes the determination of these related substances by thin layer cromatography (TLC). v 0. 05% LCA v 1. 5% CDCA

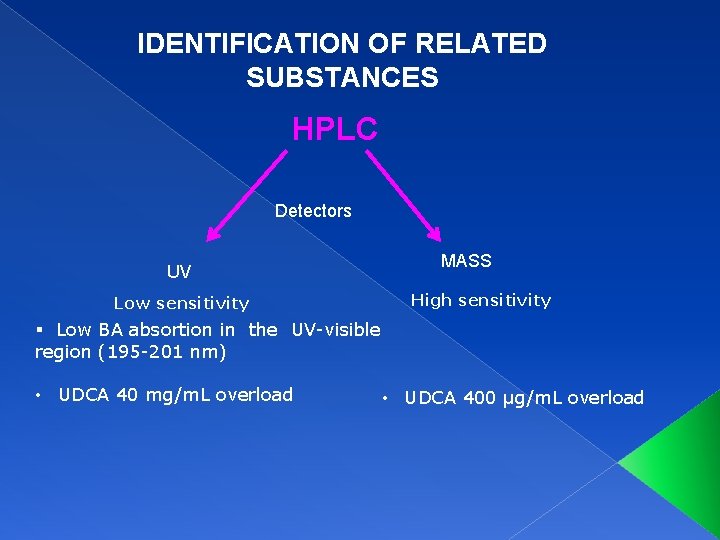
IDENTIFICATION OF RELATED SUBSTANCES HPLC Detectors UV Low sensitivity MASS High sensitivity § Low BA absortion in the UV-visible region (195 -201 nm) • UDCA 40 mg/m. L overload • UDCA 400 µg/m. L overload

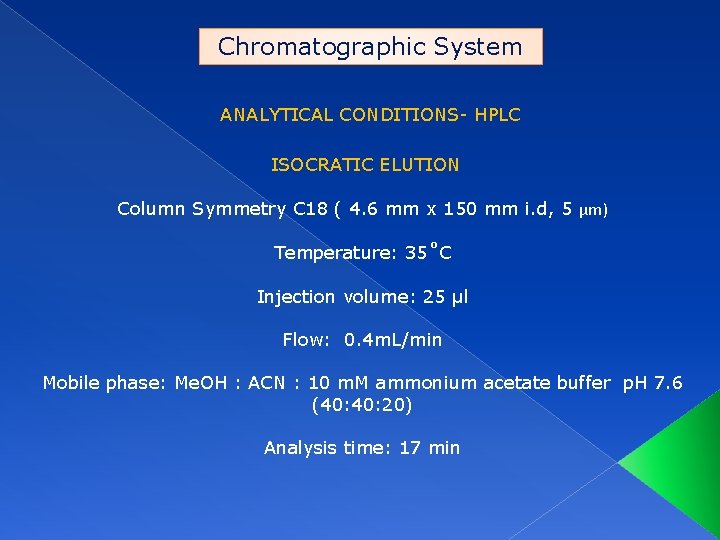
Chromatographic System ANALYTICAL CONDITIONS- HPLC ISOCRATIC ELUTION Column Symmetry C 18 ( 4. 6 mm x 150 mm i. d, 5 µm) Temperature: 35˚C Injection volume: 25 µl Flow: 0. 4 m. L/min Mobile phase: Me. OH : ACN : 10 m. M ammonium acetate buffer p. H 7. 6 (40: 20) Analysis time: 17 min

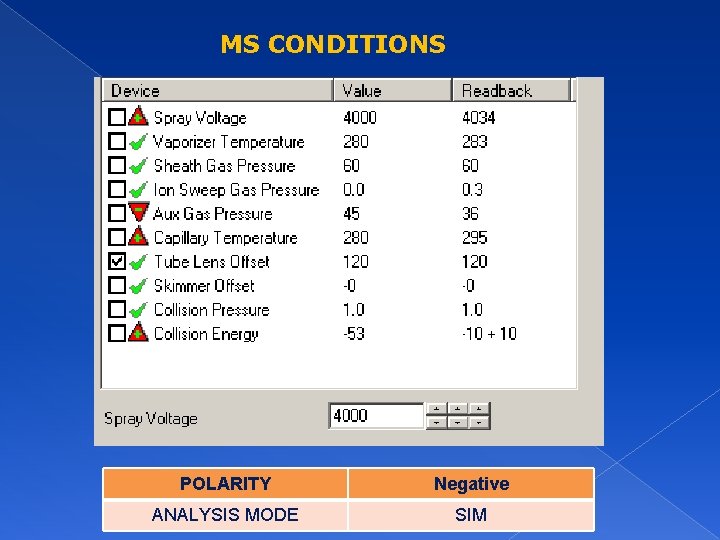
MS CONDITIONS POLARITY Negative ANALYSIS MODE SIM

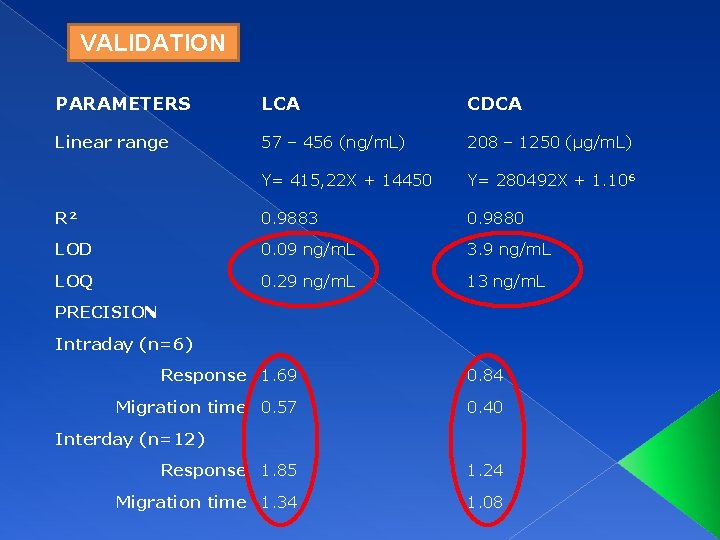
VALIDATION PARAMETERS LCA CDCA Linear range 57 – 456 (ng/m. L) 208 – 1250 (µg/m. L) Y= 415, 22 X + 14450 Y= 280492 X + 1. 10⁶ R² 0. 9883 0. 9880 LOD 0. 09 ng/m. L 3. 9 ng/m. L LOQ 0. 29 ng/m. L 13 ng/m. L PRECISION Intraday (n=6) Response 1. 69 0. 84 Migration time 0. 57 0. 40 Interday (n=12) Response 1. 85 1. 24 Migration time 1. 34 1. 08

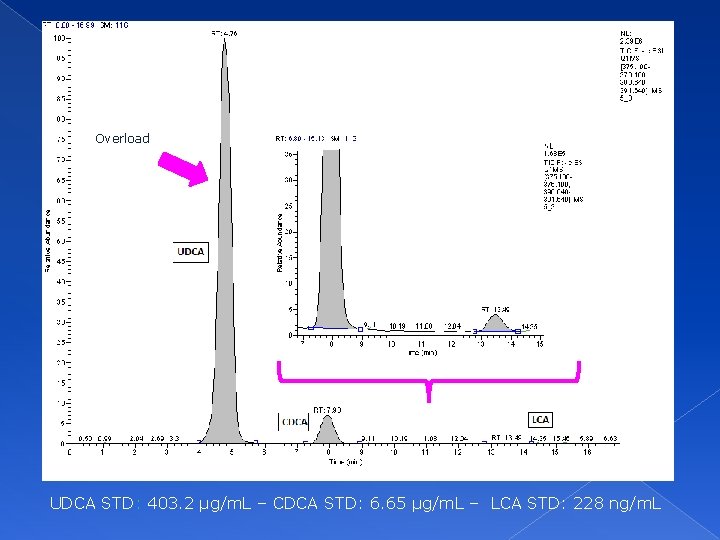
Overload UDCA STD: 403. 2 µg/m. L – CDCA STD: 6. 65 µg/m. L – LCA STD: 228 ng/m. L

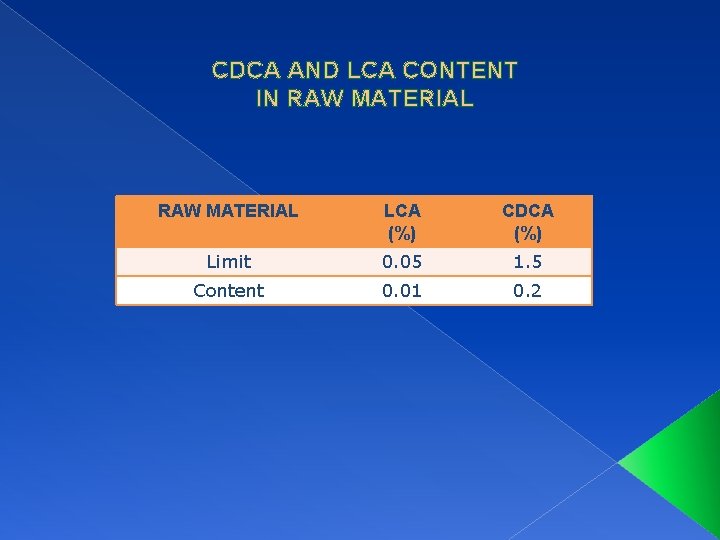
CDCA AND LCA CONTENT IN RAW MATERIAL LCA (%) CDCA (%) Limit 0. 05 1. 5 Content 0. 01 0. 2

Conclusions v An HPLC-UV method for the analysis of UDCA content in oral suspensions was developed. This method complies with ICH guidelines and is simple, fast, accurate, precise, robust and sensitive. v An HPLC-MS/MS method to quantify UDCA, CDCA and LCA was developed for the first time. It complies with USP related substances limits and validation parameters. v These methods are suitable for quality control in raw material as well as pharmaceutical formulations.


v 7 th Pharmacovigilance & Pharmaceutical Industry Conference. v Especially, Isaac Salvatore. Conference Organizer, Pharmacovigilance 2016. v. University of Buenos Aires. v. CONICET THANK YOU VERY MUCH VIELEN DANK MUCHAS GRACIAS TANTE GRAZIE

Let us meet again. . We welcome you to our future conferences of Conference Series LLC through 9 th International Conference and Exhibition on Pharmacovigilance July 17 -19, 2017 Munich, Germany http: //pharmacovigilance. pharmaceuticalconferences. com/
 Organising a seminar
Organising a seminar Star conferences inc
Star conferences inc As a result of the yalta and potsdam conferences, ________.
As a result of the yalta and potsdam conferences, ________. Meetings incentives conventions and exhibitions (mice)
Meetings incentives conventions and exhibitions (mice) Round table conference 1930-32
Round table conference 1930-32 Balmer series lyman series
Balmer series lyman series Series-shunt feedback amplifier examples
Series-shunt feedback amplifier examples Taylor vs maclaurin
Taylor vs maclaurin Series aiding and series opposing
Series aiding and series opposing Taylor frederick
Taylor frederick Sum of infinite series formula
Sum of infinite series formula Maclaurin series vs taylor series
Maclaurin series vs taylor series Ibm p series models
Ibm p series models Huawei tech (uae) fz-llc 1
Huawei tech (uae) fz-llc 1 Grizzly energy, llc
Grizzly energy, llc The nielsen company us llc
The nielsen company us llc Frank & delaney immigration law, llc
Frank & delaney immigration law, llc Fluidity operations llc
Fluidity operations llc Bsl placement pvt ltd contact number
Bsl placement pvt ltd contact number Teejan trading & contracting
Teejan trading & contracting Steinway & sons steinway asia llc.
Steinway & sons steinway asia llc. Tempur pedix
Tempur pedix Baker & mckenzie global services llc
Baker & mckenzie global services llc Trimed billing solutions llc
Trimed billing solutions llc Reconnaissance strike group
Reconnaissance strike group Globalink logistics dwc-llc
Globalink logistics dwc-llc Llc logical link control
Llc logical link control Minban llc
Minban llc Psc group llc
Psc group llc Simplify compliance llc
Simplify compliance llc Msci esg research
Msci esg research Pathstone federal street
Pathstone federal street Utku büyükşahin
Utku büyükşahin Grizzly energy llc
Grizzly energy llc Opusing llc
Opusing llc