Conference Series LLC Conferences Conference Series LLC is













- Slides: 27

Conference Series LLC Conferences Conference Series LLC is a pioneer and leading science event organizer, which publishes around 500 open access journals and conducts over 500 Medical, Clinical, Engineering, Life Sciences, Pharma scientific conferences all over the globe annually with the support of more than 1000 scientific associations and 30, 000 editorial board members and 3. 5 million followers to its credit. Conference Series LLC has organized 500 conferences, workshops and national symposiums across the major cities including San Francisco, Las Vegas, San Antonio, Omaha, Orlando, Raleigh, Santa Clara, Chicago, Philadelphia, Baltimore, United Kingdom, Valencia, Dubai, Beijing, Hyderabad, Bengaluru and Mumbai.

5 th European Biosimilars Congress Valencia, Spain, 27 -29 June 2016 Intellectual Property Issues in Global Biosimilar Programs Christoph Volpers, Ph. D, MBA 2

Disclaimer The statements and views expressed in this presentation are those of the author and do not necessarily represent the views or opinions of Michalski Hüttermann & Partners, their staff or their clients. The author does not assume any legal responsibility or liability for information, statements or opinions expressed in this presentation, albeit they are accurate to his best knowledge and belief. None of the information, data, statements or opinions contained or expressed in this presentation are meant or to be construed as regulatory, legal or patent legal advice. 5 th European Biosimilars Congress © 2016 Christoph Volpers, Ph. D, MBA 3

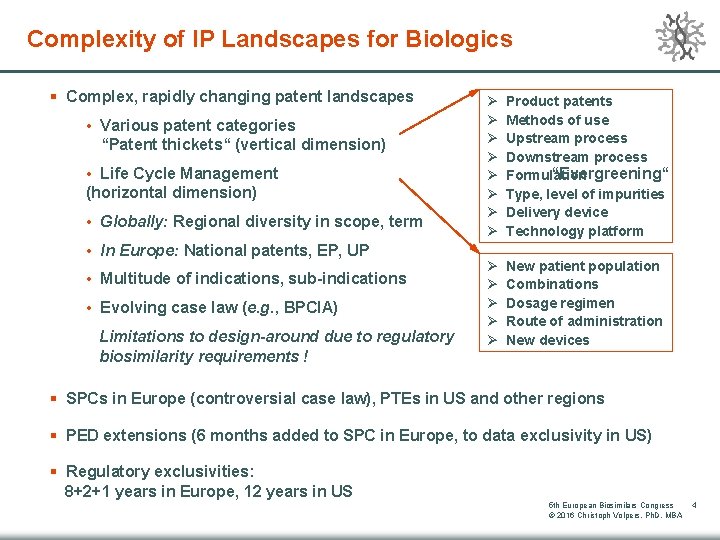
Complexity of IP Landscapes for Biologics § Complex, rapidly changing patent landscapes • Various patent categories P. “Patent thickets“ (vertical dimension) • Life Cycle Management (horizontal dimension) • Globally: Regional diversity in scope, term • In Europe: National patents, EP, UP • Multitude of indications, sub-indications • Evolving case law (e. g. , BPCIA) Limitations to design-around due to regulatory biosimilarity requirements ! Ø Ø Ø Ø Product patents Methods of use Upstream process Downstream process P. “Evergreening“ Formulation Type, level of impurities Delivery device Technology platform Ø Ø Ø New patient population Combinations Dosage regimen Route of administration New devices § SPCs in Europe (controversial case law), PTEs in US and other regions § PED extensions (6 months added to SPC in Europe, to data exclusivity in US) § Regulatory exclusivities: 8+2+1 years in Europe, 12 years in US 5 th European Biosimilars Congress © 2016 Christoph Volpers, Ph. D, MBA 4

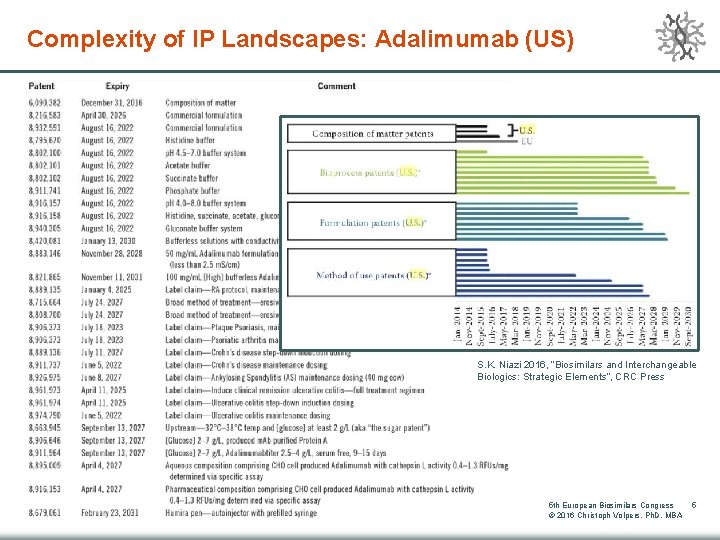
Complexity of IP Landscapes: Adalimumab (US) S. K. Niazi 2016, “Biosimilars and Interchangeable Biologics: Strategic Elements“, CRC Press 5 th European Biosimilars Congress © 2016 Christoph Volpers, Ph. D, MBA 5

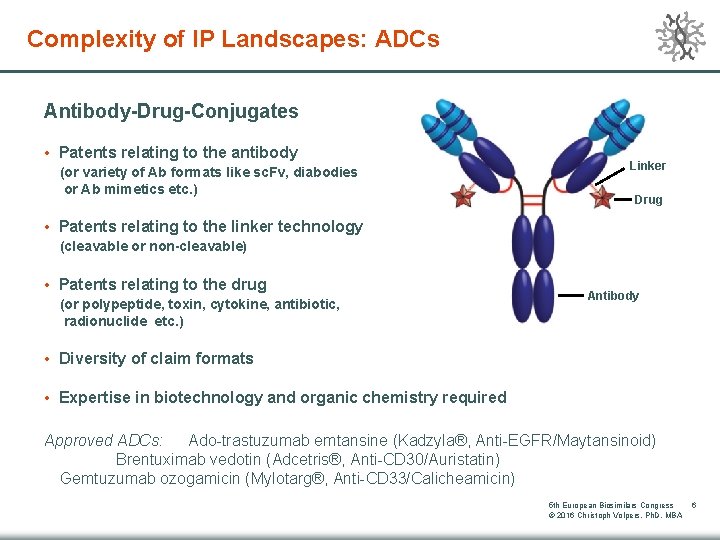
Complexity of IP Landscapes: ADCs Antibody-Drug-Conjugates • Patents relating to the antibody (or variety of Ab formats like sc. Fv, diabodies or Ab mimetics etc. ) Linker Drug • Patents relating to the linker technology (cleavable or non-cleavable) • Patents relating to the drug (or polypeptide, toxin, cytokine, antibiotic, radionuclide etc. ) Antibody • Diversity of claim formats • Expertise in biotechnology and organic chemistry required Approved ADCs: Ado-trastuzumab emtansine (Kadzyla®, Anti-EGFR/Maytansinoid) Brentuximab vedotin (Adcetris®, Anti-CD 30/Auristatin) Gemtuzumab ozogamicin (Mylotarg®, Anti-CD 33/Calicheamicin) 5 th European Biosimilars Congress © 2016 Christoph Volpers, Ph. D, MBA 6

Third Wave Biosimilars and Beyond: IP Issues Increasing number of originator products in development or regulatory approval or market phase: selection of best targets more relevant but difficult Ø Need for comprehensive, integrated FTO analysis, Requirement for more standardized, multi-factor IP risk assessment (in order to allow for optimized resource allocation) Higher complexity of products (e. g. , ADCs, glycoengineered products) Ø Platform patents might become increasingly relevant barriers (e. g. , drug conjugation, glycoengineering, antibody derivatization) Advanced biosimilar development skills, possibly accelerated timelines, start of biosimilar projects earlier in relation to reference product lifecycle Ø SPCs as market entry barriers might play even more important role 5 th European Biosimilars Congress © 2016 Christoph Volpers, Ph. D, MBA 7

Third Wave Biosimilars and Beyond: IP Issues Increasing number of originator products in development or regulatory approval or market phase: selection of best targets more relevant but difficult Ø Need for comprehensive, integrated FTO analysis, Requirement for more standardized, multi-factor IP risk assessment (in order to allow for optimized resource allocation) Higher complexity of products (e. g. , ADCs, glycoengineered products) Ø Platform patents might become increasingly relevant barriers (e. g. , drug conjugation, glycoengineering, antibody derivatization) Advanced biosimilar development skills, possibly accelerated timelines, start of biosimilar projects earlier in relation to reference product lifecycle Ø SPCs as market entry barriers might play even more important role 5 th European Biosimilars Congress © 2016 Christoph Volpers, Ph. D, MBA 8

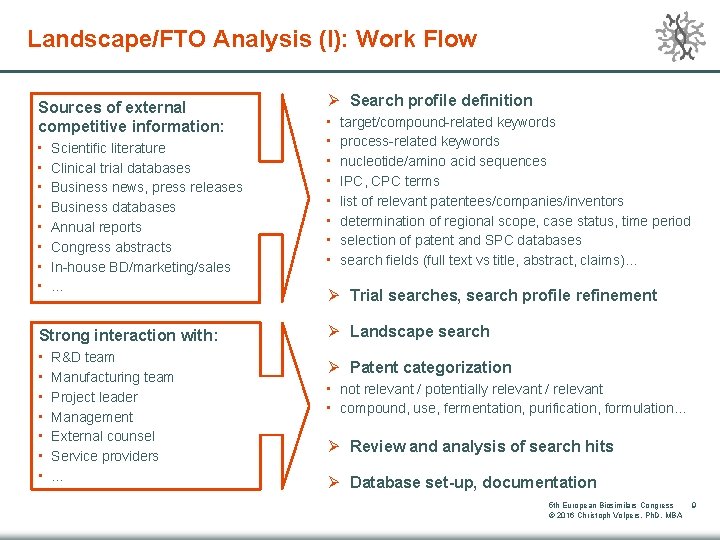
Landscape/FTO Analysis (I): Work Flow Sources of external competitive information: • • Scientific literature Clinical trial databases Business news, press releases Business databases Annual reports Congress abstracts In-house BD/marketing/sales … Strong interaction with: • • R&D team Manufacturing team Project leader Management External counsel Service providers … Ø Search profile definition • • target/compound-related keywords process-related keywords nucleotide/amino acid sequences IPC, CPC terms list of relevant patentees/companies/inventors determination of regional scope, case status, time period selection of patent and SPC databases search fields (full text vs title, abstract, claims)… Ø Trial searches, search profile refinement Ø Landscape search Ø Patent categorization • not relevant / potentially relevant / relevant • compound, use, fermentation, purification, formulation… Ø Review and analysis of search hits Ø Database set-up, documentation 5 th European Biosimilars Congress © 2016 Christoph Volpers, Ph. D, MBA 9

Landscape/FTO Analysis (II): Regular Update Ø Periodic search updates for new publications (including divisionals, continuations etc. ), adjustment of search profile as necessary Ø Monitoring of legal status of patent applications Ø Evaluation of recently granted patents 5 th European Biosimilars Congress 10 © 2016 Christoph Volpers, Ph. D, MBA

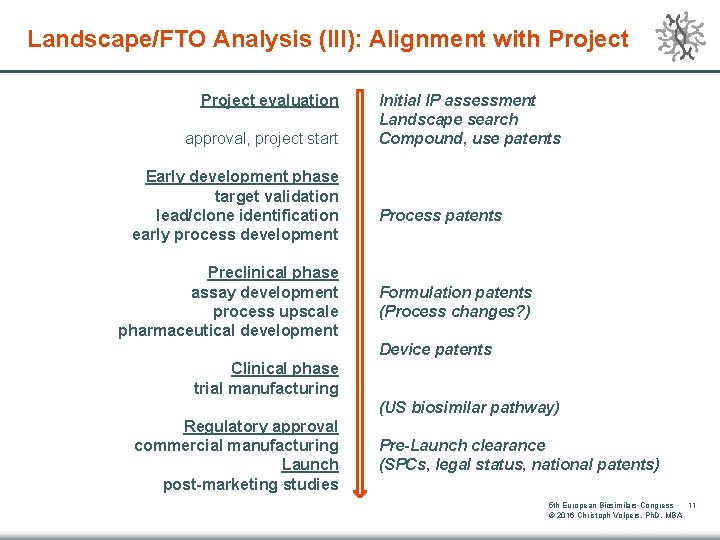
Landscape/FTO Analysis (III): Alignment with Project evaluation approval, project start Early development phase target validation lead/clone identification early process development Preclinical phase assay development process upscale pharmaceutical development Initial IP assessment Landscape search Compound, use patents Process patents Formulation patents (Process changes? ) Device patents Clinical phase trial manufacturing (US biosimilar pathway) Regulatory approval commercial manufacturing Launch post-marketing studies Pre-Launch clearance (SPCs, legal status, national patents) 5 th European Biosimilars Congress 11 © 2016 Christoph Volpers, Ph. D, MBA

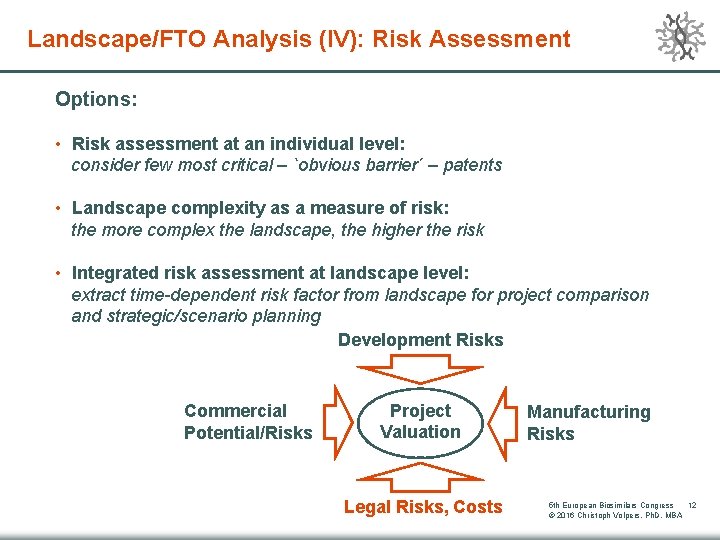
Landscape/FTO Analysis (IV): Risk Assessment Options: • Risk assessment at an individual level: consider few most critical – `obvious barrier´ – patents • Landscape complexity as a measure of risk: the more complex the landscape, the higher the risk • Integrated risk assessment at landscape level: extract time-dependent risk factor from landscape for project comparison and strategic/scenario planning Development Risks Commercial Potential/Risks Project Valuation Legal Risks, Costs Manufacturing Risks 5 th European Biosimilars Congress 12 © 2016 Christoph Volpers, Ph. D, MBA

Landscape/FTO Analysis (IV): Risk Assessment Probability/risk that… …international application will be regionalized (in relevant territories) …third party observation could influence prosecution …broad critical claims of application will get granted …patent can be circumvented …license might be obtained …patent (or patent owner) could be acquired …granted patent can be expected to be enforced (patentee, licensee; territory) …opposition will be (partly) successful (strength of invalidity arguments) …national nullification action could be successful …preliminary injunction is granted (in respective territory) …infringement trial on the merits (non-infringement, invalidity arguments) Standardized evaluation of each particular case (as far as feasible) Empirical data Statistics for respective patent system/jurisdiction 5 th European Biosimilars Congress 13 © 2016 Christoph Volpers, Ph. D, MBA

Landscape/FTO Analysis (IV): Risk Assessment Opler et al. 2014 Valuation Analysis in Pharmaceutical Licensing and M&A Transactions 5 th European Biosimilars Congress 14 © 2016 Christoph Volpers, Ph. D, MBA

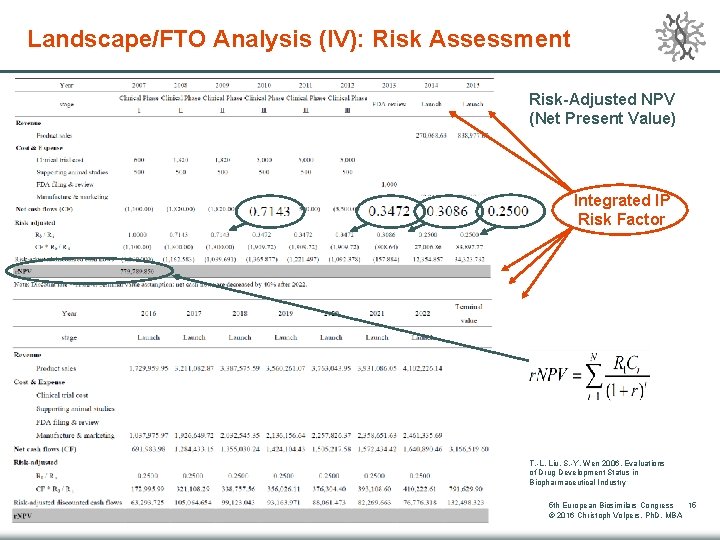
Landscape/FTO Analysis (IV): Risk Assessment Risk-Adjusted NPV (Net Present Value) Integrated l. P Risk Factor T. -L. Liu, S. -Y. Wen 2006, Evaluations of Drug Development Status in Biopharmaceutical Industry 5 th European Biosimilars Congress 15 © 2016 Christoph Volpers, Ph. D, MBA

Third Wave Biosimilars and Beyond: IP Issues Increasing number of originator products in development or regulatory approval or market phase: selection of best targets more relevant but difficult Ø Need for comprehensive, integrated FTO analysis, Requirement for more standardized, multi-factor IP risk assessment (in order to allow for optimized resource allocation) Higher complexity of products (e. g. , ADCs, glycoengineered products) Ø Platform patents might become increasingly relevant barriers (e. g. , drug conjugation, glycoengineering, antibody derivatization) Advanced biosimilar development skills, possibly accelerated timelines, start of biosimilar projects earlier in relation to reference product lifecycle Ø SPCs as market entry barriers might play even more important role 5 th European Biosimilars Congress 16 © 2016 Christoph Volpers, Ph. D, MBA

Platform Patents: Upstream / Cell Culture EP 2 041 270 B 1 (Claim 1) 5 th European Biosimilars Congress 17 © 2016 Christoph Volpers, Ph. D, MBA

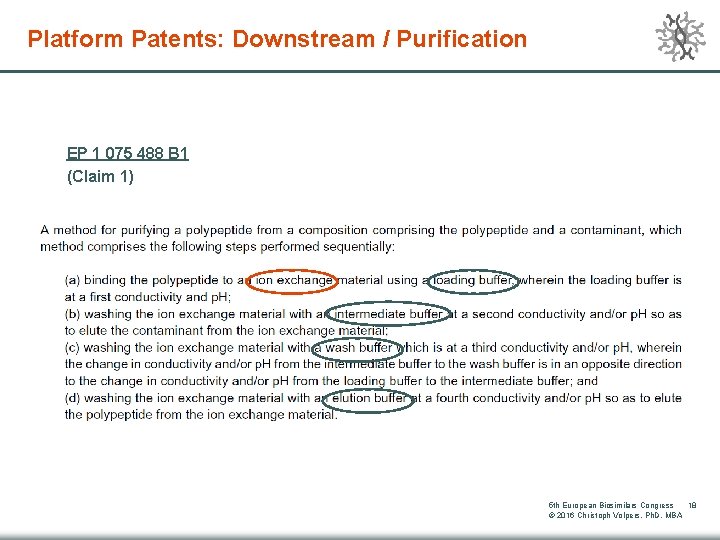
Platform Patents: Downstream / Purification EP 1 075 488 B 1 (Claim 1) 5 th European Biosimilars Congress 18 © 2016 Christoph Volpers, Ph. D, MBA

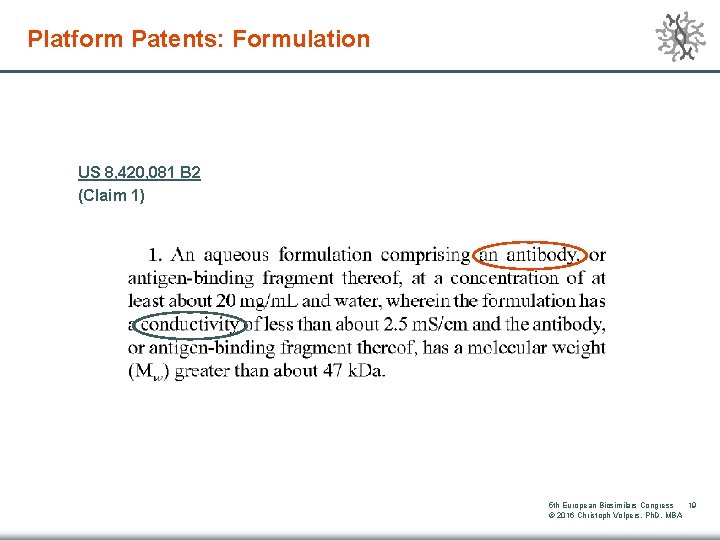
Platform Patents: Formulation US 8, 420, 081 B 2 (Claim 1) 5 th European Biosimilars Congress 19 © 2016 Christoph Volpers, Ph. D, MBA

Platform Patents: Options from Biosimilar Perspective Ø Third Party Observation (EP, PCT) Ø Opposition (EP), Post-Grant Review (US) Ø Inter Partes Review (US) Ø Nullity Action (national level for EP, in the future: centralized level for (EP and) UP) Ø Early/Late Stage Litigation according to BPCIA (US) Ø Circumvention if possible Ø (Cross-)Licensing if possible 5 th European Biosimilars Congress 20 © 2016 Christoph Volpers, Ph. D, MBA

Third Wave Biosimilars and Beyond: IP Issues Increasing number of originator products in development or regulatory approval or market phase: selection of best targets more relevant but difficult Ø Need for comprehensive, integrated FTO analysis, Requirement for more standardized, multi-factor IP risk assessment (in order to allow for optimized resource allocation) Higher complexity of products (e. g. , ADCs, glycoengineered products) Ø Platform patents might become increasingly relevant barriers (e. g. , drug conjugation, glycoengineering, antibody derivatization) Advanced biosimilar development skills, possibly accelerated timelines, start of biosimilar projects earlier in relation to reference product lifecycle Ø SPCs as market entry barriers might play even more important role 5 th European Biosimilars Congress 21 © 2016 Christoph Volpers, Ph. D, MBA

SPC: Supplementary Protection Certificate • IP right sui generis that enters into force after expiry of its basic patent • intended to compensate for the long time period needed to obtain regulatory approval • available for human and veterinary medicinal products and plant protection products (e. g. , insecticides, herbicides) • term = date of first MA in EU – date of filing of basic patent – 5 years but: max. 5 years (plus 6 months for pediatric extension) • applied for and granted on national level • `recalibration´ of the SPC regulatory framework under Unitary Patent system is being considered 5 th European Biosimilars Congress 22 © 2016 Christoph Volpers, Ph. D, MBA

SPC Regulation (EC) 469/2009 (6 May 2009) Article 1 Definitions (b) `product´ means the active ingredient or combination of active ingredients of a medicinal product (c) `basic patent´ means a patent which protects a product as such, a process to obtain a product or an application of a product, and which is designated by its holder for the purpose of the procedure for grant of a certificate Article 3 Conditions for obtaining a certificate A certificate shall be granted if, in the Member State in which the application referred to in Article 7 is submitted and at the date of that application: (a) the product is protected by a basic patent in force; (b) a valid authorization to place the product on the market as a medicinal product has been granted in accordance with Directive 2001/83/EC or Directive 2001/82/EC, as appropriate; Article 4 Subject matter of protection Within the limits of the protection conferred by the basic patent, the protection conferred by a certificate shall extend only to the product covered by the authorization to place the corresponding medicinal product on the market and for any use of the product as a medicinal product that has been authorized before the expiry of the certificate. (c) the product has not already been the subject of a certificate; (d) the authorization referred to in point (b) is the first authorization to place the product on the market as a medicinal product. 5 th European Biosimilars Congress 23 © 2016 Christoph Volpers, Ph. D, MBA

SPC Case Law: Recent CJEU Decisions SPC can be granted for an active ingredient when… Ø …it is `specified in the wording of the claims of the basic patent´ (Medeva 2011, strict interpretation of disclosure test) Ø …it represents `core inventive advance´/constitutes the subject-matter of the invention of the basic patent (Actavis I, 2013; Actavis II, 2015) Ø …it is functionally defined in the basic patent if `the claims relate, implicitly but necessarily and specifically, to the API in question´ (Eli Lilly 2013) Ø …it is claimed as product-by-process in the basic patent (Queensland 2011) Ø …an SPC for different product (or combination) is based on same basic patent (Georgetown II, 2013, more than one SPC per patent) Ø …MA relates to more active ingredients A+B (Georgetown I, 2011; Daiichi Sankyo, 2011) Ø …it is a combination A+B and MA relates to more active ingredients A+B+C+D (Medeva 2011) Ø …it is a component A of a conjugate A-B to which the MA relates and has same therapeutic effect as provided for in MA (Forsgren 2015) Ø …MA has been granted to a third party (other than patentee) 5 th European Biosimilars Congress 24 © 2016 Christoph Volpers, Ph. D, MBA

Conclusions: Global Biosimilar IP Strategy § Comprehensive, diligent landscape/freedom-to-operate analysis for all relevant regions § Platform patents to be included § Regular updates, alignment with development activities § Risk assessment on individual and integrated level § Coordinated opposition/litigation strategy in various territories § Case law developments (e. g. , SPCs in Europe, BPCIA in US) 5 th European Biosimilars Congress 25 © 2016 Christoph Volpers, Ph. D, MBA

Michalski Hüttermann & Partner Patentanwälte mb. H Hafenspitze, Speditionstr. 21 40221 Düsseldorf, Germany Tel +49 -(0)211 -159249 -0 Fax +49 -(0)211 -159249 -20 Thank you for your attention ! 5 th European Biosimilars Congress 26 © 2016 Christoph Volpers, Ph. D, MBA

LET US MEET AGAIN. . We welcome you to our future conferences of Conference Series LLC through 6 th International Conference and Exhibition on Biologics and Biosimilars October 19 -21, 2016 Houston, TX, USA http: //biosimilarsbiologics. pharmaceuticalconferences. com/europe
 Meetings incentives conferences and exhibitions (mice)
Meetings incentives conferences and exhibitions (mice) Three round table conferences
Three round table conferences Organizing seminars and conferences
Organizing seminars and conferences Star conferences inc
Star conferences inc As a result of the yalta and potsdam conferences, ________.
As a result of the yalta and potsdam conferences, ________. Taylor frederick
Taylor frederick Arithmetic sequence sum formula
Arithmetic sequence sum formula Maclaurin series vs taylor series
Maclaurin series vs taylor series P series server
P series server Heisenberg 1925 paper
Heisenberg 1925 paper Series-series feedback amplifier examples
Series-series feedback amplifier examples Serie de taylor
Serie de taylor Series aiding and series opposing
Series aiding and series opposing Prudent group
Prudent group Gateway travel agency
Gateway travel agency Collaborative neuroscience research llc
Collaborative neuroscience research llc 1o1 pest control, llc
1o1 pest control, llc Hewitt human resources
Hewitt human resources Alliance for better care
Alliance for better care Industrial asset management llc
Industrial asset management llc Llc
Llc Wlaw llc
Wlaw llc American transmission company llc
American transmission company llc Woodfield pharmaceutical
Woodfield pharmaceutical Teepa snow's positive-approach
Teepa snow's positive-approach Peridot solutions llc
Peridot solutions llc I-plast
I-plast Dtelix
Dtelix