CHEMISTRY POWERPOINT SLIDESHOW Covering concepts in the Grade




- Slides: 38

CHEMISTRY POWERPOINT SLIDESHOW Covering concepts in the Grade 9 Science Program of Studies MATTER & CHEMICAL CHANGE Supporting Science Textbook Content while enriching the Learning Process in Junior High/Middle School

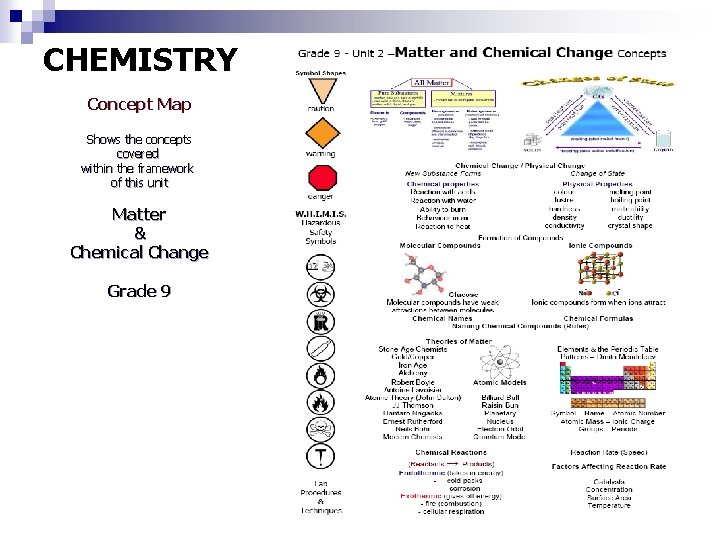
CHEMISTRY Concept Map Shows the concepts covered within the framework of this unit Matter & Chemical Change Grade 9

CHEMISTRY - Lab Safety First A good science lab is a safe one. All of the procedures, equipment and chemicals you use have been designed to help you understand the science principles you are investigating. Go over the safety notes provided using the Safety Power. Point or online @ edquest. ca and be prepared to take the safety test in class (do the practice test – linked to in the Power. Point - to help prepare you – the test in class will be slightly different). Notes: Access Safety Notes for viewing or printing @ http: //edquest. ca Practice Test: Access Safety Practice Test for viewing or printing @ http: //edquest. ca

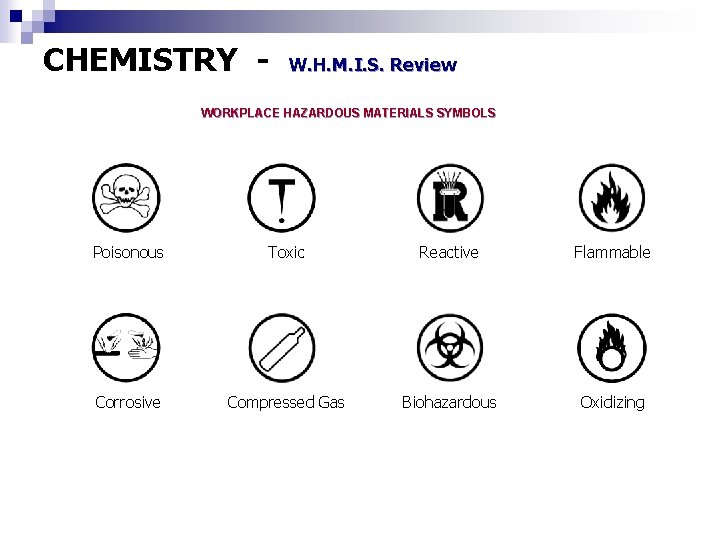
CHEMISTRY - W. H. M. I. S. Review WORKPLACE HAZARDOUS MATERIALS SYMBOLS Poisonous Toxic Reactive Flammable Corrosive Compressed Gas Biohazardous Oxidizing

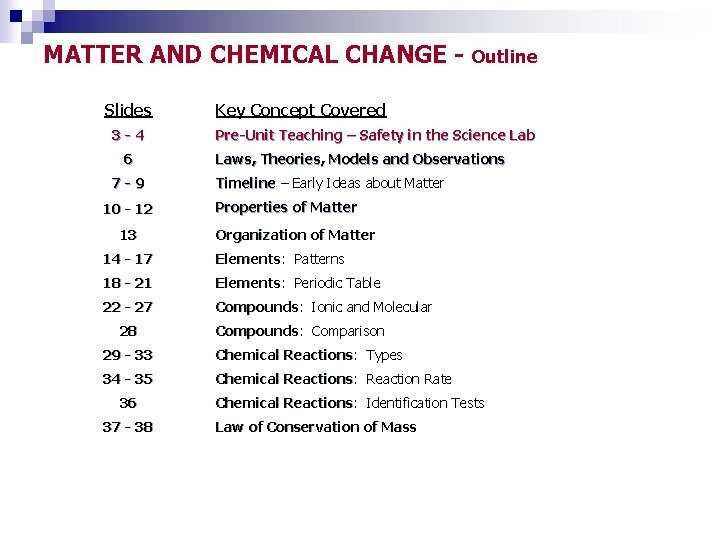
MATTER AND CHEMICAL CHANGE Slides 3 -4 6 7 -9 10 - 12 13 Key Concept Covered Pre-Unit Teaching – Safety in the Science Lab Laws, Theories, Models and Observations Timeline – Early Ideas about Matter Properties of Matter Organization of Matter 14 - 17 Elements: Elements Patterns 18 - 21 Elements: Elements Periodic Table 22 - 27 Compounds: Compounds Ionic and Molecular 28 Compounds: Compounds Comparison 29 - 33 Chemical Reactions: Reactions Types 34 - 35 Chemical Reactions: Reactions Reaction Rate 36 37 - 38 Outline Chemical Reactions: Reactions Identification Tests Law of Conservation of Mass

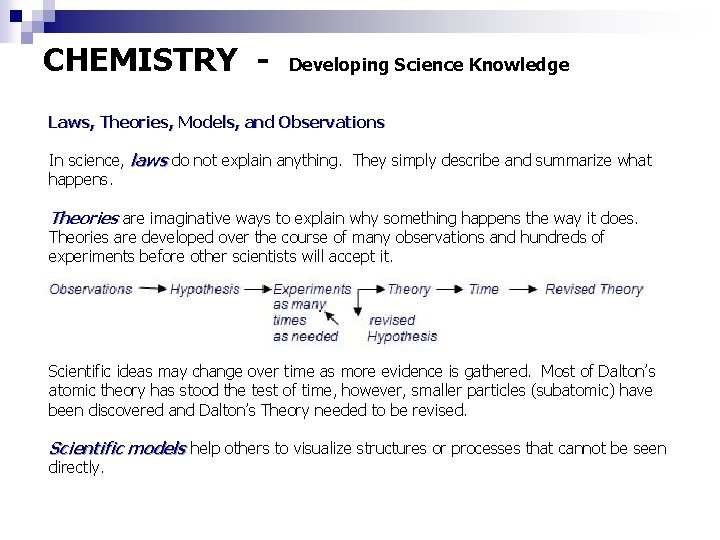
CHEMISTRY - Developing Science Knowledge Laws, Theories, Models, and Observations In science, laws do not explain anything. They simply describe and summarize what happens. Theories are imaginative ways to explain why something happens the way it does. Theories are developed over the course of many observations and hundreds of experiments before other scientists will accept it. Scientific ideas may change over time as more evidence is gathered. Most of Dalton’s atomic theory has stood the test of time, however, smaller particles (subatomic) have been discovered and Dalton’s Theory needed to be revised. Scientific models help others to visualize structures or processes that cannot be seen directly.

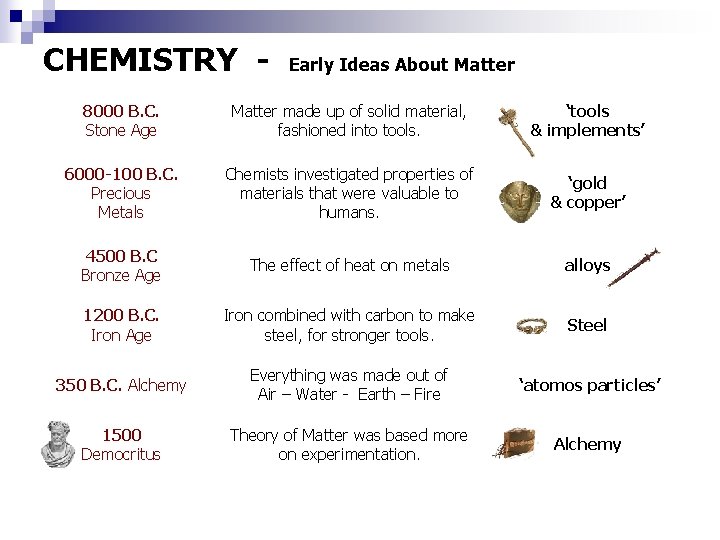
CHEMISTRY - Early Ideas About Matter 8000 B. C. Stone Age Matter made up of solid material, fashioned into tools. ‘tools & implements’ 6000 -100 B. C. Precious Metals Chemists investigated properties of materials that were valuable to humans. ‘gold & copper’ 4500 B. C Bronze Age The effect of heat on metals alloys 1200 B. C. Iron Age Iron combined with carbon to make steel, for stronger tools. Steel 350 B. C. Alchemy Everything was made out of Air – Water - Earth – Fire ‘atomos particles’ 1500 Democritus Theory of Matter was based more on experimentation. Alchemy

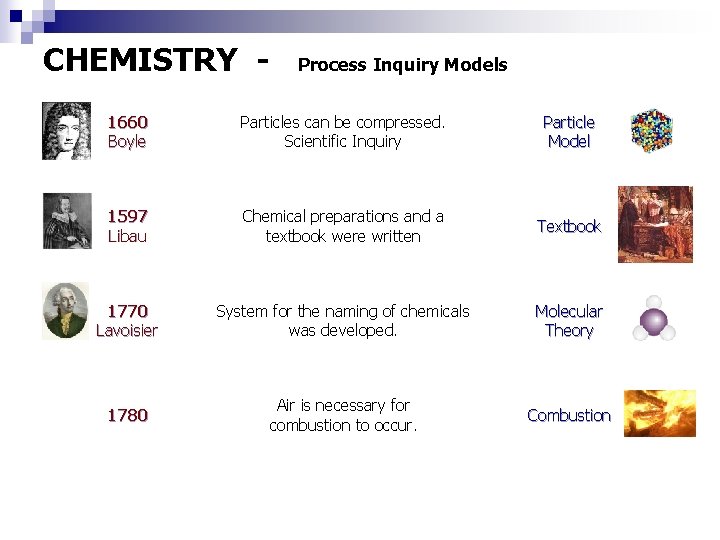
CHEMISTRY - Process Inquiry Models 1660 Boyle Particles can be compressed. Scientific Inquiry Particle Model 1597 Libau Chemical preparations and a textbook were written Textbook 1770 Lavoisier System for the naming of chemicals was developed. Molecular Theory 1780 Air is necessary for combustion to occur. Combustion

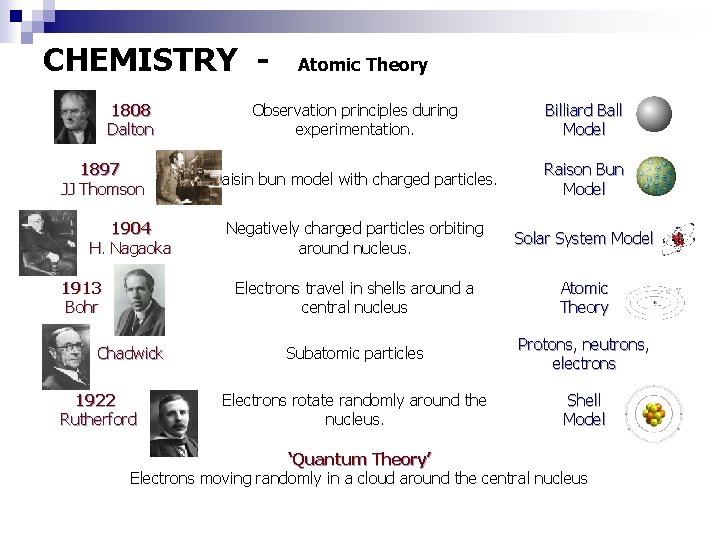
CHEMISTRY 1808 Dalton 1897 JJ Thomson 1904 H. Nagaoka 1913 Bohr Chadwick 1922 Rutherford Atomic Theory Observation principles during experimentation. Billiard Ball Model Raisin bun model with charged particles. Raison Bun Model Negatively charged particles orbiting around nucleus. Solar System Model Electrons travel in shells around a central nucleus Atomic Theory Subatomic particles Protons, neutrons, electrons Electrons rotate randomly around the nucleus. Shell Model ‘Quantum Theory’ Electrons moving randomly in a cloud around the central nucleus

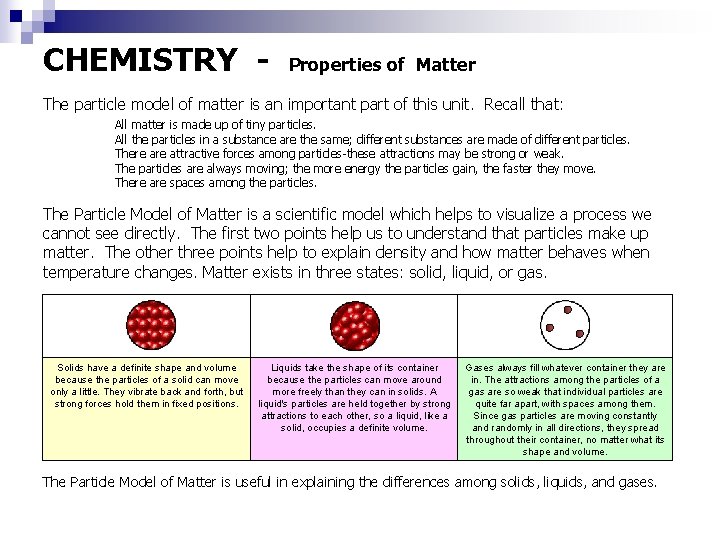
CHEMISTRY - Properties of Matter The particle model of matter is an important part of this unit. Recall that: All matter is made up of tiny particles. All the particles in a substance are the same; different substances are made of different particles. There attractive forces among particles-these attractions may be strong or weak. The particles are always moving; the more energy the particles gain, the faster they move. There are spaces among the particles. The Particle Model of Matter is a scientific model which helps to visualize a process we cannot see directly. The first two points help us to understand that particles make up matter. The other three points help to explain density and how matter behaves when temperature changes. Matter exists in three states: solid, liquid, or gas. Solids have a definite shape and volume because the particles of a solid can move only a little. They vibrate back and forth, but strong forces hold them in fixed positions. Liquids take the shape of its container because the particles can move around more freely than they can in solids. A liquid's particles are held together by strong attractions to each other, so a liquid, like a solid, occupies a definite volume. Gases always fill whatever container they are in. The attractions among the particles of a gas are so weak that individual particles are quite far apart, with spaces among them. Since gas particles are moving constantly and randomly in all directions, they spread throughout their container, no matter what its shape and volume. The Particle Model of Matter is useful in explaining the differences among solids, liquids, and gases.

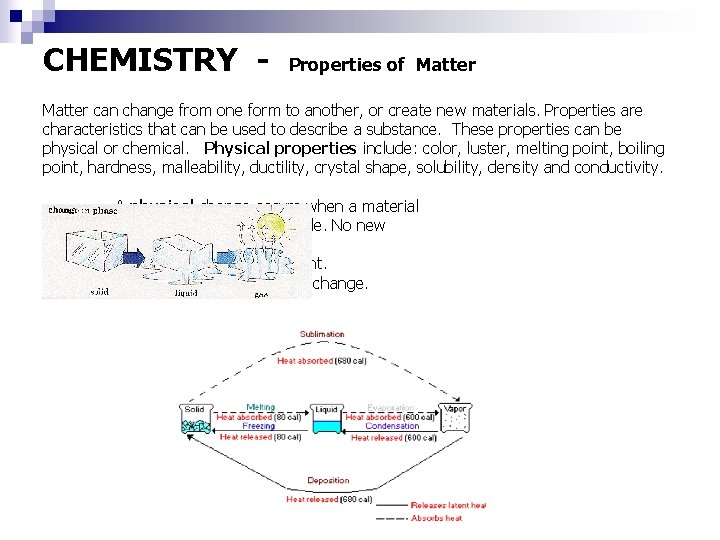
CHEMISTRY - Properties of Matter can change from one form to another, or create new materials. Properties are characteristics that can be used to describe a substance. These properties can be physical or chemical. Physical properties include: color, luster, melting point, boiling point, hardness, malleability, ductility, crystal shape, solubility, density and conductivity. A physical change occurs when a material changes state. It is reversible. No new substances are formed. The change is not permanent. Dissolving is also a physical change.

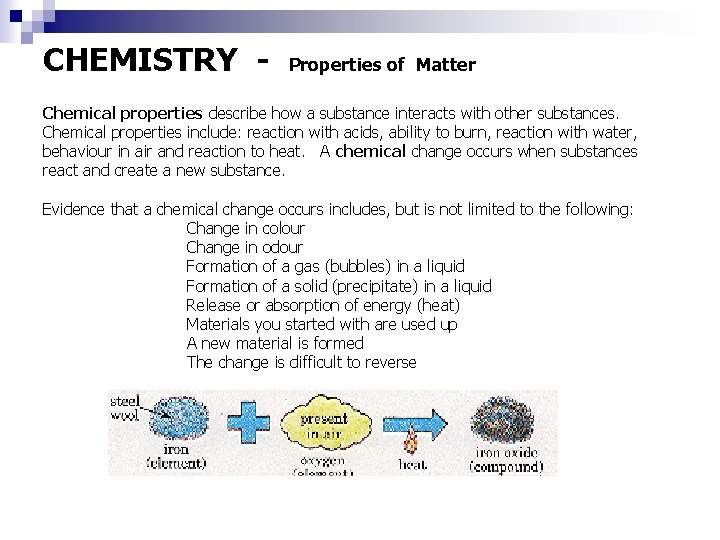
CHEMISTRY - Properties of Matter Chemical properties describe how a substance interacts with other substances. Chemical properties include: reaction with acids, ability to burn, reaction with water, behaviour in air and reaction to heat. A chemical change occurs when substances react and create a new substance. Evidence that a chemical change occurs includes, but is not limited to the following: Change in colour Change in odour Formation of a gas (bubbles) in a liquid Formation of a solid (precipitate) in a liquid Release or absorption of energy (heat) Materials you started with are used up A new material is formed The change is difficult to reverse

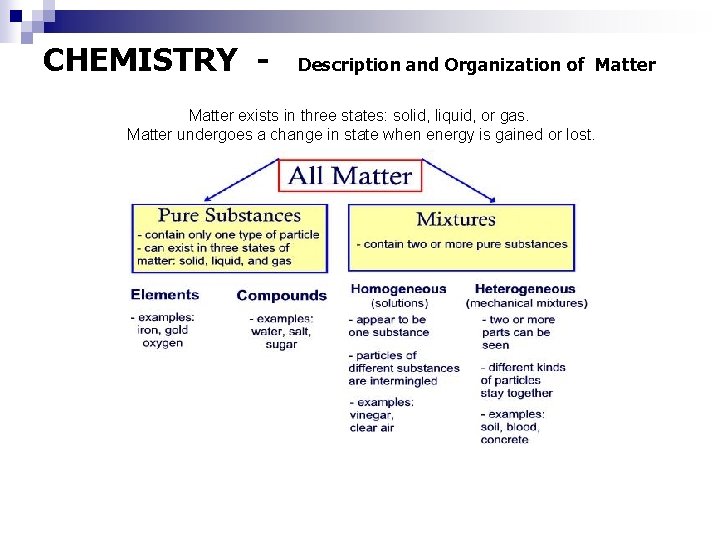
CHEMISTRY - Description and Organization of Matter exists in three states: solid, liquid, or gas. Matter undergoes a change in state when energy is gained or lost.

CHEMISTRY - Elements: Patterns and Order Finding a pattern and an order in an unknown helps scientists to organize ideas and information. It also helps them to interpret what the information means and explain these ideas, based on what they have learned – developing theories The original ‘elements’ were earth, air, fire and water Ancient Greek philosophers thought matter was made out of these four ‘elements’. They thought all things were made from these four elements with varying degrees of hotness, coldness, dryness and wetness. Alchemists (part pharmacist and part mystic) developed many useful procedures, including distillation, and they described the properties of many different materials. They also thought they could change lead and copper into gold. They used special symbols to prevent others from finding out their secrets. The current view of matter began with Sir Francis Bacon, Bacon who stated that all science should be based on experimental evidence, rather than thought. Robert Boyle recognized that elements could combine to form compounds. Bacon and Boyle motivated others to search for elements. Scientists began using heating, burning, mixing, and cooling to take matter down until it could not be broken down any further, to determine if a substance was a pure substance or a mixture.

CHEMISTRY - Elements: Patterns and Order Antoine Lavoisier defined elements as pure substances that could not be decomposed into simpler substances by means of a chemical change. In this way he identified 23 pure substances as elements. Lavoisier was one of the first chemists to use a balanced view of chemical change. Elements were then listed in order of their atomic mass. Atomic mass is the mass of one atom of an element. It is represented in atomic mass units (amu). Early chemists used symbols of the sun and the planets to identify the elements. This became a problem, when more elements were discovered than planets. John Dalton developed a new set of symbols in the early 1800’s to improve communication between chemists. Berzelius revised Dalton’s symbols by replacing them with letters, instead of pictures and representing each element by their first letter (capitalized), or their first two letters (first one capitalized and the second letter lower case).

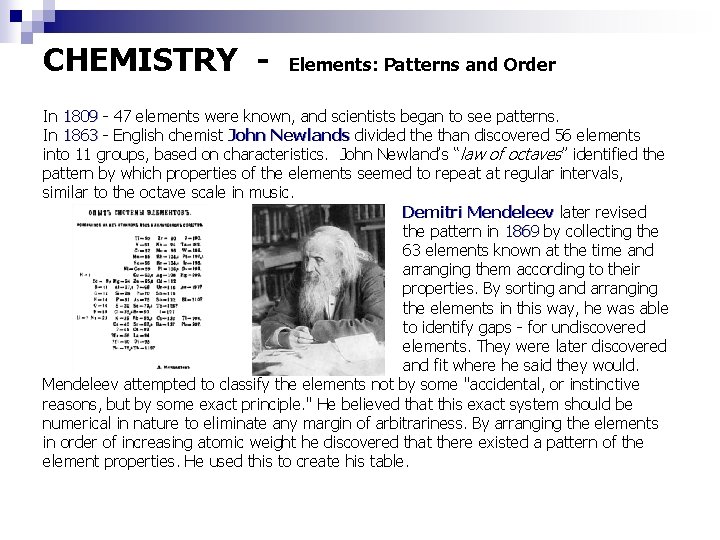
CHEMISTRY - Elements: Patterns and Order In 1809 - 47 elements were known, and scientists began to see patterns. In 1863 - English chemist John Newlands divided the than discovered 56 elements into 11 groups, based on characteristics. John Newland’s “law of octaves” identified the pattern by which properties of the elements seemed to repeat at regular intervals, similar to the octave scale in music. Demitri Mendeleev later revised the pattern in 1869 by collecting the 63 elements known at the time and arranging them according to their properties. By sorting and arranging the elements in this way, he was able to identify gaps - for undiscovered elements. They were later discovered and fit where he said they would. Mendeleev attempted to classify the elements not by some "accidental, or instinctive reasons, but by some exact principle. " He believed that this exact system should be numerical in nature to eliminate any margin of arbitrariness. By arranging the elements in order of increasing atomic weight he discovered that there existed a pattern of the element properties. He used this to create his table.

CHEMISTRY - Elements: Patterns and Order The Periodic Table continued to develop as new information about the elements was learned. 1886 - Ernest Rutherford named three types of radiation; alpha, beta and gamma rays. Marie and Pierre Curie started working on the radiation of uranium and thorium, and subsequently discovered radium and polonium. 1894 - Sir William Ramsay and Lord Rayleigh discovered the noble gases, which were added to the periodic table as group 0. 1897 - English physicist J. J. Thomson discovered electrons; small negatively charged particles in an atom. 1903 - Rutherford announced that radioactivity is caused by the breakdown of atoms. 1911 - Rutherford and German physicist Hans Geiger discovered that electrons orbit the nucleus of an atom. 1913 - Bohr discovered that electrons move around a nucleus in discrete energy called orbitals. Radiation is emitted during movement from one orbital to another. 1914 - Rutherford first identified protons in the atomic nucleus. 1932 - James Chadwick first discovered neutrons, and isotopes were identified. This was the complete basis for the periodic table. 1945 - Glenn Seaborg identified lanthanides and actinides (atomic number >92), which are usually placed below the periodic table.

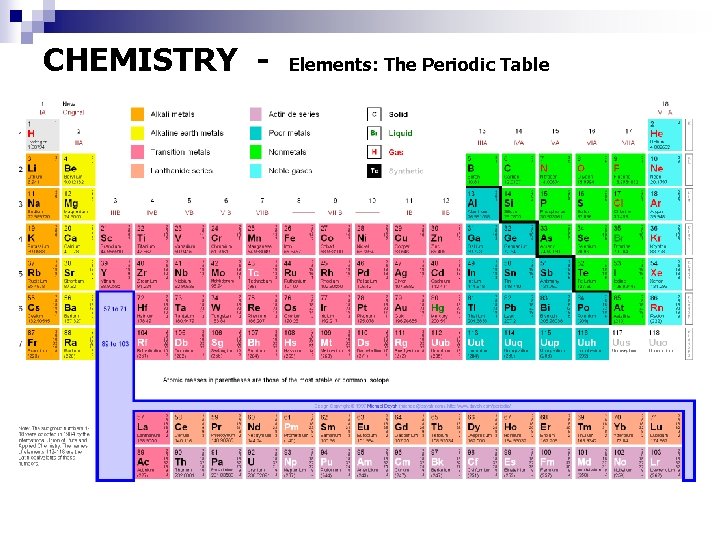
CHEMISTRY - Elements: The Periodic Table

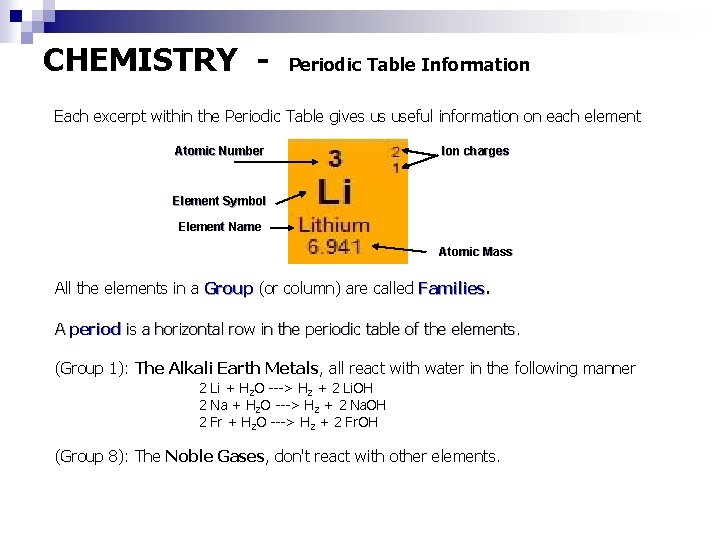
CHEMISTRY - Periodic Table Information Each excerpt within the Periodic Table gives us useful information on each element Atomic Number Ion charges Element Symbol Element Name Atomic Mass All the elements in a Group (or column) are called Families A period is a horizontal row in the periodic table of the elements. (Group 1): The Alkali Earth Metals, all react with water in the following manner 2 Li + H 2 O ---> H 2 + 2 Li. OH 2 Na + H 2 O ---> H 2 + 2 Na. OH 2 Fr + H 2 O ---> H 2 + 2 Fr. OH (Group 8): The Noble Gases, don't react with other elements.

CHEMISTRY - Periodic Table Groupings Group 1 - Alkali Metals – Sodium - softer than most metals, good heat conductors and can explode if exposed to water. Group 2 - Alkaline Earth Metals – Radium - extremely reactive, and aren't found freely in nature. Group 3 -6 -7 - Rare Earth Elements - There are 30 rare earth elements. - many are synthetic or man-made. Groups 3 -12 - Transition Metals - Iron, cobalt and nickel - the only elements known to produce a magnetic field. Groups 13 -15 - Other Metals - tin, aluminum and lead -solid with a high density Metalloids - have metal and non-metal properties. Some are semi-conductors, meaning they can carry an electrical charge under special conditions. They are great for computers and calculators.

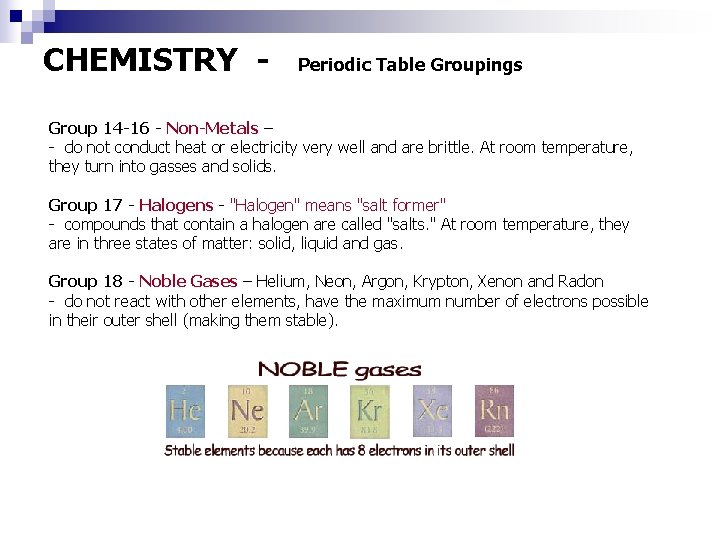
CHEMISTRY - Periodic Table Groupings Group 14 -16 - Non-Metals – - do not conduct heat or electricity very well and are brittle. At room temperature, they turn into gasses and solids. Group 17 - Halogens - "Halogen" means "salt former" - compounds that contain a halogen are called "salts. " At room temperature, they are in three states of matter: solid, liquid and gas. Group 18 - Noble Gases – Helium, Neon, Argon, Krypton, Xenon and Radon - do not react with other elements, have the maximum number of electrons possible in their outer shell (making them stable).

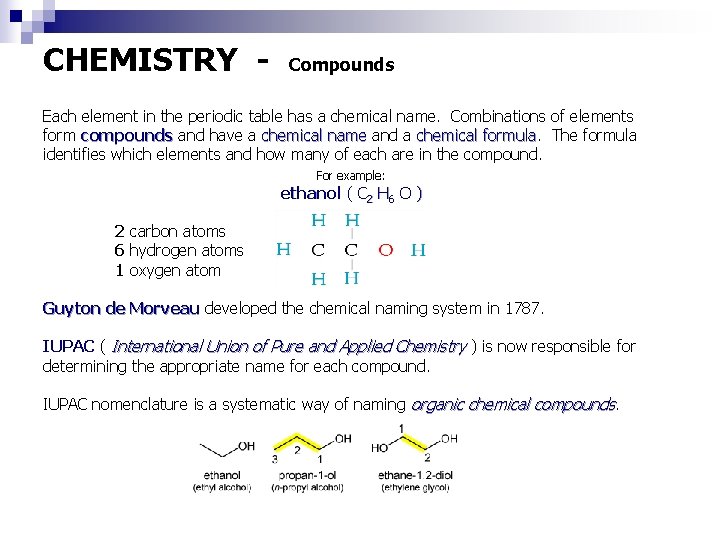
CHEMISTRY - Compounds Each element in the periodic table has a chemical name. Combinations of elements form compounds and have a chemical name and a chemical formula The formula identifies which elements and how many of each are in the compound. For example: ethanol ( C 2 H 6 O ) 2 carbon atoms 6 hydrogen atoms 1 oxygen atom Guyton de Morveau developed the chemical naming system in 1787. IUPAC ( International Union of Pure and Applied Chemistry ) is now responsible for determining the appropriate name for each compound. IUPAC nomenclature is a systematic way of naming organic chemical compounds.

CHEMISTRY - Compounds Molecular Compounds A molecule is the smallest independent unit of a pure substance. Diatomic molecules are molecules made up of 2 atoms of the same element (oxygen O 2, nitrogen N 2, hydrogen H 2). Most molecular compounds do not form large structures. Of the 10 million compounds discovered so far, about 9 million are molecular compounds. When non-metals combine, they produce a pure substance called a molecule, or molecular compound. They can be solids, liquids, or gases at room temperature. The bonding between atoms is strong, but the attraction between the molecules is weak. Examples: sugar ( C 12 H 22 O 11 (s) ), acetylene, water. Properties of molecular compounds include: low melting point, low boiling point, good insulators, poor conductors, distinct crystal shape A chemical formula tells how many of each type of atom is present in the molecule. A compound made from two elements is called a binary compound.

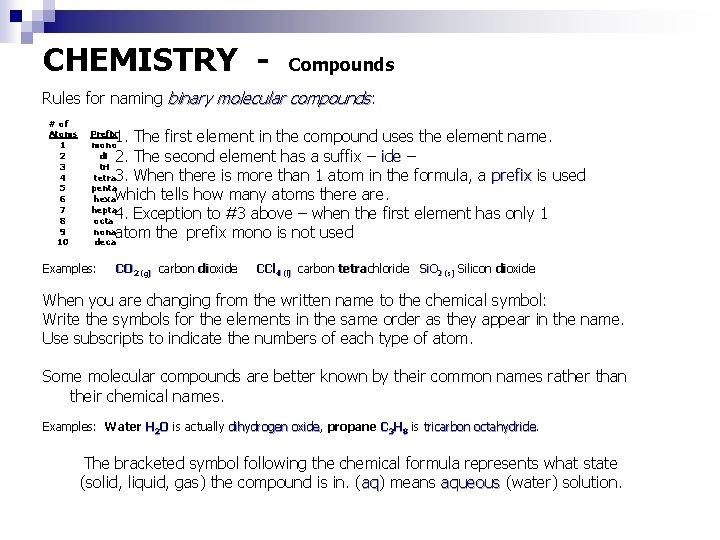
CHEMISTRY - Compounds Rules for naming binary molecular compounds: # of Atoms 1 2 3 4 5 6 7 8 9 10 1. The first element in the compound uses the element name. 2. The second element has a suffix – ide – 3. When there is more than 1 atom in the formula, a prefix is used which tells how many atoms there are. 4. Exception to #3 above – when the first element has only 1 atom the prefix mono is not used Prefix mono di tri tetra penta hexa hepta octa nona deca Examples: CO 2 (g) carbon dioxide di CCl 4 (l) carbon tetrachloride Si. O 2 (s) Silicon dioxide tetra di When you are changing from the written name to the chemical symbol: Write the symbols for the elements in the same order as they appear in the name. Use subscripts to indicate the numbers of each type of atom. Some molecular compounds are better known by their common names rather than their chemical names. Examples: Water H 2 O is actually dihydrogen oxide, oxide propane C 3 H 8 is tricarbon octahydride The bracketed symbol following the chemical formula represents what state (solid, liquid, gas) the compound is in. (aq) aq means aqueous (water) solution.

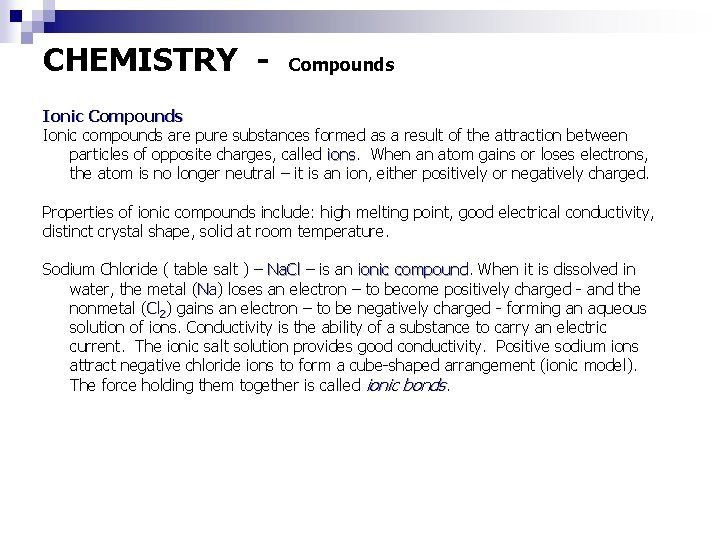
CHEMISTRY - Compounds Ionic compounds are pure substances formed as a result of the attraction between particles of opposite charges, called ions When an atom gains or loses electrons, the atom is no longer neutral – it is an ion, either positively or negatively charged. Properties of ionic compounds include: high melting point, good electrical conductivity, distinct crystal shape, solid at room temperature. Sodium Chloride ( table salt ) – Na. Cl – is an ionic compound When it is dissolved in water, the metal (Na) Na loses an electron – to become positively charged - and the nonmetal (Cl 2) gains an electron – to be negatively charged - forming an aqueous solution of ions. Conductivity is the ability of a substance to carry an electric current. The ionic salt solution provides good conductivity. Positive sodium ions attract negative chloride ions to form a cube-shaped arrangement (ionic model). The force holding them together is called ionic bonds.

CHEMISTRY - Rules for Naming Ionic Compounds 1. The chemical name of the metal or positive ion goes first, followed by the name of the non-metal or negative ion. 2. The name of the non-metal negative ion changes its ending to ide. Sodium Chloride Some ions are called polyatomic ions (meaning “many”). Polyatomic ions are a group of atoms acting as one. Example: calcium carbonate (limestone) “ate” and “ite” endings indicate polyatomic ions with oxygen. The “hypo…. ite” has one less oxygen than the “ite” for the same elements The “per…ate” has one more oxygen than the “ate” for the same elements. Hydroxide OH-1 Carbonate CO 2 -2 Phosphate PO 4 -3 Nitrite NO 2 -1 Ammonium NH 4+1 Sulfite SO 3 -2 Nitrate NO 3 -1 Sulfate SO 4 -2 Generally, elements in a group all have the same ion charge

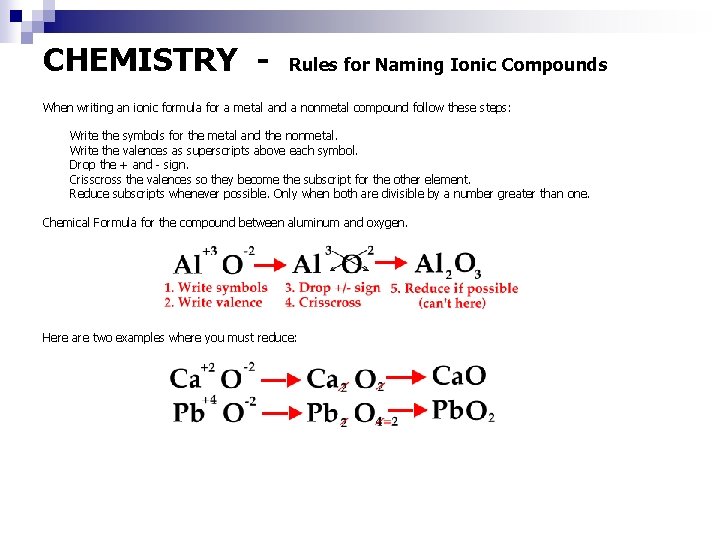
CHEMISTRY - Rules for Naming Ionic Compounds When writing an ionic formula for a metal and a nonmetal compound follow these steps: Write the symbols for the metal and the nonmetal. Write the valences as superscripts above each symbol. Drop the + and - sign. Crisscross the valences so they become the subscript for the other element. Reduce subscripts whenever possible. Only when both are divisible by a number greater than one. Chemical Formula for the compound between aluminum and oxygen. Here are two examples where you must reduce:

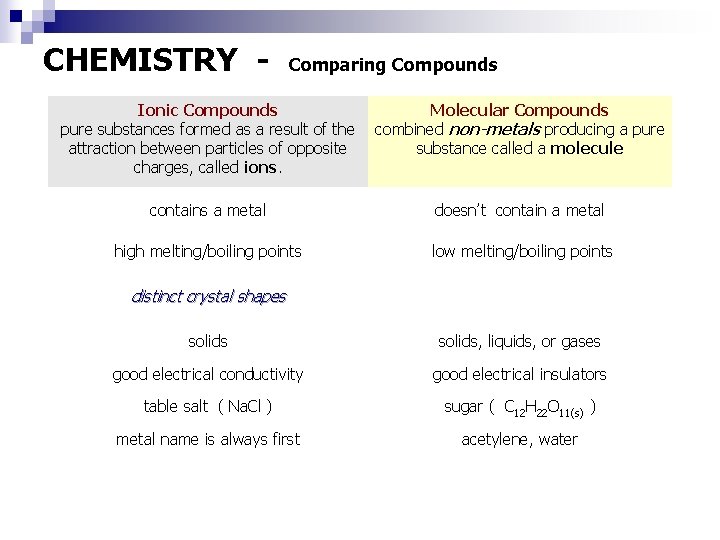
CHEMISTRY - Comparing Compounds Ionic Compounds pure substances formed as a result of the attraction between particles of opposite charges, called ions. Molecular Compounds combined non-metals producing a pure substance called a molecule contains a metal doesn’t contain a metal high melting/boiling points low melting/boiling points distinct crystal shapes solids, liquids, or gases good electrical conductivity good electrical insulators table salt ( Na. Cl ) sugar ( C 12 H 22 O 11(s) ) metal name is always first acetylene, water

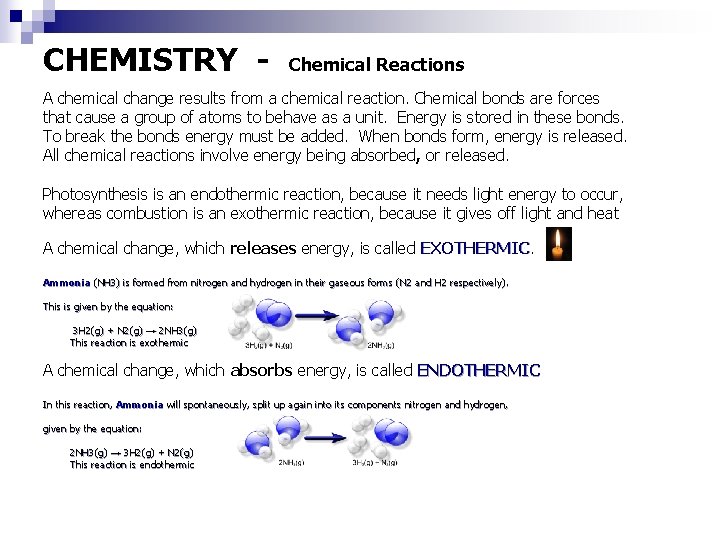
CHEMISTRY - Chemical Reactions A chemical change results from a chemical reaction. Chemical bonds are forces that cause a group of atoms to behave as a unit. Energy is stored in these bonds. To break the bonds energy must be added. When bonds form, energy is released. All chemical reactions involve energy being absorbed, or released. Photosynthesis is an endothermic reaction, because it needs light energy to occur, whereas combustion is an exothermic reaction, because it gives off light and heat A chemical change, which releases energy, is called EXOTHERMIC Ammonia (NH 3) is formed from nitrogen and hydrogen in their gaseous forms (N 2 and H 2 respectively). This is given by the equation: 3 H 2(g) + N 2(g) → 2 NH 3(g) This reaction is exothermic A chemical change, which absorbs energy, is called ENDOTHERMIC In this reaction, Ammonia will spontaneously, split up again into its components nitrogen and hydrogen, given by the equation: 2 NH 3(g) → 3 H 2(g) + N 2(g) This reaction is endothermic

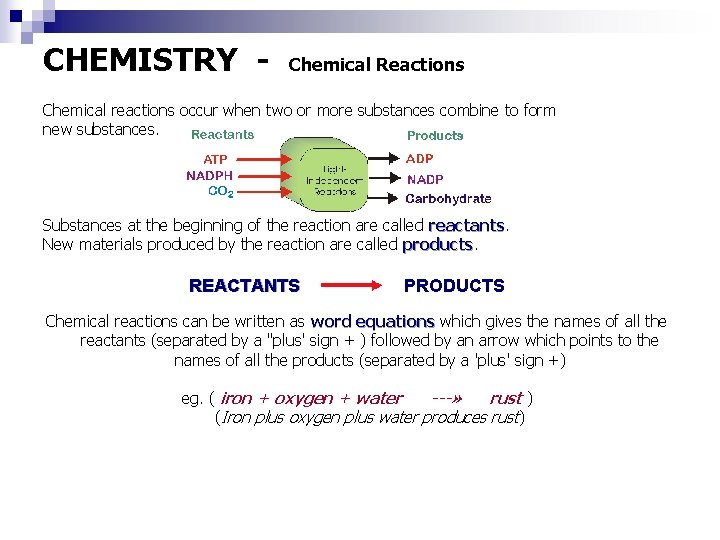
CHEMISTRY - Chemical Reactions Chemical reactions occur when two or more substances combine to form new substances. Substances at the beginning of the reaction are called reactants New materials produced by the reaction are called products REACTANTS PRODUCTS Chemical reactions can be written as word equations which gives the names of all the reactants (separated by a "plus' sign + ) followed by an arrow which points to the names of all the products (separated by a 'plus' sign +) eg. ( iron + oxygen + water ---» rust ) (Iron plus oxygen plus water produces rust)

CHEMISTRY - Chemical Reactions There are four types of chemical reactions: Combination (reactants combine) - Combustion is a chemical reaction that occurs when oxygen reacts with a substance to form a new substance and gives off energy. Combustion is the highly exothermic combination of a substance with oxygen. Combustion requires heat, oxygen, and fuel. The burning of propane ( C 3 H 8 ) in a barbeque is an exothermic reaction that produces heat to cook the food. If the heat is too intense, the products being cooked will be changed into pure carbon (the meat will be burnt). The products of combustion are not always beneficial. Burning fossil fuels (such as propane) produces carbon monoxide, carbon dioxide, sulfur oxides, nitrogen oxides, smoke, soot, ash and heat.

CHEMISTRY - Chemical Reactions Decomposition (reactants break down) - Corrosion is a slow chemical change that occurs when oxygen in the air reacts with a metal. Corrosion is a chemical reaction in which the metal is decomposed (eaten away), when it reacts with other substances in the environment. Preventing Corrosion protection (e. g. painting the metal) involves protecting metal from contact with the environment and the factors that affect the reaction rate of this chemical reaction. Coating a corrosive metal with a thin layer of zinc is called galvanization. The process of coating a corrosive metal with another metal through electrolysis is called electroplating.

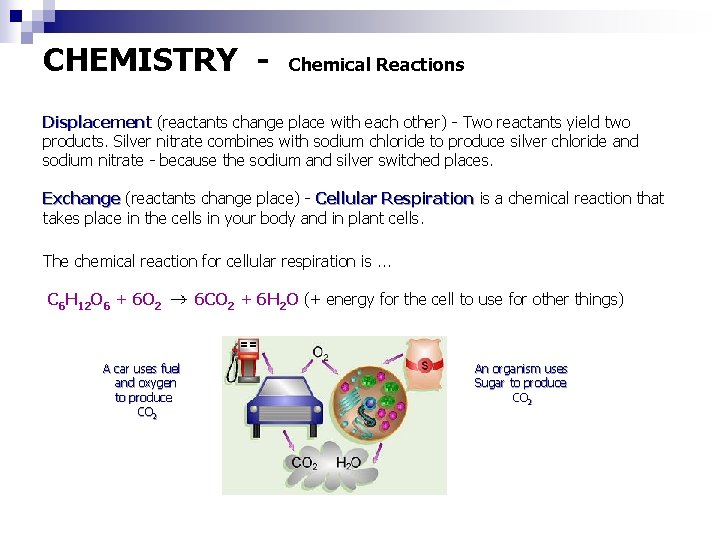
CHEMISTRY - Chemical Reactions Displacement (reactants change place with each other) - Two reactants yield two products. Silver nitrate combines with sodium chloride to produce silver chloride and sodium nitrate - because the sodium and silver switched places. Exchange (reactants change place) - Cellular Respiration is a chemical reaction that takes place in the cells in your body and in plant cells. The chemical reaction for cellular respiration is. . . C 6 H 12 O 6 + 6 O 2 A car uses fuel and oxygen to produce CO 2 6 CO 2 + 6 H 2 O (+ energy for the cell to use for other things) An organism uses Sugar to produce CO 2

CHEMISTRY - Reaction Rate The speed of a chemical reaction is called the reaction rate. Factors affecting the reaction rate include: v Temperature -The higher the temperature the faster the reaction rate v Surface Area -The more surface in contact, the faster the reaction rate v Concentration - The higher the concentration, the faster the reaction v Catalysts - The presence of a catalyst (substances that help a reaction proceed faster) also affects the reaction rate. Catalysts are not consumed in the reaction. Types of reactions involving catalysts can be found in living and non-living things. Enzymes are catalysts, present in the body, that speed up reactions which break down food. They also help to rid the body of poison. Enzymes are organic proteins known as amino acids and are found in all living things. They are Without enzymes, there would be no life. When there is a partial reduction of availability of enzymes, then life is reduced. They are "activists. " All activity of life depends on them. The greening of leaves in spring, the ripening of foods, the digestion and absorption of food, all require enzymes. Without enzymes, seeds could not sprout and the soil could not produce, therefore fruits and crops would not ripen or grow.

CHEMISTRY - Chemical Reactions Speeding Up a Reaction With Catalysts A catalyst is a substance that help a reaction proceed faster and are not consumed in the reaction. Types of reactions involving catalysts can be found in living and non-living things. Enzymes are natural catalysts that help in the reactions in the body, which break down food. They also get rid of poison in the body. Catalase (an enzyme found in plant and animal cells) speeds up the breaking down of hydrogen peroxide into harmless oxygen and water. Slowing Down a Reaction With Inhibitors are substances that slow down chemical reactions. Plants have natural inhibitors in their seeds to prevent germination until the right conditions are present. Inhibitors are added to foods to slow down their decomposition.

CHEMISTRY - Chemical Reaction - Identification Tests OXYGEN Light a wooden splint. Blow out the flame, allowing the splint to continue glowing. Hold the glowing splint in a small amount of the unknown gas. If the splint bursts into flame, then the gas being tested is oxygen. HYDROGEN Light a wooden splint. Hold the glowing splint in a small amount of the unknown gas. If you hear a "pop", then the gas being tested is Hydrogen. CARBON DIOXIDE The test for the presence of Carbon Dioxide uses limewater (a clear colorless solution of calcium hydroxide, or slaked lime). Bubble the unknown gas (carbon dioxide) through the limewater solution, or add a few drops of the limewater solution to the gas and swirl it around. If the limewater turns milky, the gas is Carbon Dioxide. A solid precipitate of calcium carbonate is formed. Calcium carbonate is chalk or limestone, and it is this, that makes the lime water cloudy. calcium hydroxide + carbon dioxide Ca(OH)2(aq) + CO 2(g) calcium carbonate + water. Ca. CO 3(s) + H 2 O(l)

CHEMISTRY - Law of Conservation of Mass In a chemical reaction, the total mass of the reactants, is always equal to the total mass of the products. This law goes well with the atomic theory Atoms (matter) are never created or destroyed In a chemical reaction the atoms and molecules are simply rearranged. This law of conservation of mass does not apply to nuclear reactions, because there is some loss of mass: mass is changed into energy. This was first suggested by Albert Einstein in his famous equation: E =MC 2 (E Is Energy, M is Mass, C 2 is a large number) A very tiny amount of mass is equal to a very large amount of energy In an open system some of the mass seems to disappear, when it is in the form of a gas.

CHEMISTRY - Law of Conservation of Mass Other scientists followed up on the law of conservation of mass by stating the … Law of Definite Composition Compounds are pure substances that contain two or more elements combined together in fixed (or definite) proportions. Water is an example of this law. Pure water always contains 11% Hydrogen and 89% Oxygen. Law of Multiple Proportions. . . states that the masses of one element, which combine with a fixed mass of the second element, are in a ratio of whole numbers. Pure substances have constant composition and properties. An unknown substance can be identified by measuring a property of the substance (eg. density) and compare it to known values of other substances. If the test property matches a known value, it is likely that substance, because each substance has its own distinguishing properties unique to that substance.
 Tommy's window slideshow prayer
Tommy's window slideshow prayer The tale of three trees slideshow
The tale of three trees slideshow Power point sekolah sabat
Power point sekolah sabat Hinata slideshow
Hinata slideshow Dust bowl slideshow
Dust bowl slideshow Dividing negative numbers
Dividing negative numbers Chapter 8 lord of flies summary
Chapter 8 lord of flies summary Farenheight 451 part 2 summary
Farenheight 451 part 2 summary Franck condon principle
Franck condon principle Slideshow
Slideshow Webnode stránky
Webnode stránky Probability slideshow
Probability slideshow Open house slideshow
Open house slideshow Which term is used to describe bitmap images?
Which term is used to describe bitmap images? Electricity grade 9 ppt
Electricity grade 9 ppt Jacob and esau slideshow
Jacob and esau slideshow 19032007 colour
19032007 colour Martin luther king slides
Martin luther king slides Introductory chemistry concepts and critical thinking
Introductory chemistry concepts and critical thinking Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Cigna not covering vitamin d testing
Cigna not covering vitamin d testing Cigna policy for cpt 82306
Cigna policy for cpt 82306 Continuous pyrolysis plant
Continuous pyrolysis plant Heart membranes
Heart membranes Sequential covering
Sequential covering Medulla of hair
Medulla of hair Letter of placing an order
Letter of placing an order Menenges
Menenges Thick clear jelly that helps give the eyeball its shape
Thick clear jelly that helps give the eyeball its shape Set covering machine
Set covering machine Signed statement for head covering
Signed statement for head covering To safely transport cargo use
To safely transport cargo use Animals in phylum chordata
Animals in phylum chordata Whats a covering letter
Whats a covering letter The scale structure covering the exterior of the hair
The scale structure covering the exterior of the hair Processus vaginalis anatomy
Processus vaginalis anatomy Covering tracks
Covering tracks Phylum chordata
Phylum chordata